Image-Based Phenotyping for Non-Destructive In Situ Rice (Oryza sativa L.) Tiller Counting Using Proximal Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
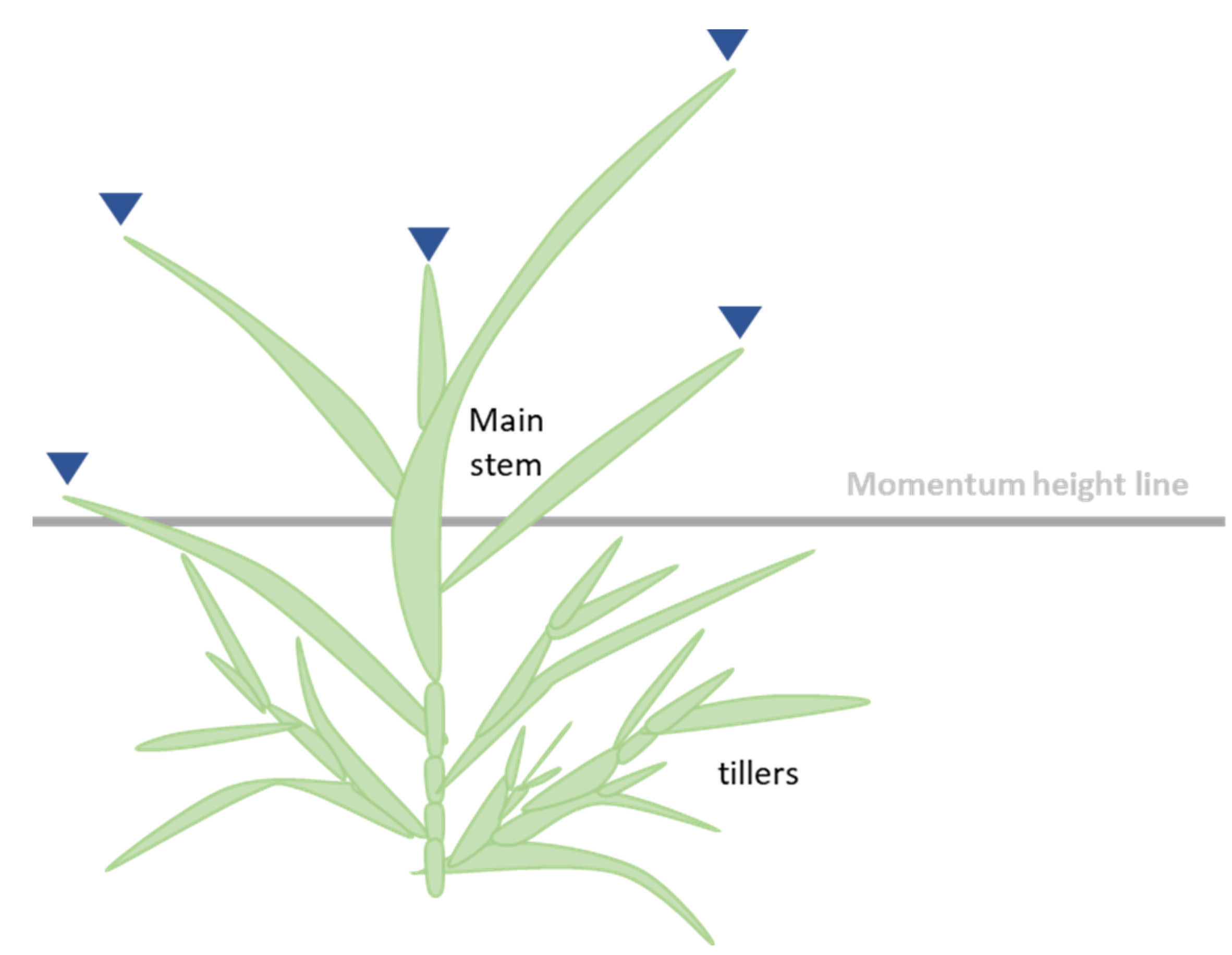
2.2. Analysis Method
- In the image of this experiment, the width of the leaf appeared to be at least 12 pixels. The distance from the centerline of the leaf to the leaf edge would be at least six pixels. Therefore, two leaves that do not overlap should have a centerline distance of at least 6 + 6 pixels. The window size was set to 25 pixels in the horizontal direction to observe 12 pixels on each side.
- The window size in the horizontal direction was set to 12 pixels. The tip of the skeleton has a maximum distance of six pixels from the actual leaf tip, owing to the setting of the line-following method. Therefore, the distance between the skeleton tip of the non-overlapping leaf tip and the centerline of another leaf that interrupts the leaf tip is approximately 6 + 6 pixels.
