Impact of EEG Frequency Bands and Data Separation on the Performance of Person Verification Employing Neural Networks
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Feature Extraction
- the normalized peak frequency (the peak frequency divided by the sampling frequency);
- the peak power in the frequency band divided by the mean power in this frequency band;
- the total power in the frequency band divided by the total signal power.
2.3. Neural Networks
2.4. Results Evaluation
- true positive (TP) is the number of segments (feature set vectors) adequately recognized as genuine (a verified person);
- true negative (TN) is the number of segments correctly classified as an impostor (a person who pretends to be the verified person);
- false positive (FP) is the number of segments incorrectly classified as genuine;
- false negative (FN) is the number of segments incorrectly classified as an impostor.
3. Results
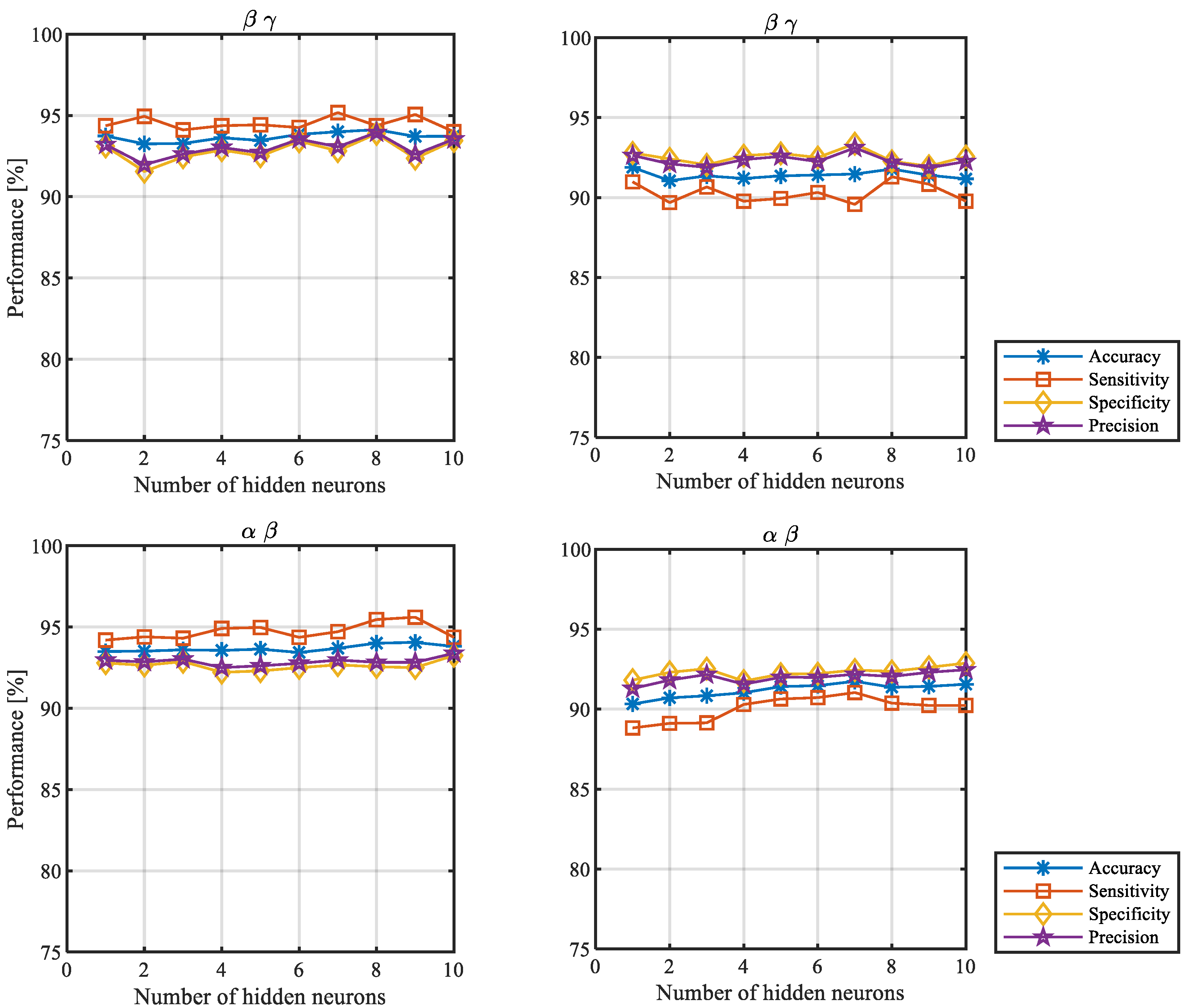
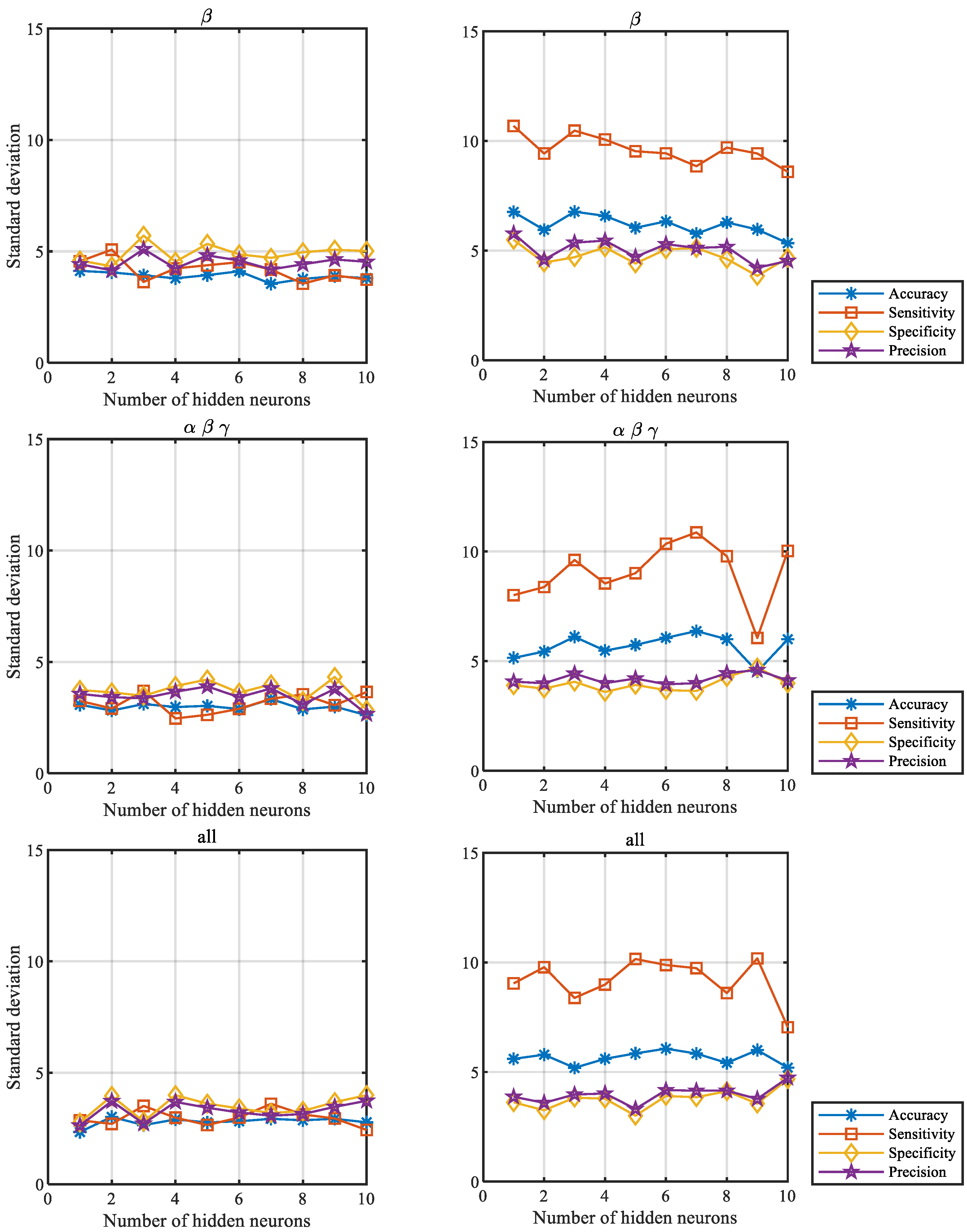
3.1. Influence of the Number of Hidden Neurons on the Verification Performance for Both Scenarios
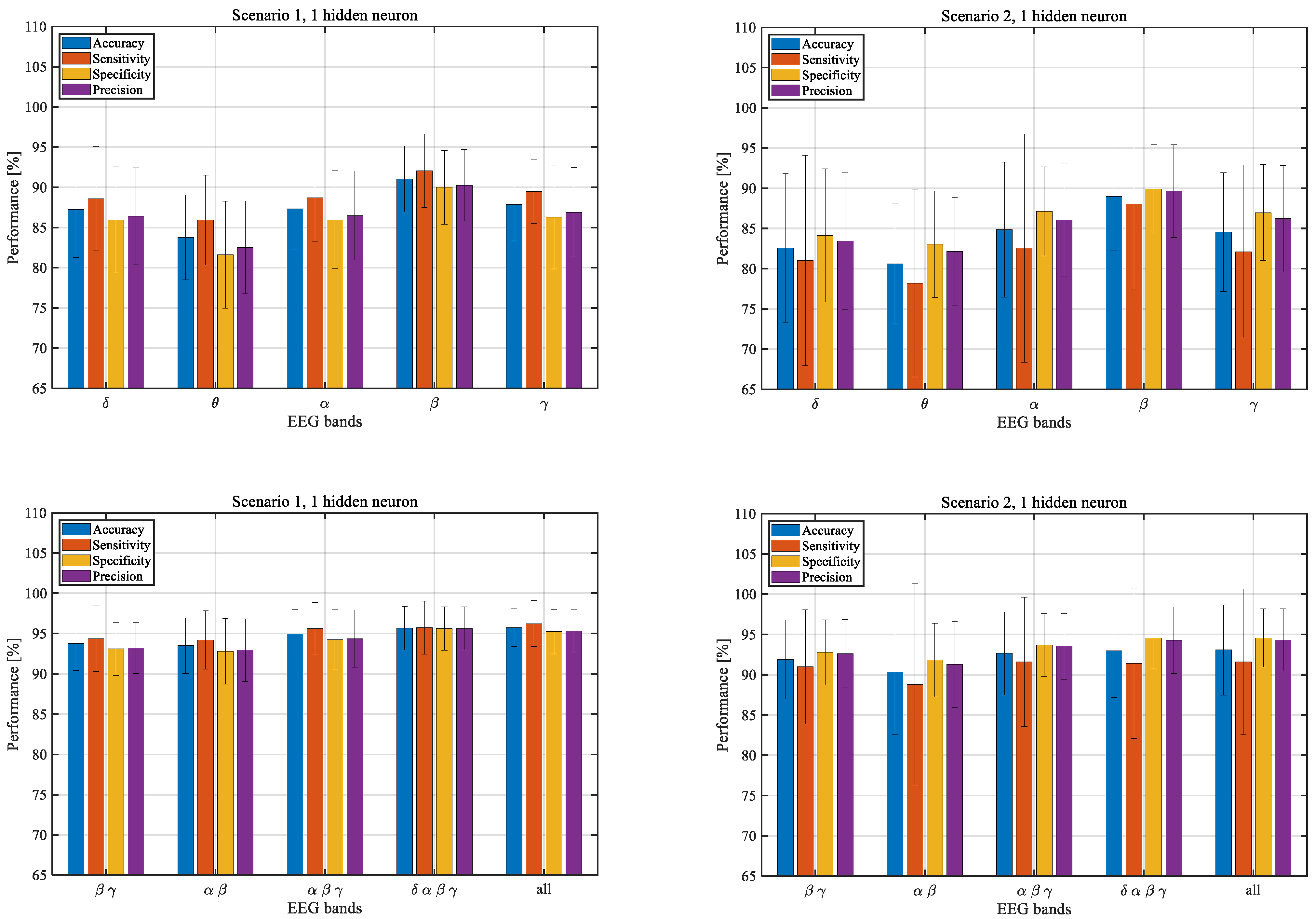
3.2. Performance of the Models Trained with One Hidden Neuron
3.3. Differences between EEG Frequency Bands
3.4. External Impostor Attack
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Wang, Y.; Nakanishi, M.; Zhang, D. EEG-Based Brain-Computer Interfaces. In Neural Interface: Frontiers and Applications. Advances in Experimental Medicine and Biology, vol 1101; Zheng, X., Ed.; Springer: Singapore, 2019; pp. 41–65. ISBN 9789811320507. [Google Scholar]
