Determination of Bio-Based Fertilizer Composition Using Combined NIR and MIR Spectroscopy: A Model Averaging Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Analysis
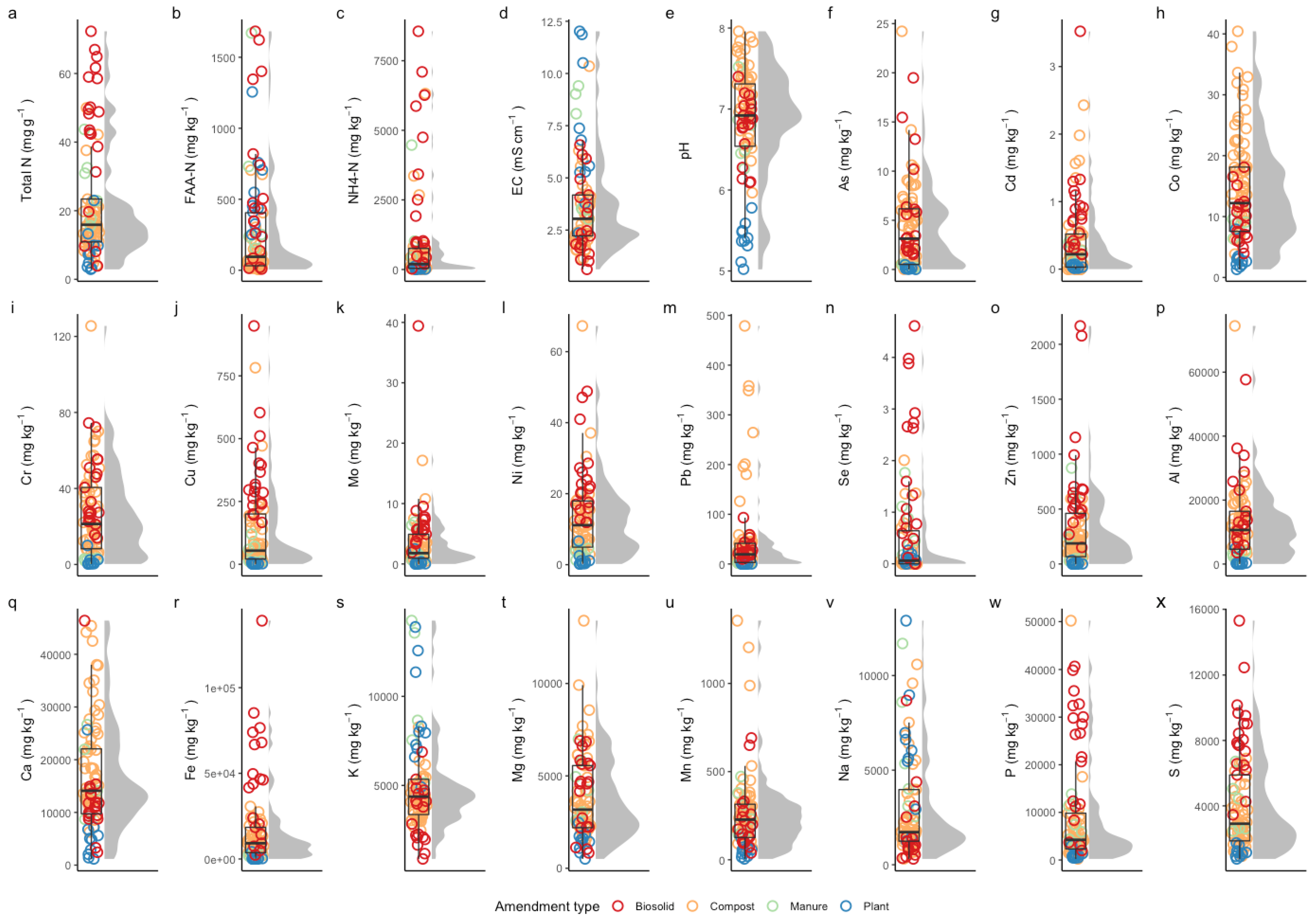
2.2. Sample Characterization
3. Model Development
3.1. Data Prepossessing
3.2. Optimal Wavelength Selection
| Algorithm 1 Wavelength selection algorithm |
| 1: Fit PLS model on NIR/MIR spectral data |
| 2: Find MSE of cross-validation (CV) |
| 3: Store MSE as MSE(0) for the start of the loop |
| 4: Find all the regression coefficients(B) |
| 5: Arrange B in ascending order |
| 6: Arrange spectra accordingly |
| 7: procedure wavelength selection() |
| 8: initialize i = 1 |
| 9: Discard one wavelength at time |
| 10: Fit PLS on remaining wavelengths |
| 11: Find MSE of CV |
| 12: if then |
| 13: Discard Wavelength |
| 14: i = i + 1 |
| 15: Repeat step 9 |
| 16: else |
| 17: Stop |
| 18: Print all the discarded wavelengths |
| 19: Print all the remaining wavelengths |
| 20: Selected wavelengths = remaining wavelengths |
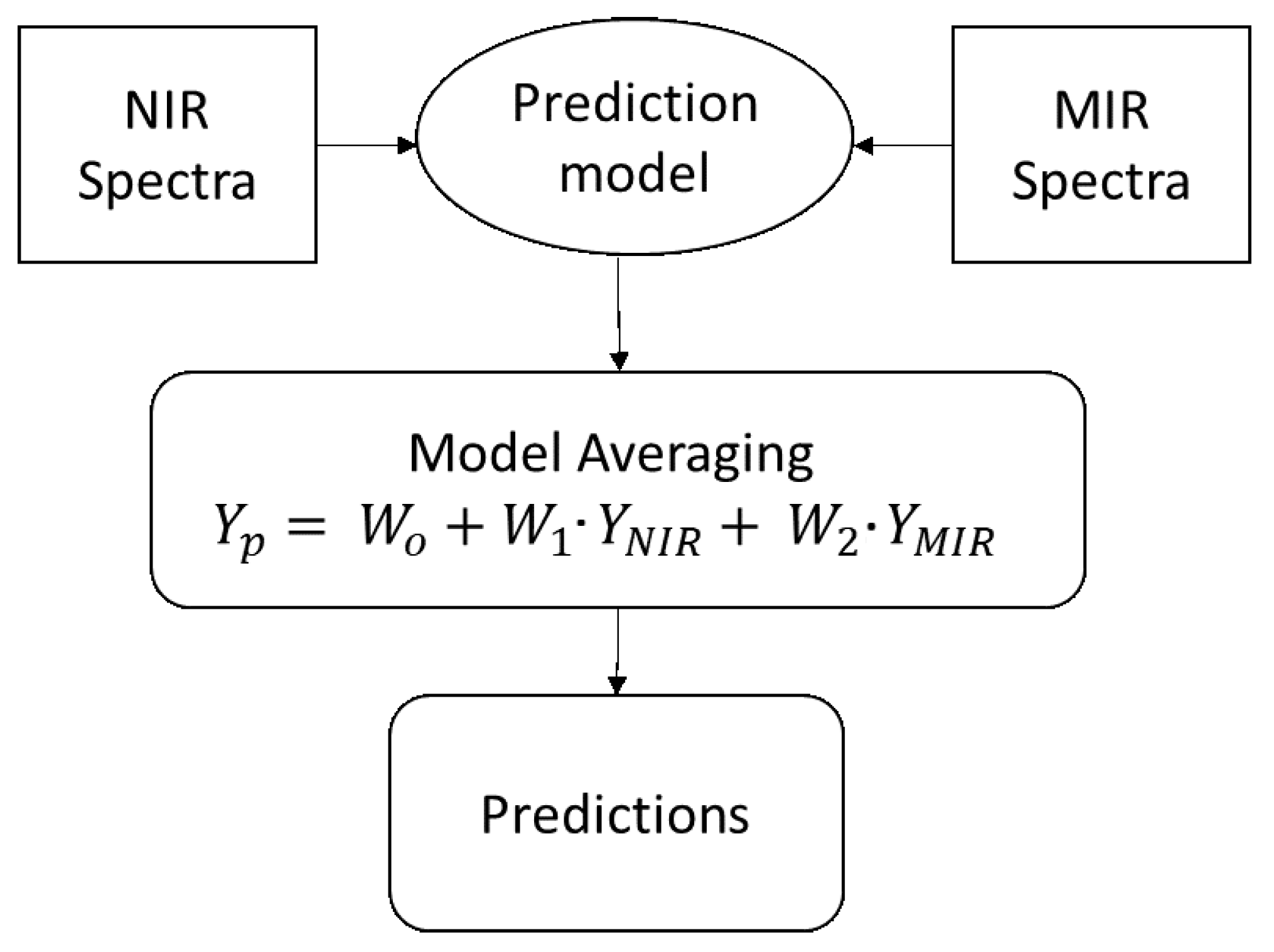
3.3. Model Averaging
3.4. Model Assessment Criteria
4. Results
4.1. Near-Infrared (NIR) and Mid-Infrared (MIR) Predictions
4.2. Prediction of Elements Using Model Averaging NIR and MIR Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dogliotti, S.; Giller, K.; van Ittersum, M. Achieving global food security whilst reconciling demands on the environment: Report of the First International Conference on Global Food Security. Food Secur. 2014, 6, 299–302. [Google Scholar] [CrossRef]
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- Msangi, S.; Rosegrant, M.W. Energy and Agriculture: Evolving Dynamics and Future Implications. In Sustainable Economic Development; Elsevier: Amsterdam, The Netherlands, 2015; pp. 261–291. [Google Scholar]
- Savci, S. Investigation of effect of chemical fertilizers on environment. Apcbee Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Paungfoo-Lonhienne, C.; Visser, J.; Lonhienne, T.G.; Schmidt, S. Past, present and future of organic nutrients. Plant Soil 2012, 359, 1–18. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Ge, L.; Anten, N.P.; van Dixhoorn, I.D.; Feindt, P.H.; Kramer, K.; Leemans, R.; Meuwissen, M.P.; Spoolder, H.; Sukkel, W. Why we need resilience thinking to meet societal challenges in bio-based production systems. Curr. Opin. Environ. Sustain. 2016, 23, 17–27. [Google Scholar] [CrossRef]
