An Infrared Laser Sensor for Monitoring Gas-Phase CO2 in the Headspace of Champagne Glasses under Wine Swirling Conditions
Abstract
1. Introduction
2. Instrument Design
3. Instrument Control and Data Processing
- L: cavity length ( cm);
- : operative wavelength in µm.
| • : wavenumber (cm) | • : air broadening coefficient (cm·atm) |
| • L: optical path (cm) | • : CO broadening coefficient (cm·atm) |
| • : Boltzmann constant (J·K) | • : line center (cm) |
| • T: temperature (K) | • : line strength (cm·(molecule·cm)−1) |
| • P: pressure (Pa) | • : CO mixing ratio |
| • : atmospheric pressure (Pa) |
4. Wine-Swirling Application
4.1. Champagne Samples
4.2. Glass Shape and Standardized Effervescence
4.3. Wine-Swirling Motion Parameters
4.4. Gas-Phase CO Monitoring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charters, S. Drinking Sparkling Wine: An Exploratory Investigation. Int. J. Wine Mark. 2005, 17, 54–68. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Cilindre, C. Recent Progress in the Analytical Chemistry of Champagne and Sparkling Wines. Annu. Rev. Anal. Chem. 2021, 14, 21–46. [Google Scholar] [CrossRef] [PubMed]
- Meusel, T.; Negoias, S.; Scheibe, M.; Hummel, T. Topographical differences in distribution and responsiveness of trigeminal sensitivity within the human nasal mucosa. Pain 2010, 151, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Saint-Eve, A.; Déléris, I.; Feron, G.; Ibarra, D.; Guichard, E.; Souchon, I. How trigeminal, taste and aroma perceptions are affected in mint-flavored carbonated beverages. Food Qual. Prefer. 2010, 21, 1026–1033. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Santos, M.; Martín-Álvarez, P.J.; Reineccius, G. Influence of carbonation on aroma release from liquid systems using an artificial throat and a proton transfer reaction-mass spectrometric technique (PTR-MS). Flavour Fragr. J. 2009, 24, 226–233. [Google Scholar] [CrossRef]