- Viviani, G.; Vallesi, A. EEG-neurofeedback and executive function enhancement in healthy adults: A systematic review. Psychophysiology 2021, 58, e13874. [Google Scholar] [CrossRef] [PubMed]
- Kappel, S.L.; Looney, D.; Mandic, D.P.; Kidmose, P. Physiological artifacts in scalp EEG and ear-EEG. Biomed. Eng. Online 2017, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kawala-Sterniuk, A.; Podpora, M.; Pelc, M.; Blaszczyszyn, M.; Gorzelanczyk, E.J.; Martinek, R.; Ozana, S. Comparison of Smoothing Filters in Analysis of EEG Data for the Medical Diagnostics Purposes. Sensors 2020, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Van Den Broek, S.P.; Reinders, F.; Donderwinkel, M.; Peters, M.J. Volume conduction effects in EEG and MEG. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 522–534. [Google Scholar] [CrossRef]
- Malekzadeh, A.; Zare, A.; Yaghoobi, M.; Kobravi, H.-R.; Alizadehsani, R. Epileptic Seizures Detection in EEG Signals Using Fusion Handcrafted and Deep Learning Features. Sensors 2021, 21, 1–28. [Google Scholar] [CrossRef]
- Kautzky, A.; Vanicek, T.; Philippe, C.; Kranz, G.S.; Wadsak, W.; Mitterhauser, M.; Hartmann, A.; Hahn, A.; Hacker, M.; Rujescu, D.; et al. Machine learning classification of ADHD and HC by multimodal serotonergic data. Transl. Psychiatry 2020, 10, 104. [Google Scholar] [CrossRef]
- Phan, T.-D.T.; Kim, S.-H.; Yang, H.-J.; Lee, G.-S. EEG-Based Emotion Recognition by Convolutional Neural Network with Multi-Scale Kernels. Sensors 2021, 21, 5052. [Google Scholar] [CrossRef]
- ISO/IEC 2382-37:2022 Information Technology—Vocabulary—Part 37: Biometrics, 3rd ed.; ISO/IEC: Geneva, Switzerland, 2022.