- Nookabkaew, S.; Rangkadilok, N.; Prachoom, N.; Satayavivad, J. Concentrations of trace elements in organic fertilizers and animal manures and feeds and cadmium contamination in herbal tea (Gynostemma pentaphyllum Makino). J. Agric. Food Chem. 2016, 64, 3119–3126. [Google Scholar] [CrossRef]
- Sager, M. Trace and nutrient elements in manure, dung and compost samples in Austria. Soil Biol. Biochem. 2007, 39, 1383–1390. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Grunert, O.; Meers, E.; Jablonowski, N.D. Evaluating the Fertilising Potential of Blended Recovered Nutrients in Horticultural Growing Medium on Viola × wittrockiana L. Agronomy 2022, 12, 182. [Google Scholar] [CrossRef]
- Metson, A.J. Methods of chemical analysis for soil survey samples. Soil Sci. 1957, 83, 245. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, D. Recent trends in industrial and other engineering applications of non destructive testing: A review. Int. J. Sci. Eng. Res. 2013, 4, 3. [Google Scholar]
- Carra, J.B.; Fabris, M.; Dieckow, J.; Brito, O.R.; Vendrame, P.R.S.; Macedo Dos Santos Tonial, L. Near-infrared spectroscopy coupled with chemometrics tools: A rapid and non-destructive alternative on soil evaluation. Commun. Soil Sci. Plant Anal. 2019, 50, 421–434. [Google Scholar] [CrossRef]
- Gholizadeh, S. A review of non-destructive testing methods of composite materials. Procedia Struct. Integr. 2016, 1, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Galvez-Sola, L.; Moral, R.; Perez-Murcia, M.; Perez-Espinosa, A.; Bustamante, M.; Martinez-Sabater, E.; Paredes, C. The potential of near infrared reflectance spectroscopy (NIRS) for the estimation of agroindustrial compost quality. Sci. Total. Environ. 2010, 408, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Sirisomboon, P. NIR Spectroscopy for quality evaluation of fruits and vegetables. Mater. Today Proc. 2018, 5, 22481–22486. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Rigby, H.; Clarke, B.O.; Pritchard, D.L.; Meehan, B.; Beshah, F.; Smith, S.R.; Porter, N.A. A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment. Sci. Total. Environ. 2016, 541, 1310–1338. [Google Scholar] [CrossRef] [PubMed]
- Recena, R.; Fernández-Cabanás, V.M.; Delgado, A. Soil fertility assessment by Vis-NIR spectroscopy: Predicting soil functioning rather than availability indices. Geoderma 2019, 337, 368–374. [Google Scholar] [CrossRef]