- Cain, W.S.; Murphy, C.L. Interaction between chemoreceptive modalities of odour and irritation. Nature 1980, 284, 255–257. [Google Scholar] [CrossRef]
- Wise, P.M.; Wysocki, C.J.; Radil, T. Time-intensity ratings of nasal irritation from carbon dioxide. Chem. Senses 2003, 28, 751–760. [Google Scholar] [CrossRef][Green Version]
- Cilindre, C.; Conreux, A.; Liger-Belair, G. Simultaneous monitoring of gaseous CO2 and ethanol above champagne glasses via micro-gas chromatography (μGC). J. Agric. Food Chem. 2011, 59, 7317–7323. [Google Scholar] [CrossRef]
- Mulier, M.; Zeninari, V.; Joly, L.; Decarpenterie, T.; Parvitte, B.; Jeandet, P.; Liger-Belair, G. Development of a compact CO2 sensor based on near-infrared laser technology for enological applications. Appl. Phys. B Lasers Opt. 2009, 94, 725–733. [Google Scholar] [CrossRef]
- Moriaux, A.L.; Vallon, R.; Cilindre, C.; Parvitte, B.; Liger-Belair, G.; Zeninari, V. Development and validation of a diode laser sensor for gas-phase CO2 monitoring above champagne and sparkling wines. Sens. Actuators B Chem. 2018, 257, 745–752. [Google Scholar] [CrossRef]
- Moriaux, A.L.; Vallon, R.; Parvitte, B.; Zeninari, V.; Liger-Belair, G.; Cilindre, C. Monitoring gas-phase CO2 in the headspace of champagne glasses through combined diode laser spectrometry and micro-gas chromatography analysis. Food Chem. 2018, 264, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Moriaux, A.L.; Vallon, R.; Lecasse, F.; Chauvin, N.; Parvitte, B.; Zéninari, V.; Liger-Belair, G.; Cilindre, C. How does gas-phase CO2 evolve in the headspace of champagne glasses? J. Agric. Food Chem. 2021, 69, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, J.F.; Pelchat, M.L. Influence of glass shape on wine aroma. J. Sens. Stud. 2002, 17, 19–28. [Google Scholar] [CrossRef]