- Jijomon, C.M.; Vinod, A.P. EEG-based Biometric Identification using Frequently Occurring Maximum Power Spectral Features. In Proceedings of the 2018 IEEE Applied Signal Processing Conference, Kolkata, India, 7–9 December 2018; pp. 249–252. [Google Scholar] [CrossRef]
- Abo-Zahhad, M.; Ahmed, S.M.; Abbas, S.N. State-of-the-art methods and future perspectives for personal recognition based on electroencephalogram signals. IET Biom. 2015, 4, 179–190. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, L.; Mao, X.; Hu, C.; Liu, P. Review on EEG-Based Authentication Technology. Comput. Intell. Neurosci. 2021, 2021, 5229576. [Google Scholar] [CrossRef]
- Jalaly Bidgoly, A.; Jalaly Bidgoly, H.; Arezoumand, Z. A survey on methods and challenges in EEG based authentication. Comput. Secur. 2020, 93, 101788. [Google Scholar] [CrossRef]
- DelPozo-Banos, M.; Travieso, C.M.; Weidemann, C.T.; Alonso, J.B. EEG biometric identification: A thorough exploration of the time-frequency domain. J. Neural Eng. 2015, 12, 056019. [Google Scholar] [CrossRef]
- Yang, S.; Deravi, F. On the Usability of Electroencephalographic Signals for Biometric Recognition: A Survey. IEEE Trans. Hum.-Mach. Syst. 2017, 47, 958–969. [Google Scholar] [CrossRef]
- Arias-Cabarcos, P.; Habrich, T.; Becker, K.; Becker, C.; Strufe, T. Inexpensive Brainwave Authentication: New Techniques and Insights on User Acceptance. In Proceedings of the 30th USENIX Security Symposium (USENIX Security 21), Virtual, 11–13 August 2021; pp. 55–72. [Google Scholar]
- Palaniappan, R.; Mandic, D.P. Biometrics from brain electrical activity: A machine learning approach. IEEE Trans. Pattern Anal. Mach. Intell. 2007, 29, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Jayarathne, I.; Cohen, M.; Amarakeerthi, S. BrainID: Development of an EEG-based biometric authentication system. In Proceedings of the 2016 IEEE 7th Annual Information Technology, Electronics and Mobile Communication Conference (IEMCON), Vancouver, BC, Canada, 13–15 October 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Smit, D.J.A.; Stam, C.J.; Posthuma, D.; Boomsma, D.I.; de Geus, E.J.C. Heritability of “Small-World” Networks in the Brain: A Graph Theoretical Analysis of Resting-State EEG Functional Connectivity. Hum. Brain Mapp. 2008, 29, 1368–1378. [Google Scholar] [CrossRef]
- Van Beijsterveldt, C.E.M.; Molenaar, P.C.M.; de Geus, E.J.C.; Boomsma, D.I. Heritability of human brain functioning as assessed by electroencephalosraphy. Am. J. Hum. Genet. 1996, 58, 562–573. [Google Scholar] [PubMed]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Poulos, M.; Rangoussi, M.; Alexandris, N. Neural network based person identification using EEG features. In Proceedings of the 1999 IEEE International Conference on Acoustics, Speech, and Signal Processing, ICASSP99 (Cat. No.99CH36258), Phoenix, AZ, USA, 15–19 March 1999; pp. 1117–1120. [Google Scholar] [CrossRef]
- Abdullah, M.K.; Subari, K.S.; Loong, J.L.C.; Ahmad, N.N. Analysis of the EEG Signal for a Practical Biometric System. World Acad. Sci. Eng. Technol. 2010, 44, 1133–1137. [Google Scholar]
- Thomas, K.P.; Vinod, A.P. EEG-Based Biometric Authentication Using Gamma Band Power During Rest State. Circuits Syst. Signal Process. 2018, 37, 277–289. [Google Scholar] [CrossRef]