- ◦
- The line-following method sets a parameter for the interval between the placement of decision points. Each unevenness caused by a single shade is recognized as a tiller of the skeleton if the parameter value is significantly small. Conversely, the skeleton is drawn from multiple leaves if the set value is considerably large. For the images captured in this experiment, the best parameter setting in the preliminary study was seven pixels. When placing the decision point at a point seven pixels off, the tip of the skeleton will have a maximum of six-pixel separation from the actual leaf tip.
3. Results
- a.
- True tiller number (A)—Manually counted leaf-tip number (B)
- b.
- Manually counted leaf-tip number (B)—Automatically counted leaf-tip number (C)
- c.
- True tiller number (A)—Automatically counted leaf-tip number (C)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horie, T.; Yajima, M.; Nakagawa, H. Yield Forecasting. Agric. Syst. 1992, 40, 211–236. [Google Scholar] [CrossRef]
- Horie, T. [Basics of Crop Cultivation] Sakumotsu Saibai No Kiso; Rural Culture Association Japan: Tokyo, Japan, 2004. (In Japanese) [Google Scholar]
- Guo, W.; Fukatsu, T.; Ninomiya, S. Automated Characterization of Flowering Dynamics in Rice Using Field-Acquired Time-Series RGB Images. Plant Methods 2015, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Hayat, M.A.; Wu, J.; Cao, Y. Unsupervised Bayesian Learning for Rice Panicle Segmentation with UAV Images. Plant Methods 2020, 16, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, H.; Cao, Z.; Lu, H.; Madec, S.; Liu, L.; Shen, C. TasselNetv2: In-Field Counting of Wheat Spikes with Context-Augmented Local Regression Networks. Plant Methods 2019, 15, 150. [Google Scholar] [CrossRef]
- Zhou, C.; Ye, H.; Hu, J.; Shi, X.; Hua, S.; Yue, J.; Xu, Z.; Yang, G. Automated Counting of Rice Panicle by Applying Deep Learning Model to Images from Unmanned Aerial Vehicle Platform. Sensors 2019, 19, 3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Gao, S.; Xiao, F.; Li, G.; Ding, Y.; Guo, Q.; Paul, M.J.; Liu, Z. Leaf to Panicle Ratio (LPR): A New Physiological Trait Indicative of Source and Sink Relation in Japonica Rice Based on Deep Learning. Plant Methods 2020, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Tehran, P.; Sabermanesh, K.; Virlet, N.; Hawkesford, M.J. Automated Method to Determine Two Critical Growth Stages of Wheat: Heading and Flowering. Front. Plant Sci. 2017, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Cao, Z.; Lu, H.; Li, Y.; Xiao, Y. In-Field Automatic Observation of Wheat Heading Stage Using Computer Vision. Biosyst. Eng. 2016, 143, 28–41. [Google Scholar] [CrossRef]
- Duan, L.; Huang, C.; Chen, G.; Xiong, L.; Liu, Q.; Yang, W. Determination of Rice Panicle Numbers During Heading by Multi-angle Imaging. Crop J. 2015, 3, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Liang, D.; Yang, X.; Yang, H.; Yue, J.; Yang, G. Wheat Ears Counting in Field Conditions Based on Multi-feature Optimization and TWSVM. Front. Plant Sci. 2018, 9, 1024. [Google Scholar] [CrossRef]
- Xiong, X.; Duan, L.; Liu, L.; Tu, H.; Yang, P.; Wu, D.; Chen, G.; Xiong, L.; Yang, W.; Liu, Q. Panicle-SEG: A Robust Image Segmentation Method for Rice Panicles in the Field Based on Deep Learning and Superpixel Optimization. Plant Methods 2017, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, S.V.; Balasubramanian, V.N.; Fukatsu, T.; Ninomiya, S.; Guo, W. Automatic Estimation of Heading Date of Paddy Rice Using Deep Learning. Plant Methods 2019, 15, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Cao, Z.; Zhao, L.; Zhang, J.; Lv, C.; Li, C.; Xie, J. Rice Heading Stage Automatic Observation by Multi-classifier Cascade Based Rice Spike Detection Method. Agric. For. Meteorol. 2018, 259, 260–270. [Google Scholar] [CrossRef]