- Vilanova, M.; Vidal, P.; Cortés, S. Effect of the glass shape on flavor perception of “toasted wine” from ribeiro (NW Spain). J. Sens. Stud. 2008, 23, 114–124. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Tasting: A Professional Handbook (Food Science and Technology, Internacional Series); Academic Press: Waltham, MA, USA, 2009; p. 68. [Google Scholar]
- Gerlach, G.; Guth, U.; Oelßner, W. Carbon Dioxide Sensing: Fundamentals, Principles, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Durry, G.; Megie, G. Atmospheric CH4 and H2O monitoring with near-infrared InGaAs laser diodes by the SDLA, a balloonborne spectrometer for tropospheric and stratospheric in situ measurements. Appl. Opt. 1999, 38, 7342. [Google Scholar] [CrossRef]
- Richard, E.C.; Kelly, K.K.; Winkler, R.H.; Wilson, R.; Thompson, T.L.; McLaughlin, R.J.; Schmeltekopf, A.L.; Tuck, A.F. A fast-response near-infrared tunable diode laser absorption spectrometer for in situ measurements of CH4 in the upper troposphere and lower stratosphere. Appl. Phys. B Lasers Opt. 2002, 75, 183–194. [Google Scholar] [CrossRef]
- Zéninari, V.; Vicet, A.; Parvitte, B.; Joly, L.; Durry, G. In situ sensing of atmospheric CO2 with laser diodes near 2.05 μm: A spectroscopic study. Infrared Phys. Technol. 2004, 45, 229–237. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Li, J.; Yu, B.; Zhao, W.; Chen, W. A review of signal enhancement and noise reduction techniques for tunable diode laser absorption spectroscopy. Appl. Spectrosc. Rev. 2014, 49, 666–691. [Google Scholar] [CrossRef]