- Ma, L.; Minett, J.W.; Blu, T.; Wang, W.S.-Y. Resting State EEG-based biometrics for individual identification using convolutional neural networks. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 2848–2851. [Google Scholar] [CrossRef]
- Nakanishi, I.; Baba, S.; Miyamoto, C. EEG based biometric authentication using new spectral features. In Proceedings of the 2009 International Symposium on Intelligent Signal Processing and Communication Systems, Kanazawa, Japan, 7–9 January 2009; pp. 651–654. [Google Scholar] [CrossRef]
- Cheng, C.-Y. EEG-Based Person Identification System and Its Longitudinal Adaptation. Master’s Thesis, National Chiao Tung University, Taiwan, China, 2013. [Google Scholar]
- Maiorana, E. Learning deep features for task-independent EEG-based biometric verification. Pattern Recognit. Lett. 2021, 143, 122–129. [Google Scholar] [CrossRef]
- Maiorana, E.; La Rocca, D.; Campisi, P. On the Permanence of EEG Signals for Biometric Recognition. IEEE Trans. Inf. Forensics Secur. 2016, 11, 163–175. [Google Scholar] [CrossRef]
- Maiorana, E.; Campisi, P. Longitudinal Evaluation of EEG-Based Biometric Recognition. IEEE Trans. Inf. Forensics Secur. 2018, 13, 1123–1138. [Google Scholar] [CrossRef]
- Tangkraingkij, P.; Lursinsap, C.; Sanguansintukul, S.; Desudchit, T. Personal Identification by EEG Using ICA and Neural Network. In Proceedings of the Computational Science and Its Applications—ICCSA 2010, Fukuoka, Japan, 23–26 March 2010; Taniar, D., Gervasi, O., Murgante, B., Pardede, E., Apduhan, B.O., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6018 LNCS, pp. 419–430. [Google Scholar]
- Chan, H.-L.; Kuo, P.-C.; Cheng, C.-Y.; Chen, Y.-S. Challenges and Future Perspectives on Electroencephalogram-Based Biometrics in Person Recognition. Front. Neuroinform. 2018, 12, 66. [Google Scholar] [CrossRef]
- Campisi, P.; Rocca, D. La Brain waves for automatic biometric-based user recognition. IEEE Trans. Inf. Forensics Secur. 2014, 9, 782–800. [Google Scholar] [CrossRef]
- Kaufman, S.; Rosset, S.; Perlich, C.; Stitelman, O. Leakage in data mining: Formulation, detection, and avoidance. ACM Trans. Knowl. Discov. Data 2012, 6, 1–21. [Google Scholar] [CrossRef]
- Jasper, H.H. Report of the committee on methods of clinical examination in electroencephalography: 1957. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar] [CrossRef]
- Jijomon, C.M.; Vinod, A.P. EEG-based biometric identification using frequency-weighted power feature. IET Biom. 2020, 9, 251–258. [Google Scholar] [CrossRef]
- Basheer, I.A.; Hajmeer, M. Artificial neural networks: Fundamentals, computing, design, and application. J. Microbiol. Methods 2000, 43, 3–31. [Google Scholar] [CrossRef]
- Hagan, M.T.; Menhaj, M.B. Training feedforward networks with the Marquardt algorithm. IEEE Trans. Neural. Netw. 1994, 5, 989–993. [Google Scholar] [CrossRef]
- Wróbel, A. Beta activity: A carrier for visual attention. Acta Neurobiol. Exp. 2000, 60, 247–260. [Google Scholar]
- Kamiński, J.; Brzezicka, A.; Gola, M.; Wróbel, A. Beta band oscillations engagement in human alertness process. Int. J. Psychophysiol. 2012, 85, 125–128. [Google Scholar] [CrossRef]