- Kuang, B.; Mahmood, H.S.; Quraishi, M.Z.; Hoogmoed, W.B.; Mouazen, A.M.; van Henten, E.J. Sensing soil properties in the laboratory, in situ, and on-line: A review. Adv. Agron. 2012, 114, 155–223. [Google Scholar]
- Sánchez, M.T.; Entrenas, J.A.; Torres, I.; Vega, M.; Pérez-Marín, D. Monitoring texture and other quality parameters in spinach plants using NIR spectroscopy. Comput. Electron. Agric. 2018, 155, 446–452. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Tewari, J.; Gopinathan, N.; Stolee, J.; Strong, R.; Boulas, P.; Cho, B.K. Quality assessment of pharmaceutical tablet samples using Fourier transform near infrared spectroscopy and multivariate analysis. Infrared Phys. Technol. 2017, 85, 300–306. [Google Scholar] [CrossRef]
- Ye, W.; Lorimor, J.C.; Hurburgh, C.R., Jr.; Zhang, H.; Hattey, J. Application of Near-Infrared Reflectance Spectroscopy for Determination of Nutrient. Trans. ASAE 2005, 48, 1911–1918. [Google Scholar] [CrossRef]
- Huang, G.; Han, L.; Yang, Z.; Wang, X. Evaluation of the nutrient metal content in Chinese animal manure compost using near infrared spectroscopy (NIRS). Bioresour. Technol. 2008, 99, 8164–8169. [Google Scholar] [CrossRef]
- Bedin, F.C.B.; Faust, M.V.; Guarneri, G.A.; Assmann, T.S.; Lafay, C.B.B.; Soares, L.F.; de Oliveira, P.A.V.; dos Santos-Tonial, L.M. NIR associated to PLS and SVM for fast and non-destructive determination of C, N, P, and K contents in poultry litter. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118834. [Google Scholar] [CrossRef]
- Baldock, J.; Creamer, C.; Szarvas, S.; McGowan, J.; Carter, T.; Farrell, M. Linking decomposition rates of soil organic amendments to their chemical composition. Soil Res. 2021, 59, 6. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X. A new approach to near-infrared spectral data analysis using independent component analysis. J. Chem. Inf. Comput. Sci. 2001, 41, 992–1001. [Google Scholar] [CrossRef] [Green Version]
- Malley, D.; Martin, P.; Ben-Dor, E. Application in analysis of soils. Near-Infrared Spectrosc. Agric. 2004, 44, 729–784. [Google Scholar]
- Wu, Y.; Chen, J.; Ji, J.; Gong, P.; Liao, Q.; Tian, Q.; Ma, H. A mechanism study of reflectance spectroscopy for investigating heavy metals in soils. Soil Sci. Soc. Am. J. 2007, 71, 918–926. [Google Scholar] [CrossRef]
- Wu, C.Y.; Jacobson, A.R.; Laba, M.; Kim, B.; Baveye, P.C. Surrogate correlations and near-infrared diffuse reflectance sensing of trace metal content in soils. Water Air Soil Pollut. 2010, 209, 377–390. [Google Scholar] [CrossRef]
