Nanosensor Based on Thermal Gradient and Machine Learning for the Detection of Methanol Adulteration in Alcoholic Beverages and Methanol Poisoning
Abstract
1. Introduction
2. Materials and Methods
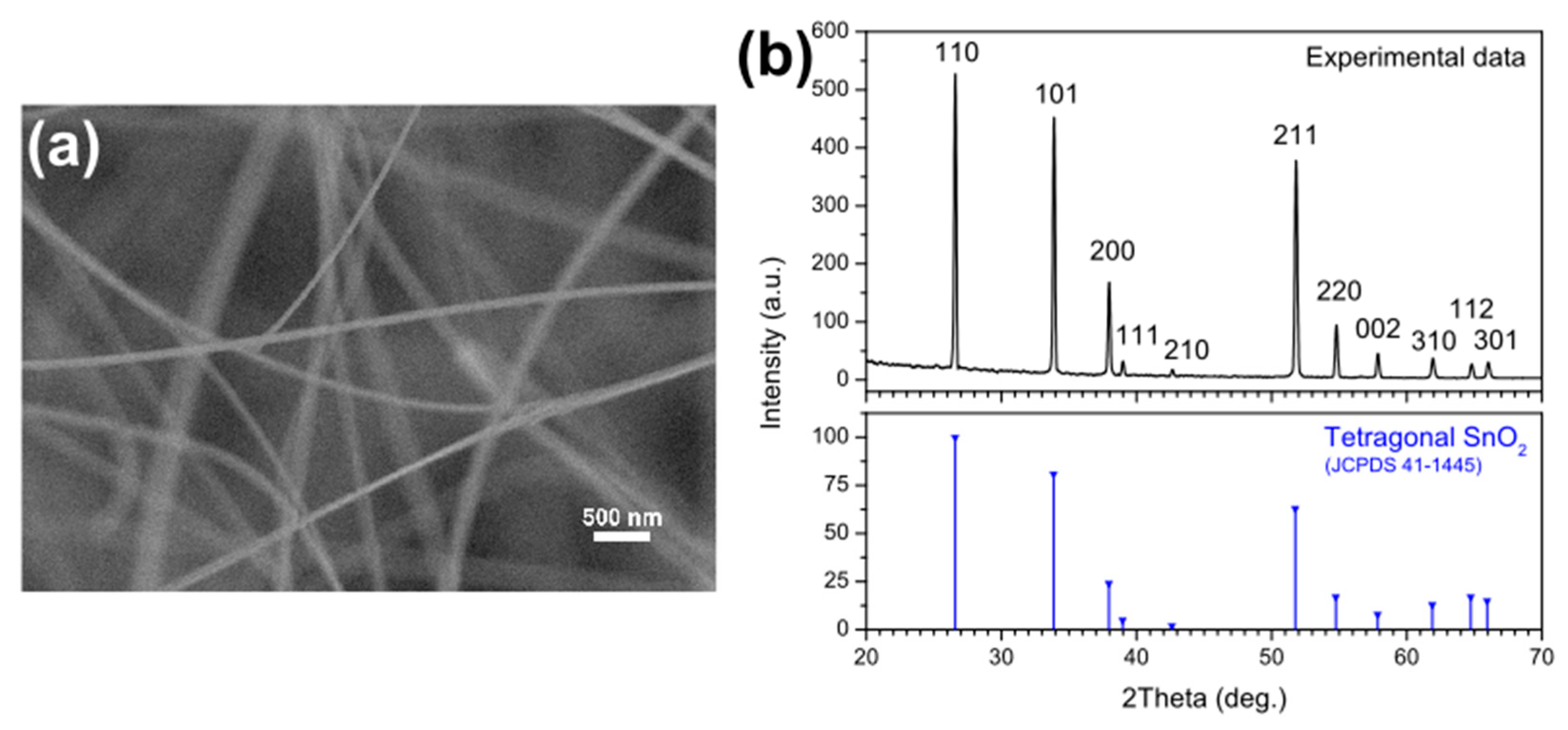
2.1. Synthesis of SnO2 Nanowires
2.2. Nanowires Characterization
2.3. Sensor Fabrication
2.4. Gas Sensor Measurements
2.5. Machine Learning Techniques
3. Results and Discussion
3.1. Nanowires Characterization
3.2. Traditional Gas Measurements
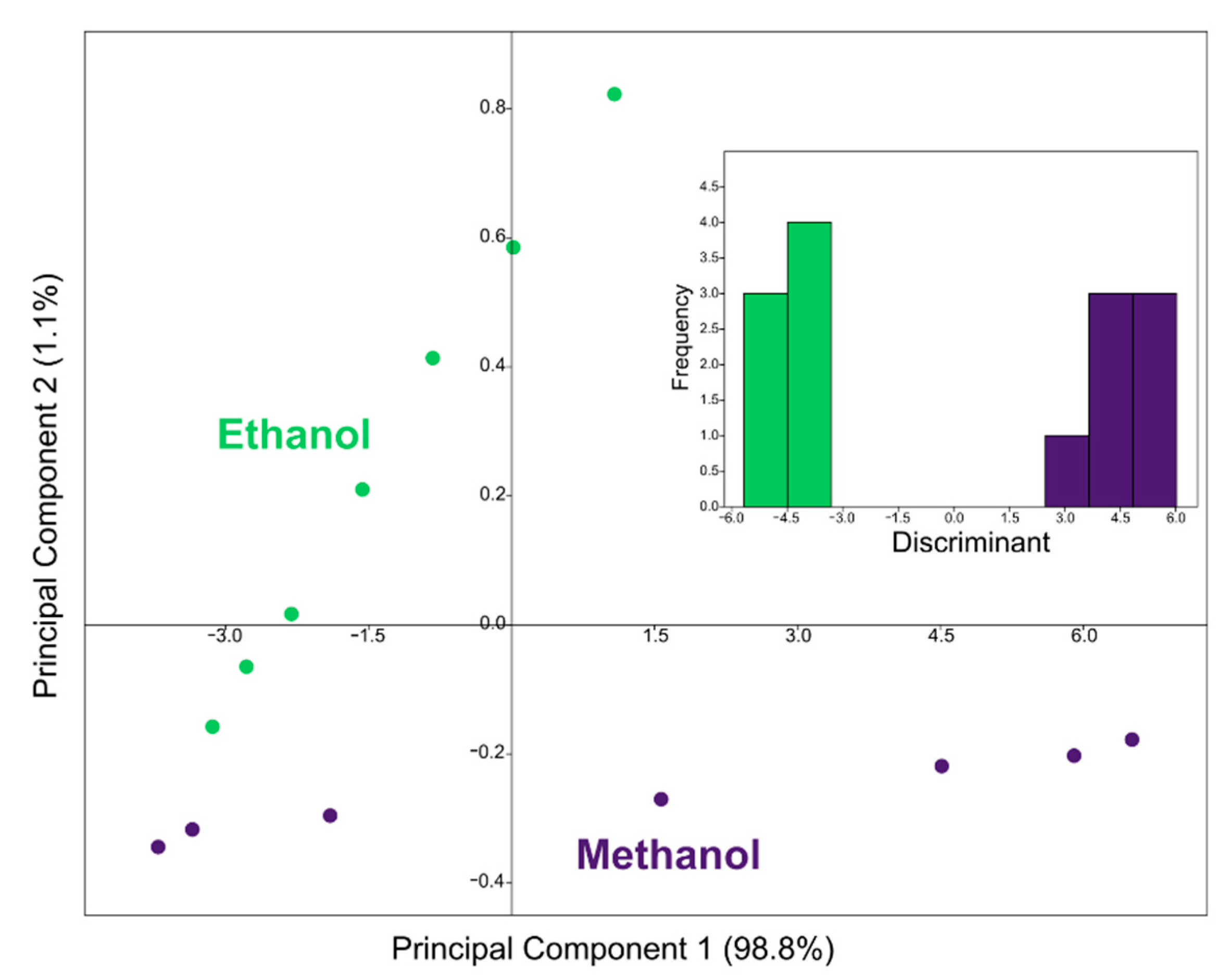
3.3. Machine Learning: Visualization and Classification
3.4. Machine Learning: Quantification
3.5. Relative Humidity
3.6. Experimental Measurements in Realistic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barceloux, D.G.; Bond, G.R.; Krenzelok, E.P.; Cooper, H.; Vale, J.A. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J. Toxicol. Clin. Toxicol. 2002, 40, 415–446. [Google Scholar] [CrossRef] [PubMed]
- Hovda, K.E.; McMartin, K.; Jacobsen, D. Methanol and Formaldehyde. In Critical Care Toxicology; Brent, J., Megarbane, B., Palmer, R., Hatten, B., Burkhart, K., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Hoyte, C.; Schimmel, J.; Hadianfar, A.; Banerji, S.; Nakhaee, S.; Mehrpour, O. Toxic alcohol poisoning characteristics and treatments from 2000 to 2017 at a United States regional poison center. DARU J. Pharm. Sci. 2021, 29, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.; Kowalska, A. Illicit Alcohol: Public Health Risk of Methanol Poisoning and Policy Mitigation Strategies. Foods 2021, 10, 1625. [Google Scholar] [CrossRef] [PubMed]
- Agence France-Presse. Voice of America News: Death Toll in Iran Alcohol Poisoning Jumps to 84. Available online: https://www.voanews.com/a/death-toll-in-iranalcohol-poisoning-jumps-to-84/4633468.html (accessed on 13 June 2022).