- Banayo, N.P.M.C.; Kato, Y. Farmer Participatory Research in Agricultural Extension Programs: A Case Study of Fertilizer Management in Tropical Rice. Exp. Agric. 2020, 56, 710–721. [Google Scholar] [CrossRef]
- Fang, W.; Feng, H.; Yang, W.; Duan, L.; Chen, G.; Xiong, L.; Liu, Q. High-Throughput Volumetric Reconstruction for 3D Wheat Plant Architecture Studies. J. Innov. Opt. Health Sci. 2016, 9, 1650037. [Google Scholar] [CrossRef]
- Boyle, R.D.; Corke, F.M.K.; Doonan, J.H. Automated Estimation of Tiller Number in Wheat by Ribbon Detection. Mach. Vis. Appl. 2016, 27, 637–646. [Google Scholar] [CrossRef]
- Deng, R.; Jiang, Y.; Tao, M.; Huang, X.; Bangura, K.; Liu, C.; Lin, J.; Qi, L. Deep Learning-Based Automatic Detection of Productive Tillers in Rice. Comput. Electron. Agric. 2020, 177, 105703. [Google Scholar] [CrossRef]
- Jin, X.; Madec, S.; Dutartre, D.; de Solan, B.; Comar, A.; Baret, F. High-Throughput Measurements of Stem Characteristics to Estimate Ear Density and Above-Ground Biomass. Plant Phenom. 2019, 2019, 4820305. [Google Scholar] [CrossRef] [Green Version]
- Katayama, T. [Studies on Rice and Wheat Tillers]. Ine Mugi No Bungetsu Kenkyu; Yokendo: Tokyo, Japan, 1951. (In Japanese) [Google Scholar]
- Nemoto, K.; Morita, S.; Baba, T. Shoot and Root Development in Rice Related to the Phyllochron. Crop Sci. 1995, 35, 24–29. [Google Scholar] [CrossRef]
- Wallace Wilhelm, G.S.M. Symposium on the Phyllochron: Importance of the Phyllochron in Studying Development and Growth in Grasses; Wiley: Hoboken, NJ, USA, 1995. [Google Scholar]
- Nagai, M. [Ecological Studies on Nutritional Growth Potential of Paddy Rice 1. Leaf Emergence Process] Suitou no eiyou seichousei ni kansuru seitaigaku teki kenkyu 1. Shutsuyou Keika ni Tsuite. In Nissakuki; Crop Science Society of Japan: Tokyo, Japan, 1966. (In Japanese) [Google Scholar]
- Katsuya, M. [Leaf Out Progression and Elongation of Leaves] Shutsuyou keika to doushinyou ni tsuite. In Nissakuki; Crop Science Society of Japan: Tokyo, Japan, 1983. (In Japanese) [Google Scholar]
- Gotou, Y. [Analysis of Mode of Increase in Tillering in Paddy Rice]. In Suitou ni Okeru Bungetsu Zouka Youshiki Kaiseki; Tohoku University: Sendai, Japan, 1992. (In Japanese) [Google Scholar]
- Yusuke, G.; Mitsuo, S. [Analysis of Rice Seedling Fertility in Rice Populations I. Stem Number Increase Patterns Under Field Conditions with Different Amounts of Nitrogen as Basal Fertilizer]. In Kotaigun Ni Okeru Suitou No Bungetsusei No Kaiseki Daiippou Kihi Chissoryou Wo Ini Suru Hojyou Jyoukenka Deno Keisuu Zouka Yousiki; Crop Science Society of Japan: Tokyo, Japan, 1992. (In Japanese) [Google Scholar]
- Liu, F.; Yang, Y.; Zeng, Y.; Liu, Z. Bending Diagnosis of Rice Seedling Lines and Guidance Line Extraction of Automatic Weeding Equipment in Paddy Field. Mech. Syst. Signal Process. 2020, 142, 106791. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, Y.; Chen, X.; Biswas, A.; Li, S.; Yuan, Z.; Wang, H.; Qi, L. Automatic Detection of Plant Rows for a Transplanter in Paddy Field Using Faster R-CNN. IEEE Access 2020, 8, 147231–147240. [Google Scholar] [CrossRef]
- Bouman, B.A.M.; Van Laar, H.H. Description and evaluation of the rice growth model ORYZA2000 under nitrogen-limited conditions. Agric. Syst. 2006, 87, 249–273. [Google Scholar] [CrossRef]
- Van Diepen, C.A.; Wolf, J.; van Keulen, H.; Rappoldt, C. WOFOST: A simulation model of crop production. Soil Use Manag. 1989, 5, 16–24. [Google Scholar] [CrossRef]