- McCarty, G.; Reeves, J.; Reeves, V.; Follett, R.; Kimble, J. Mid-infrared and near-infrared diffuse reflectance spectroscopy for soil carbon measurement. Soil Sci. Soc. Am. J. 2002, 66, 640–646. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B. Regression rules as a tool for predicting soil properties from infrared reflectance spectroscopy. Chemom. Intell. Lab. Syst. 2008, 94, 72–79. [Google Scholar] [CrossRef]
- Reeves, J.B., III; Smith, D.B. The potential of mid-and near-infrared diffuse reflectance spectroscopy for determining major-and trace-element concentrations in soils from a geochemical survey of North America. Appl. Geochem. 2009, 24, 1472–1481. [Google Scholar] [CrossRef]
- Le Guillou, F.; Wetterlind, W.; Rossel, R.V.; Hicks, W.; Grundy, M.; Tuomi, S. How does grinding affect the mid-infrared spectra of soil and their multivariate calibrations to texture and organic carbon? Soil Res. 2015, 53, 913–921. [Google Scholar] [CrossRef]
- O’Rourke, S.; Minasny, B.; Holden, N.; McBratney, A. Synergistic use of Vis-NIR, MIR, and XRF spectroscopy for the determination of soil geochemistry. Soil Sci. Soc. Am. J. 2016, 80, 888–899. [Google Scholar] [CrossRef] [Green Version]
- Javadi, S.H.; Munnaf, M.A.; Mouazen, A.M. Fusion of Vis-NIR and XRF spectra for estimation of key soil attributes. Geoderma 2021, 385, 114851. [Google Scholar] [CrossRef]
- Aldabaa, A.A.A.; Weindorf, D.C.; Chakraborty, S.; Sharma, A.; Li, B. Combination of proximal and remote sensing methods for rapid soil salinity quantification. Geoderma 2015, 239–240, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Weindorf, D.C.; Li, B.; Ali Aldabaa, A.A.; Ghosh, R.K.; Paul, S.; Nasim Ali, M. Development of a hybrid proximal sensing method for rapid identification of petroleum contaminated soils. Sci. Total. Environ. 2015, 514, 399–408. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Min, S. Data fusion strategy in quantitative analysis of spectroscopy relevant to olive oil adulteration. Vib. Spectrosc. 2019, 101, 20–27. [Google Scholar] [CrossRef]
- Granger, C.W.; Ramanathan, R. Improved methods of combining forecasts. J. Forecast. 1984, 3, 197–204. [Google Scholar] [CrossRef]
- Spiegelman, C.H.; McShane, M.J.; Goetz, M.J.; Motamedi, M.; Yue, Q.L.; Coté, G.L. Theoretical justification of wavelength selection in PLS calibration: Development of a new algorithm. Anal. Chem. 1998, 70, 35–44. [Google Scholar] [CrossRef]