- David, S. Khmer Times: More than 100 Villagers Return Home after Methanol Poisoning. Available online: https://www.khmertimeskh.com/50487671/more-than-100-villagers-return-home-after-methanol-poisoning/ (accessed on 13 June 2022).
- Rosli, J. Methanol Poisoning: Health Ministry Tracking Down Source as Deaths Hit 45, MalayMail, 2 October 2018. Available online: https://www.malaymail.com/news/malaysia/2018/10/02/methanol-poisoning-health-ministry-tracking-down-source-as-deaths-hit-45/1678523 (accessed on 13 June 2022).
- “Toxic Alcohol ‘Laced with Methanol’ Kills at Least 99 People in India”. The Guardian. Available online: https://www.theguardian.com/world/2019/feb/11/toxic-alcohol-methanol-india-uttar-pradesh-uttarakhand (accessed on 13 June 2022).
- “Assam Hooch Tragedy: Death Toll Rises to 133, over 200 Hospitalised” India Today. Available online: https://www.indiatoday.in/india/story/assam-hooch-tragedy-death-toll-rises-to-133-over-200-hospitalised-1463709-2019-02-24 (accessed on 13 June 2022).
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.-W.; et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.A. Methanol and ethylene glycol intoxication. Crit. Care Clin. 2012, 28, 661–711. [Google Scholar] [CrossRef]
- Kraut, J.A. Diagnosis of toxic alcohols: Limitations of present methods. Clin. Toxicol. 2015, 53, 589–595. [Google Scholar] [CrossRef]
- D’Silva, J. India’s problem with toxic alcohol. BMJ 2015, 351, h4536. [Google Scholar] [CrossRef]
- Laakso, O.; Haapala, M.; Jaakkola, P.; Laaksonen, R.; Luomanmäki, K.; Nieminen, J.; Pettersson, M.; Päivä, H.; Räsänen, M.; Himberg, J.-J. FT-IR breath test in the diagnosis and control of treatment of methanol intoxications. J. Anal. Toxicol. 2001, 25, 26–30. [Google Scholar] [CrossRef][Green Version]
- Lombardo, L.; Grassini, S.; Parvis, M.; Donato, N.; Gullino, A. Ethanol breath measuring system. In Proceedings of the IEEE Medical Measurements and Applications, MeMeA 2020-Conference Proceedings 2020, Bari, Italy, 1 June–1 July 2020. [Google Scholar] [CrossRef]
- Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1222.pdf (accessed on 13 June 2022).
- Thai, N.X.; Van Duy, N.; Hung, C.M.; Nguyen, H.; Tonezzer, M.; Nguyen, V.H.; Nguyen, D.H. Prototype edge-grown nanowire sensor array for the real-time monitoring and classification of multiple gases. J. Sci. Adv. Mater. Devices 2020, 5, 409–416. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Bi, D.; Liu, Q.; Yang, C.; Zhang, J. Continuously improved gas-sensing performance of Zn2SnO4 porous octahedrons by structure evolution and further ZnSnO3 nanosheets decoration. J. Alloys Compd. 2022, 901, 163744. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J.; Yao, P.; Li, X. Hollow hierarchical SnO2-ZnO composite nanofibers with heterostructure based on electrospinning method for detecting methanol. Sens. Actuators B Chem. 2014, 192, 543–549. [Google Scholar] [CrossRef]
- Huang, J.; Meng, C.; Wang, H.; Ren, H.; Lu, X.; Joo, S.W. Preparation of cross-linked porous SnO2 nanosheets using three-dimensional reduced graphene oxide as a template and their gas sensing property. J. Alloys Compd. 2022, 910, 164763. [Google Scholar] [CrossRef]
- Priya, S.; Halder, J.; Mandal, D.; Chowdhury, A.; Singh, T.; Chandra, A. Hierarchical SnO2 nanostructures for potential VOC sensor. J. Mater. Sci. 2021, 56, 9883–9893. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar]
- Tonezzer, M.; Le, D.T.T.; Duy, L.V.; Nguyen, D.H.; Gasperi, F.; Nguyen, V.D.; Biasioli, F. Electronic noses based on metal oxide nanowires: A review. Nanotechnol. Rev. 2022, 11, 897–925. [Google Scholar] [CrossRef]