- Edwin, R.P.; Dudermel, M.T.; Lamare, M. Refractive index measurements of ten germanium samples. Appl. Opt. 1982, 21, 878. [Google Scholar] [CrossRef]
- Herzberger, M.; Salzberg, C.D. Refractive Indices of Infrared Optical Materials and Color Correction of Infrared Lenses. J. Opt. Soc. Am. 1962, 52, 420. [Google Scholar] [CrossRef]
- Caputi, A.; Ueda, M.; Walter, P.; Brown, T. Titrimetric determination of carbon dioxide in wine. Am. J. Enol. Vitic. 1970, 21, 140–144. [Google Scholar]
- Liger-Belair, G. Modeling the Losses of Dissolved CO2 from Laser-Etched Champagne Glasses. J. Phys. Chem. B 2016, 120, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Reclari, M.; Dreyer, M.; Tissot, S.; Obreschkow, D.; Wurm, F.M.; Farhat, M. Surface wave dynamics in orbital shaken cylindrical containers. Phys. Fluids 2014, 26, 052104. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Bourget, M.; Pron, H.; Polidori, G.; Cilindre, C. Monitoring gaseous CO2 and ethanol above champagne glasses: Flute versus coupe, and the role of temperature. PLoS ONE 2012, 7, e30628. [Google Scholar] [CrossRef] [PubMed]
- Liger-Belair, G.; Bourget, M.; Villaume, S.; Jeandet, P.; Pron, H.; Polidori, G. On the losses of dissolved CO2 during champagne serving. J. Agric. Food Chem. 2010, 58, 8768–8775. [Google Scholar] [CrossRef]


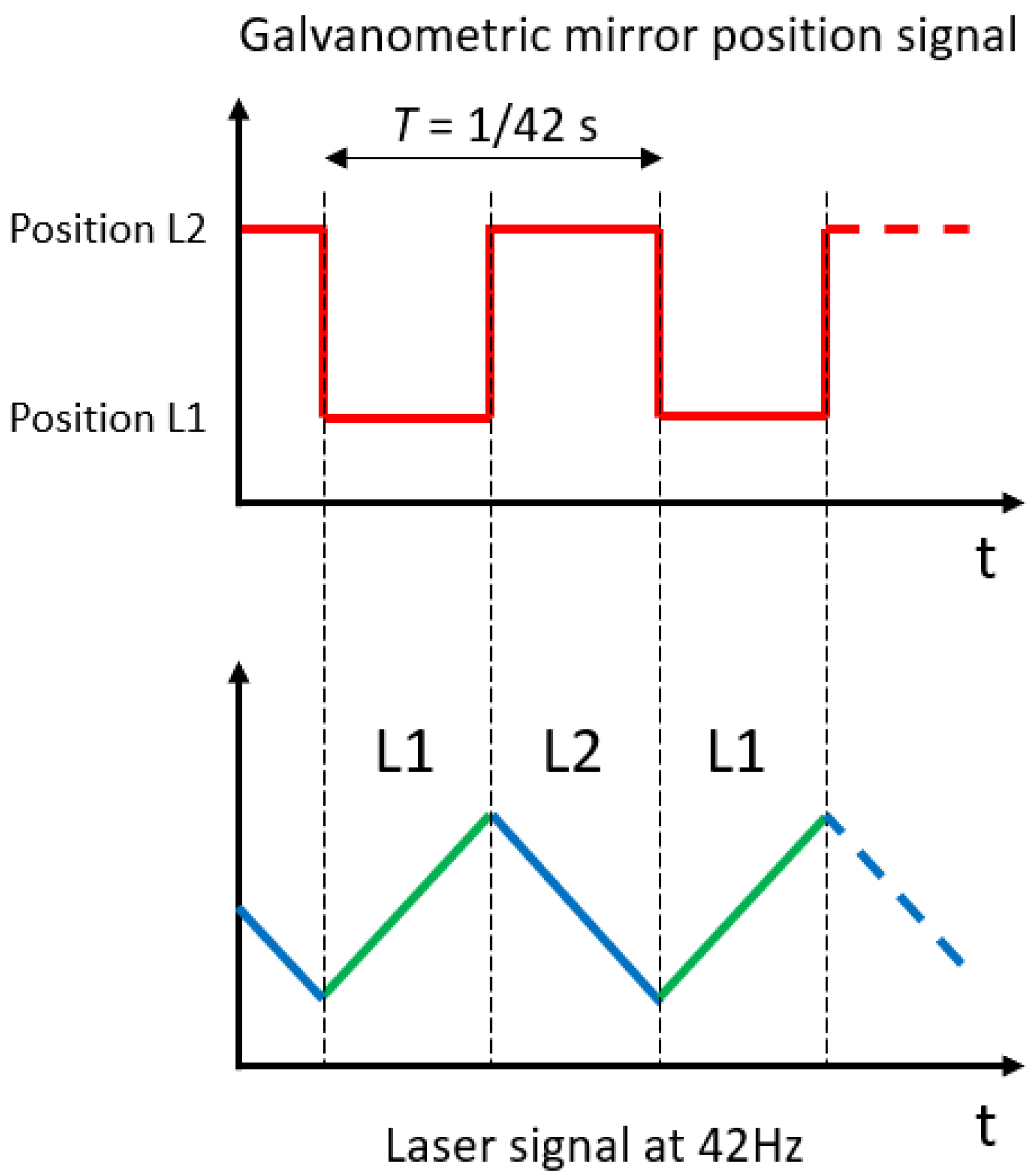
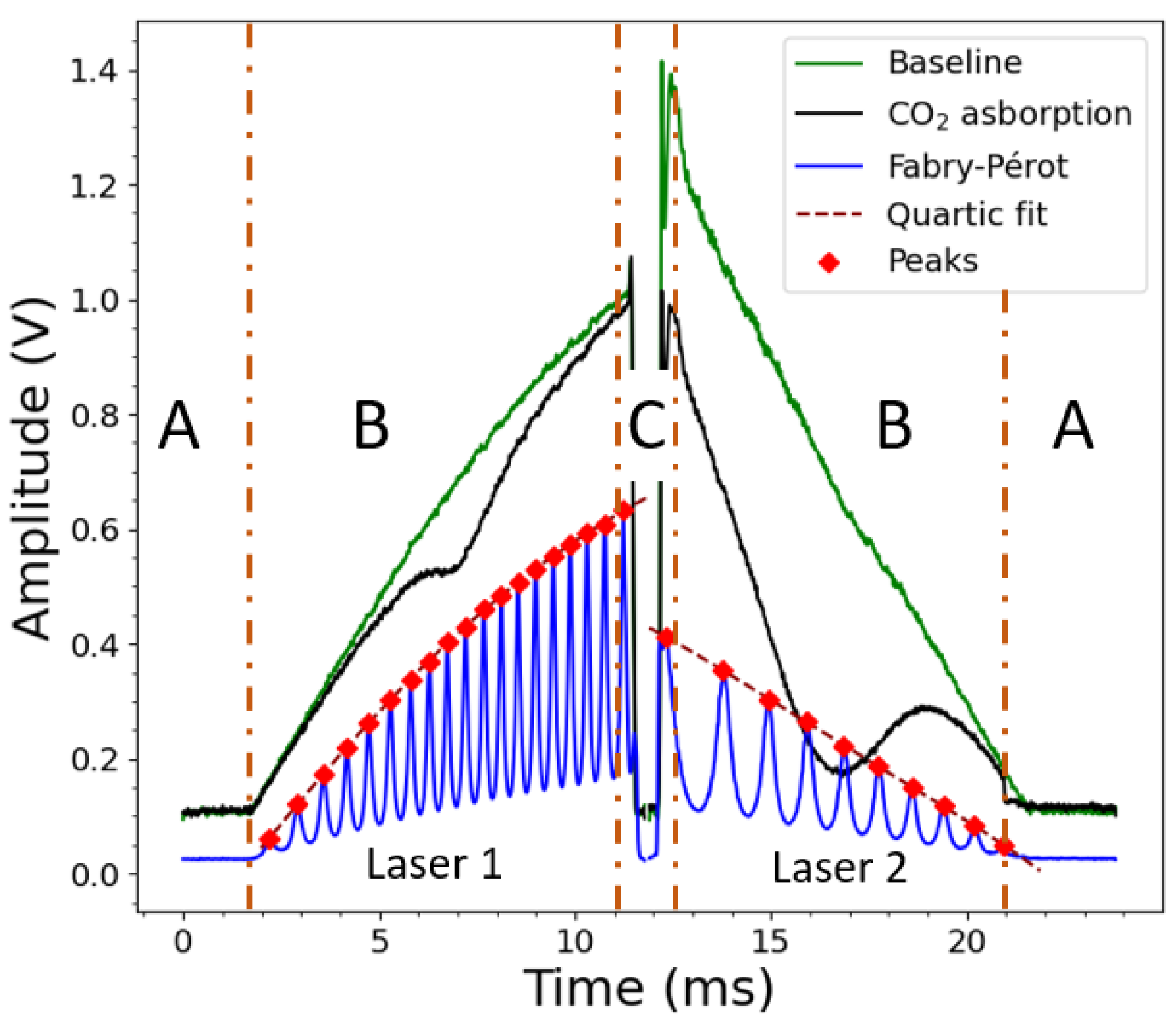

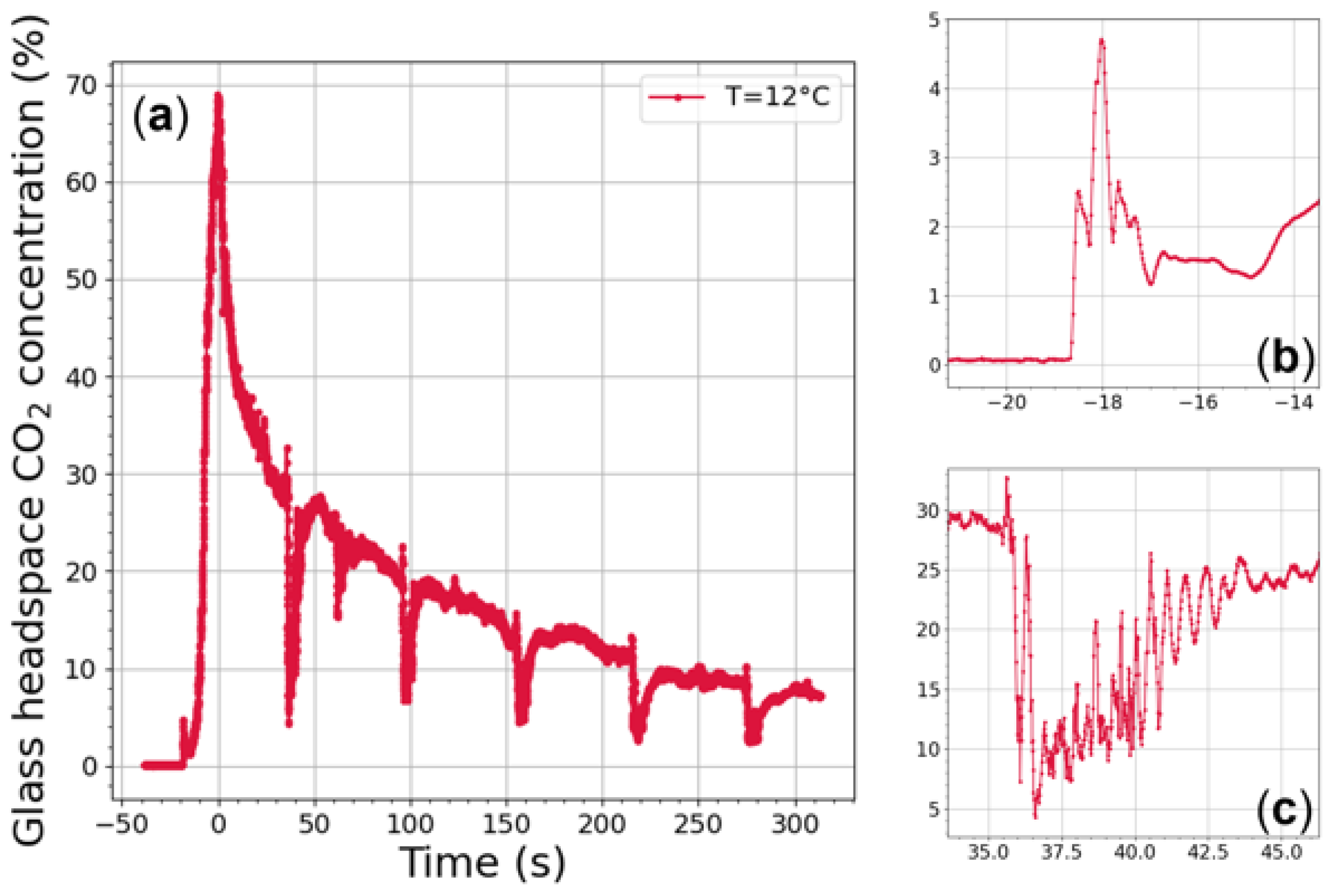

| Laser 1 @ 2.004 µm | Laser 2 @ 2.682 µm | |
|---|---|---|
| Line scanned (cm) | 4985.932174 | 3728.410105 |
| Line strength (cm·(molecule·cm)−1) | 1.156 × 10 | 5.792 × 10 |
| Concentration range (%) | 10–100 | 0.5–15 |
| Laser 1 @ 2.004 µm | Laser 2 @ 2.682 µm | ||
|---|---|---|---|
| (cm) | s (cm·(molecule·cm)−1) | (cm) | s (cm·(molecule·cm)−1) |