- Farrell, M.; Baldock, J.; Creamer, C.; Szarvas, S.; McGowan, J.; Carter, T. Spectral Chemistry and Decomposition Characteristics of a Range of Organic Amendments; Data Collection; CSIRO: East Melbourne, Australia, 2021. [Google Scholar]
- Jones, D.; Owen, A.; Farrar, J. Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biol. Biochem. 2002, 34, 1893–1902. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, R.L. Nitrogen-inorganic forms. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1123–1184. [Google Scholar]
- Candolfi, A.; De Maesschalck, R.; Jouan-Rimbaud, D.; Hailey, P.; Massart, D. The influence of data pre-processing in the pattern recognition of excipients near-infrared spectra. J. Pharm. Biomed. Anal. 1999, 21, 115–132. [Google Scholar] [CrossRef]
- Schoot, M.; Kapper, C.; van Kollenburg, G.H.; Postma, G.J.; van Kessel, G.; Buydens, L.M.; Jansen, J.J. Investigating the need for preprocessing of near-infrared spectroscopic data as a function of sample size. Chemom. Intell. Lab. Syst. 2020, 204, 104105. [Google Scholar] [CrossRef]
- Alander, J.T.; Bochko, V.; Martinkauppi, B.; Saranwong, S.; Mantere, T. A review of optical nondestructive visual and near-infrared methods for food quality and safety. Int. J. Spectrosc. 2013, 2013, 341402. [Google Scholar] [CrossRef]
- Mishra, P.; Rutledge, D.N.; Roger, J.M.; Wali, K.; Khan, H.A. Chemometric pre-processing can negatively affect the performance of near-infrared spectroscopy models for fruit quality prediction. Talanta 2021, 229, 122303. [Google Scholar] [CrossRef]
- Qiao, X.X.; Wang, C.; Feng, M.C.; Yang, W.D.; Ding, G.W.; Sun, H.; Liang, Z.Y.; Shi, C.C. Hyperspectral estimation of soil organic matter based on different spectral preprocessing techniques. Spectrosc. Lett. 2017, 50, 156–163. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Y.; Shao, X. A variable selection method based on uninformative variable elimination for multivariate calibration of near-infrared spectra. Chemom. Intell. Lab. Syst. 2008, 90, 188–194. [Google Scholar] [CrossRef]
- Mamouei, M.; Budidha, K.; Baishya, N.; Qassem, M.; Kyriacou, P. Comparison of wavelength selection methods for in-vitro estimation of lactate: A new unconstrained, genetic algorithm-based wavelength selection. Sci. Rep. 2020, 10, 16905. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, S.; Rossel, R.V.; Biswas, A.; Li, S.; Zhou, Y.; Shi, Z. X-ray fluorescence and visible near infrared sensor fusion for predicting soil chromium content. Geoderma 2019, 352, 61–69. [Google Scholar] [CrossRef]