- Lu, T.; Al-Hamry, A.; Rosolen, J.M.; Hu, Z.; Hao, J.; Wang, Y.; Adiraju, A.; Yu, T.; Matsubara, E.Y.; Kanoun, O. Flexible impedimetric electronic nose for high-accurate determination of individual volatile organic compounds by tuning the graphene sensitive properties. Chemosensors 2021, 9, 360. [Google Scholar] [CrossRef]
- Liu, H.; Wu, R.; Guo, Q.; Hua, Z.; Wu, Y. Electronic Nose Based on Temperature Modulation of MOS Sensors for Recognition of Excessive Methanol in Liquors. ACS Omega 2021, 6, 30598–30606. [Google Scholar] [CrossRef]
- Tonezzer, M.; Izidoro, S.C.; Moraes, J.P.A.; Dang, L.T.T. Improved Gas Selectivity Based on Carbon Modified SnO2 Nanowires. Front. Mater. 2019, 6, 277. [Google Scholar] [CrossRef]
- Tonezzer, M.; Thai, N.X.; Gasperi, F.; Duy, N.V.; Biasioli, F. Quantitative Assessment of Trout Fish Spoilage with a Single Nanowire Gas Sensor in a Thermal Gradient. Nanomaterials 2021, 11, 1604. [Google Scholar] [CrossRef]
- Tonezzer, M. Single nanowire gas sensor able to distinguish fish and meat and evaluate their degree of freshness. Chemosensors 2021, 9, 249. [Google Scholar] [CrossRef]
- Güniat, L.; Caroff, P.; Morral, A.F.i. Vapor Phase Growth of Semiconductor Nanowires: Key Developments and Open Questions. Chem. Rev. 2019, 119, 8958–8971. [Google Scholar] [CrossRef] [PubMed]
- Thai, N.X.; Tonezzer, M.; Masera, L.; Nguyen, H.; Duy, N.V.; Hoa, N.D. Multi gas sensors using one nanomaterial, temperature gradient, and machine learning algorithms for discrimination of gases and their concentration. Anal. Chim. Acta 2020, 1124, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Paleczek, A.; Rydosz, A. Review of the algorithms used in exhaled breath analysis for the detection of diabetes. J. Breath Res. 2022, 16, 026003. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, D.; McMartin, K.E. Methanol and ethylene glycol poisonings. Mechanism of toxicity, clinical course, diagnosis and treatment. Med. Toxicol. 1986, 1, 309–334. [Google Scholar] [CrossRef]




| Estimated | |||||||
|---|---|---|---|---|---|---|---|
| Ethanol | Methanol | ||||||
| 1 ppm | 10 ppm | 100 ppm | 1 ppm | 10 ppm | 100 ppm | ||
| True | 1 ppm | 8 | |||||
| 10 ppm | 8 | ||||||
| 100 ppm | 8 | ||||||
| 1 ppm | 8 | ||||||
| 10 ppm | 8 | ||||||
| 100 ppm | 8 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonezzer, M.; Bazzanella, N.; Gasperi, F.; Biasioli, F. Nanosensor Based on Thermal Gradient and Machine Learning for the Detection of Methanol Adulteration in Alcoholic Beverages and Methanol Poisoning. Sensors 2022, 22, 5554. https://doi.org/10.3390/s22155554
Tonezzer M, Bazzanella N, Gasperi F, Biasioli F. Nanosensor Based on Thermal Gradient and Machine Learning for the Detection of Methanol Adulteration in Alcoholic Beverages and Methanol Poisoning. Sensors. 2022; 22(15):5554. https://doi.org/10.3390/s22155554
Chicago/Turabian StyleTonezzer, Matteo, Nicola Bazzanella, Flavia Gasperi, and Franco Biasioli. 2022. "Nanosensor Based on Thermal Gradient and Machine Learning for the Detection of Methanol Adulteration in Alcoholic Beverages and Methanol Poisoning" Sensors 22, no. 15: 5554. https://doi.org/10.3390/s22155554
APA StyleTonezzer, M., Bazzanella, N., Gasperi, F., & Biasioli, F. (2022). Nanosensor Based on Thermal Gradient and Machine Learning for the Detection of Methanol Adulteration in Alcoholic Beverages and Methanol Poisoning. Sensors, 22(15), 5554. https://doi.org/10.3390/s22155554