- Huang, J.; Tiana, L.; Shunlin, L.; Maa, H.; Becker-Reshefc, I.; Huangd, Y.; Su, W.; Zhang, X.; Zhu, D.; Wu, W. Improving Winter Wheat Yield Estimation by Assimilation of the Leaf Area Index from Landsat TM and MODIS Data into the WOFOST model. Agric. For. Meteorol. 2015, 204, 106–121. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Huang, J.; Wu, W.; Fan, J.; Zou, J.; Wu, S. Assimilation of MODIS-LAI into the WOFOST Model for Forecasting Regional Winter Wheat Yield. Math. Comput. Modell. 2013, 58, 634–643. [Google Scholar] [CrossRef]
- Cheng, Z.; Meng, J.; Shang, J.; Liu, J.; Huang, J.; Qiao, Y.; Qian, B.; Jing, Q.; Dong, T.; Yu, L. Generating Time-Series LAI Estimates of Maize Using Combined Methods Based on Multispectral UAV Observations and WOFOST Model. Sensors 2020, 20, 6006. [Google Scholar] [CrossRef] [PubMed]










| Accuracy | Method | Reference | ||
|---|---|---|---|---|
| Recall | Precision | Imaging | Analysis | |
| 50% | Not Available | fixed-point camera | SVM | [3] |
| 88% | 87% | UAV | improved R-FCN | [6] |
| 95% | 75% | UAV | Unsupervised Bayesian learning | [4] |
| 82.50% | Not Available | field scanner | SVM | [8] |
| 95% | 56% | fixed-point camera | SVM | [9] |
| 94% | Not Available | turntable camera | ANN | [10] |
| 73% | 82% | GAV | SVM | [11] |
| 89% | 82% | fixed-point camera | CNN | [12] |
| 70% | 85% | fixed-point camera | CNN | [13] |
| 62% | 99.6% | fixed-point camera | CNN | [14] |
| True Tiller Number—Manual Leaf Tip Counting | Manual Leaf Tip Counting—Automatic Leaf Tip Counting | True Tiller Number—Automatic Leaf Tip Counting | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 July | 23 July | 7 August | 3 July | 23 July | 7 August | 3 July | 23 July | 7 August | ||
| R (correlation coefficient) | 0.73 | 0.83 | 0.73 | 0.49 | 0.47 | 0.30 | 0.27 | 0.25 | 0.21 | |
| error average | −1.12 | −1.16 | −1.14 | 2.15 | 3.68 | 1.70 | 1.03 | 2.53 | 0.56 | |
| error variance | 1.53 | 1.55 | 2.06 | 2.18 | 2.52 | 3.46 | 2.55 | 2.99 | 3.68 | |
| t-test p value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | |
| Histogram of errors (sample number for each error) | −7 or less | 1 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 3 |
| −6 | 1 | 1 | 3 | 0 | 0 | 1 | 1 | 0 | 3 | |
| −5 | 3 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 4 | |
| −4 | 12 | 2 | 6 | 1 | 0 | 2 | 6 | 1 | 5 | |
| −3 | 18 | 11 | 22 | 5 | 0 | 2 | 16 | 1 | 5 | |
| −2 | 63 | 29 | 21 | 9 | 1 | 4 | 16 | 9 | 10 | |
| −1 | 71 | 19 | 17 | 12 | 4 | 9 | 25 | 3 | 11 | |
| 0 | 61 | 18 | 22 | 26 | 5 | 11 | 40 | 8 | 15 | |
| 1 | 21 | 9 | 13 | 49 | 11 | 16 | 41 | 17 | 15 | |
| 2 | 7 | 5 | 4 | 40 | 11 | 23 | 38 | 12 | 15 | |
| 3 | 0 | 0 | 7 | 47 | 10 | 10 | 33 | 10 | 5 | |
| 4 | 1 | 0 | 0 | 38 | 16 | 13 | 21 | 11 | 7 | |
| 5 | 0 | 0 | 0 | 16 | 17 | 6 | 12 | 6 | 8 | |
| 6 | 0 | 0 | 0 | 10 | 6 | 3 | 3 | 5 | 5 | |
| 7 or more | 0 | 0 | 0 | 6 | 14 | 10 | 5 | 12 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamagishi, Y.; Kato, Y.; Ninomiya, S.; Guo, W. Image-Based Phenotyping for Non-Destructive In Situ Rice (Oryza sativa L.) Tiller Counting Using Proximal Sensing. Sensors 2022, 22, 5547. https://doi.org/10.3390/s22155547
Yamagishi Y, Kato Y, Ninomiya S, Guo W. Image-Based Phenotyping for Non-Destructive In Situ Rice (Oryza sativa L.) Tiller Counting Using Proximal Sensing. Sensors. 2022; 22(15):5547. https://doi.org/10.3390/s22155547
Chicago/Turabian StyleYamagishi, Yuki, Yoichiro Kato, Seishi Ninomiya, and Wei Guo. 2022. "Image-Based Phenotyping for Non-Destructive In Situ Rice (Oryza sativa L.) Tiller Counting Using Proximal Sensing" Sensors 22, no. 15: 5547. https://doi.org/10.3390/s22155547
APA StyleYamagishi, Y., Kato, Y., Ninomiya, S., & Guo, W. (2022). Image-Based Phenotyping for Non-Destructive In Situ Rice (Oryza sativa L.) Tiller Counting Using Proximal Sensing. Sensors, 22(15), 5547. https://doi.org/10.3390/s22155547