- Frenich, A.G.; Jouan-Rimbaud, D.; Massart, D.; Kuttatharmmakul, S.; Galera, M.M.; Vidal, J.M. Wavelength selection method for multicomponent spectrophotometric determinations using partial least squares. Analyst 1995, 120, 2787–2792. [Google Scholar] [CrossRef]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.; Arcenegui, V.; Mataix-Beneyto, J. Near infrared spectroscopy for determination of various physical, chemical and biochemical properties in Mediterranean soils. Soil Biol. Biochem. 2008, 40, 1923–1930. [Google Scholar] [CrossRef] [Green Version]
- Lucasius, C.B.; Beckers, M.L.; Kateman, G. Genetic algorithms in wavelength selection: A comparative study. Anal. Chim. Acta 1994, 286, 135–153. [Google Scholar] [CrossRef]
- He, Z.; Honeycutt, C.W.; Zhang, T.; Bertsch, P.M. Preparation and FT-IR characterization of metal phytate compounds. J. Environ. Qual. 2006, 35, 1319–1328. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Sharma, N.; Singh, V.K. Application of X-ray fluorescence spectrometry in plant science: Solutions, threats, and opportunities. X-ray Spectrom. 2021, 51, 304–327. [Google Scholar] [CrossRef]




| Type | Total | Train | Test |
|---|---|---|---|
| composts. | 50 | 40 | 10 |
| manure. | 6 | 4 | 2 |
| plants residues. | 10 | 8 | 2 |
| bio-solids. | 19 | 15 | 4 |
| Prediction Results without Wavelength Selection | Prediction Results with Wavelength Selection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIR | MIR | NIR | MIR | ||||||||||
| Element | RMSE | R2 | RPD | RMSE | R2 | RPD | RMSE | R2 | RPD | RMSE | R2 | RPD | Ranking |
| N | 3.92 | 0.88 | 2.86 | 4.87 | 0.85 | 2.3 | 2.88 | 0.94 | 6.94 | 3.56 | 0.92 | 5.4 | NIR |
| FAA-N | 0.46 | 0.68 | 1.96 | 0.54 | 0.63 | 1.81 | 0.24 | 0.79 | 2.21 | 0.27 | 0.78 | 2.12 | NIR |
| NO3-N | 0.60 | 0.66 | 1.74 | 0.62 | 0.61 | 1.58 | 0.4 | 0.73 | 2.48 | 0.59 | 0.70 | 2.18 | NIR |
| NH4-N | 0.71 | 0.82 | 2.43 | 0.63 | 0.87 | 2.85 | 0.66 | 0.88 | 2.87 | 0.59 | 0.92 | 3.69 | MIR |
| EC | 1.23 | 0.8 | 2.31 | 1.12 | 0.82 | 2.56 | 1.13 | 0.85 | 2.61 | 1.08 | 0.86 | 2.72 | MIR |
| pH | 0.31 | 0.78 | 2.21 | 0.43 | 0.71 | 1.92 | 0.27 | 0.82 | 2.38 | 0.35 | 0.76 | 1.83 | NIR |
| As | 3.38 | 0.63 | 1.8 | 3.35 | 0.67 | 1.86 | 3.06 | 0.68 | 1.66 | 3 | 0.7 | 1.82 | MIR |