| 4984.526436 | 1.015 × 10 | 3727.070585 | 1.071 × 10 |
| 4985.334523 | 2.802 × 10 | 3727.082822 | 5.925 × 10 |
| 4985.439252 | 2.598 × 10 | 3727.798746 | 1.277 × 10 |
| 4985.932174 | 1.156 × 10 | 3728.410105 | 5.792 × 10 |
| 4986.514153 | 2.397 × 10 | 3728.557020 | 1.467 × 10 |
| 4986.542862 | 2.201 × 10 | 3729.262969 | 1.641 × 10 |
| 4987.308270 | 1.253 × 10 | 3729.712250 | 5.514 × 10 |
| Parameter | Mean ± std |
|---|---|
| Semi-major axis | mm |
| Semi-minor axis | mm |
| Angular speed | rpm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecasse, F.; Vallon, R.; Polak, F.; Cilindre, C.; Parvitte, B.; Liger-Belair, G.; Zéninari, V. An Infrared Laser Sensor for Monitoring Gas-Phase CO2 in the Headspace of Champagne Glasses under Wine Swirling Conditions. Sensors 2022, 22, 5764. https://doi.org/10.3390/s22155764
Lecasse F, Vallon R, Polak F, Cilindre C, Parvitte B, Liger-Belair G, Zéninari V. An Infrared Laser Sensor for Monitoring Gas-Phase CO2 in the Headspace of Champagne Glasses under Wine Swirling Conditions. Sensors. 2022; 22(15):5764. https://doi.org/10.3390/s22155764
Chicago/Turabian StyleLecasse, Florian, Raphaël Vallon, Frédéric Polak, Clara Cilindre, Bertrand Parvitte, Gérard Liger-Belair, and Virginie Zéninari. 2022. "An Infrared Laser Sensor for Monitoring Gas-Phase CO2 in the Headspace of Champagne Glasses under Wine Swirling Conditions" Sensors 22, no. 15: 5764. https://doi.org/10.3390/s22155764
APA StyleLecasse, F., Vallon, R., Polak, F., Cilindre, C., Parvitte, B., Liger-Belair, G., & Zéninari, V. (2022). An Infrared Laser Sensor for Monitoring Gas-Phase CO2 in the Headspace of Champagne Glasses under Wine Swirling Conditions. Sensors, 22(15), 5764. https://doi.org/10.3390/s22155764