| Cd | 0.45 | 0.47 | 1.32 | 0.39 | 0.54 | 1.51 | 0.37 | 0.54 | 1.47 | 0.32 | 0.63 | 1.66 | MIR |
| Co | 7.43 | 0.61 | 1.7 | 7.54 | 0.63 | 1.73 | 5.59 | 0.66 | 1.78 | 5.6 | 0.67 | 1.79 | MIR/NIR |
| Cr | 17.17 | 0.53 | 1.37 | 21.01 | 0.47 | 1.31 | 13.76 | 0.67 | 1.69 | 18.11 | 0.56 | 1.5 | NIR |
| Cu | 0.13 | 0.63 | 1.43 | 0.15 | 0.57 | 1.37 | 0.09 | 0.72 | 1.89 | 0.097 | 0.7 | 1.81 | NIR |
| Mo | 10.21 | 0.09 | 0.97 | 10.06 | 0.11 | 0.98 | 8.81 | 0.12 | 1.08 | 8.76 | 0.15 | 1.1 | MIR/NIR |
| Ni | 10.13 | 0.39 | 0.92 | 10.07 | 0.41 | 0.98 | 8.7 | 0.45 | 1.12 | 8.6 | 0.47 | 1.2 | MIR/NIR |
| Pb | 0.09 | 0.67 | 1.91 | 0.081 | 0.71 | 1.98 | 0.043 | 0.75 | 2.03 | 0.042 | 0.76 | 2.06 | MIR/NIR |
| Se | 0.51 | 0.79 | 2.64 | 0.54 | 0.75 | 2.61 | 0.38 | 0.87 | 2.82 | 0.39 | 0.86 | 2.72 | NIR |
| Zn | 0.36 | 0.26 | 1.11 | 0.25 | 0.34 | 1.23 | 0.27 | 0.32 | 1.5 | 0.18 | 0.41 | 1.55 | MIR |
| Al | 7.8 | 0.75 | 2.11 | 6.12 | 0.83 | 3.24 | 5.5 | 0.84 | 2.5 | 3.8 | 0.92 | 3.68 | MIR |
| Ca | 6.13 | 0.73 | 2.09 | 6.38 | 0.67 | 1.96 | 4.3 | 0.82 | 2.39 | 4.72 | 0.78 | 2.18 | NIR |
| Fe | 15.56 | 0.61 | 1.74 | 12.23 | 0.76 | 2.48 | 12.88 | 0.73 | 1.98 | 9.91 | 0.84 | 2.58 | MIR |
| K | 1.82 | 0.65 | 1.87 | 1.78 | 0.71 | 1.93 | 1.67 | 0.76 | 2.03 | 1.62 | 0.77 | 2.1 | MIR |
| Mg | 1.83 | 0.67 | 1.89 | 1.79 | 0.71 | 1.94 | 1.56 | 0.75 | 2.03 | 1.53 | 0.76 | 2.05 | MIR |
| Mn | 0.31 | 0.61 | 1.78 | 0.33 | 0.58 | 1.67 | 0.17 | 0.73 | 1.98 | 0.18 | 0.66 | 1.74 | NIR |
| Na | 2.12 | 0.43 | 1.31 | 2.21 | 0.53 | 1.39 | 2.014 | 0.56 | 1.51 | 1.95 | 0.59 | 1.56 | MIR |
| P | 3.54 | 0.86 | 3.23 | 3.92 | 0.81 | 2.89 | 2.67 | 0.93 | 3.8 | 3.12 | 0.9 | 3.24 | NIR |
| S | 1.38 | 0.76 | 2.33 | 1.36 | 0.78 | 2.45 | 1.08 | 0.83 | 2.46 | 1.05 | 0.85 | 2.57 | MIR |
| Model Averaging | Percent Improvement from NIR | Percent Improvement from MIR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | RMSE | R2 | RPD | RMSE | R2 | RPD | RMSE | R2 | RPD |
| N | 2.84 | 0.96 | 6.98 | −1.39 | 2.13 | 0.58 | −20.22 | 4.35 | 29.26 |
| FAA-N | 0.22 | 0.81 | 2.34 | −8.33 | 2.53 | 5.88 | −18.52 | 3.85 | 10.38 |
| NO3-N | 0.37 | 0.76 | 2.57 | −7.50 | 4.10 | 3.60 | −37.28 | 8.57 | 17.88 |
| NH4-N | 0.57 | 0.94 | 3.8 | −13.64 | 6.82 | 32.40 | −3.39 | 2.17 | 2.98 |
| EC | 0.99 | 0.89 | 2.97 | −12.39 | 4.71 | 13.79 | −8.33 | 3.49 | 9.19 |
| pH | 0.25 | 0.85 | 2.6 | −7.41 | 3.66 | 9.24 | −28.57 | 11.84 | 42.08 |
| As | 2.91 | 0.75 | 2.1 | −4.90 | 10.29 | 26.51 | −3.00 | 7.14 | 15.38 |
| Cd | 0.27 | 0.75 | 2.02 | −27.03 | 38.89 | 37.41 | −15.63 | 19.05 | 21.69 |
| Co | 4.84 | 0.75 | 1.98 | −13.42 | 13.64 | 11.24 | −13.57 | 11.94 | 10.61 |
| Cr | 9.82 | 0.77 | 2.08 | −28.63 | 14.93 | 23.08 | −45.78 | 37.50 | 38.67 |
| Cu | 0.079 | 0.8 | 2.21 | −12.22 | 11.11 | 16.93 | −18.56 | 14.29 | 22.10 |
| Mo | 8.34 | 0.16 | 1.2 | −5.33 | 33.33 | 11.11 | −4.79 | 6.67 | 9.09 |
| Ni | 8.58 | 0.48 | 1.21 | −1.38 | 6.67 | 8.04 | −0.23 | 2.13 | 0.83 |
| Pb | 0.038 | 0.81 | 2.32 | −11.63 | 8.00 | 14.29 | −9.52 | 6.58 | 12.62 |
| Se | 0.35 | 0.89 | 3.06 | −7.89 | 2.30 | 8.51 | −10.26 | 3.49 | 12.50 |
| Zn | 0.14 | 0.53 | 1.67 | −48.15 | 65.63 | 11.33 | −22.22 | 29.27 | 7.74 |
| Al | 3.18 | 0.94 | 4.12 | −42.18 | 11.90 | 64.80 | −16.32 | 2.17 | 11.96 |
| Ca | 3.22 | 0.9 | 3.19 | −25.12 | 9.76 | 33.47 | −31.78 | 15.38 | 46.33 |
| Fe | 9.56 | 0.85 | 2.68 | −25.78 | 16.44 | 35.35 | −3.53 | 1.19 | 3.88 |
| K | 1.54 | 0.8 | 2.2 | −7.78 | 5.26 | 8.37 | −4.94 | 3.90 | 4.76 |
| Mg | 1.36 | 0.81 | 2.28 | −12.82 | 8.00 | 12.32 | −11.11 | 6.58 | 11.22 |
| Mn | 0.16 | 0.75 | 2.02 | −5.88 | 2.74 | 2.02 | −11.11 | 13.64 | 16.09 |
| Na | 1.77 | 0.66 | 1.71 | −12.12 | 17.86 | 13.25 | −9.23 | 11.86 | 9.62 |
| P | 2.51 | 0.94 | 4.31 | −5.99 | 1.08 | 13.42 | −19.55 | 4.44 | 33.02 |
| S | 0.81 | 0.91 | 3.33 | −25.00 | 9.64 | 35.37 | −22.86 | 7.06 | 29.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wali, K.; Khan, H.A.; Farrell, M.; Henten, E.J.V.; Meers, E. Determination of Bio-Based Fertilizer Composition Using Combined NIR and MIR Spectroscopy: A Model Averaging Approach. Sensors 2022, 22, 5919. https://doi.org/10.3390/s22155919
Wali K, Khan HA, Farrell M, Henten EJV, Meers E. Determination of Bio-Based Fertilizer Composition Using Combined NIR and MIR Spectroscopy: A Model Averaging Approach. Sensors. 2022; 22(15):5919. https://doi.org/10.3390/s22155919
Chicago/Turabian StyleWali, Khan, Haris Ahmad Khan, Mark Farrell, Eldert J. Van Henten, and Erik Meers. 2022. "Determination of Bio-Based Fertilizer Composition Using Combined NIR and MIR Spectroscopy: A Model Averaging Approach" Sensors 22, no. 15: 5919. https://doi.org/10.3390/s22155919
APA StyleWali, K., Khan, H. A., Farrell, M., Henten, E. J. V., & Meers, E. (2022). Determination of Bio-Based Fertilizer Composition Using Combined NIR and MIR Spectroscopy: A Model Averaging Approach. Sensors, 22(15), 5919. https://doi.org/10.3390/s22155919