- Williams, P.E.E.G. Alpha Feedback—A Comparison of Two Control Groups. Psychosom. Med. 1977, 39, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Paluch, K.; Jurewicz, K.; Rogala, J.; Krauz, R.; Szczypińska, M.; Mikicin, M.; Wróbel, A.; Kublik, E. Beware: Recruitment of Muscle Activity by the EEG-Neurofeedback Trainings of High Frequencies. Front. Hum. Neurosci. 2017, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- DeGood, D.E.; Chisholm, R.C. Multiple Response Comparison of Parietal EEG and Frontalis EMG Biofeedback. Psychophysiology 1977, 14, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Maurizio, S.; Liechti, M.D.; Brandeis, D.; Jäncke, L.; Drechsler, R. Differential EMG Biofeedback for Children with ADHD: A Control Method for Neurofeedback Training with a Case Illustration. Appl. Psychophysiol. Biofeedback 2013, 38, 109–119. [Google Scholar] [CrossRef][Green Version]
- Ermentrout, G.B.; Kopell, N. Oscillator Death in Systems of Coupled Neural Oscillators. SIAM J. Appl. Math. 1990, 50, 125–146. [Google Scholar] [CrossRef]
- Kopell, N.; Ermentrout, G.B.; Whittington, M.A.; Traub, R.D. Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl. Acad. Sci. USA 2000, 97, 1867–1872. [Google Scholar] [CrossRef]
- Chandrasekaran, L.; Achuthan, S.; Canavier, C.C. Stability of two cluster solutions in pulse coupled networks of neural oscillators. J. Comput. Neurosci. 2011, 30, 427–445. [Google Scholar] [CrossRef][Green Version]
- Chen, J.X.; Mao, Z.J.; Yao, W.X.; Huang, Y.F. EEG-based biometric identification with convolutional neural network. Multimed. Tools Appl. 2020, 79, 10655–10675. [Google Scholar] [CrossRef]
- Özdenizci, O.; Wang, Y.; Koike-Akino, T.; Erdoğmuş, D. Adversarial Deep Learning in EEG Biometrics. IEEE Signal Process. Lett. 2019, 26, 710–714. [Google Scholar] [CrossRef]



| hn | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| fb | |||||||||||
| δ | 87.3 ±6.0 | 87.4 ±5.8 | 87.5 ±5.3 | 88.2 ±5.6 | 87.9 ±5.3 | 89.0 ±5.1 | 88.9 ±5.1 | 88.3 ±5.4 | 88.8 ±5.1 | 88.9 ±5.3 | |
| θ | 83.8 ±5.3 | 83.4 ±5.4 | 84.3 ±4.7 | 83.8 ±4.4 | 84.1 ±4.9 | 84.3 ±4.8 | 84.1 ±5.1 | 84.0 ±5.0 | 84.2 ±5.2 | 83.8 ±5.3 | |
| α | 87.3 ±5.0 | 87.6 ±5.1 | 87.5 ±4.9 | 88.0 ±5.0 | 88.0 ±4.9 | 87.8 ±5.0 | 87.5 ±4.5 | 87.6 ±4.8 | 87.6 ±4.7 | 87.3 ±5.4 | |
| β | 91.0 ±4.1 | 91.5 ±4.1 | 92.1 ±3.9 | 92.0 ±3.8 | 91.9 ±3.9 | 91.9 ±4.1 | 92.1 ±3.5 | 91.9 ±3.8 | 92.4 ±3.9 | 92.2 ±3.8 | |
| γ | 87.9 ±4.5 | 88.1 ±5.0 | 88.1 ±5.0 | 87.9 ±4.9 | 87.5 ±5.0 | 88.0 ±4.5 | 87.5 ±4.7 | 87.3 ±5.3 | 87.6 ±5.0 | 87.0 ±5.3 | |
| β γ | 93.7 ±3.3 | 93.2 ±3.7 | 93.3 ±3.3 | 93.6 ±3.3 | 93.5 ±3.5 | 93.8 ±3.1 | 94.0 ±2.8 | 94.1 ±3.0 | 93.7 ±3.1 | 93.7 ±3.1 | |
| α β | 93.5 ±3.5 | 93.5 ±3.5 | 93.6 ±3.7 | 93.6 ±3.4 | 93.6 ±3.6 | 93.4 ±4.0 | 93.7 ±3.6 | 94.0 ±3.1 | 94.1 ±3.3 | 93.8 ±3.4 | |
| α β γ | 94.9 ±3.1 | 94.7 ±2.8 | 94.9 ±3.1 | 94.8 ±3.0 | 94.6 ±3.0 | 94.9 ±2.9 | 94.8 ±3.3 | 94.3 ±2.9 | 94.4 ±3.0 | 94.6 ±2.6 | |
| δ α β γ | 95.7 ±2.7 | 95.4 ±2.7 | 95.3 ±2.6 | 95.4 ±2.6 | 95.0 ±2.9 | 95.4 ±2.8 | 95.3 ±2.8 | 95.2 ±2.5 | 95.3 ±2.5 | 95.3 ±2.8 | |
| All | 95.7 ±2.3 | 95.5 ±3.0 | 95.6 ±2.7 | 95.4 ±2.9 | 95.6 ±2.8 | 95.6 ±2.8 | 95.4 ±2.9 | 95.2 ±2.9 | 95.2 ±2.9 | 95.5 ±2.8 | |
| hn | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| fb | |||||||||||
| δ | 82.6 ±9.2 | 82.9 ±8.6 | 82.7 ±8.6 | 82.0 ±8.6 | 82.7 ±9.4 | 83.6 ±8.5 | 82.7 ±9.1 | 82.9 ±8.7 | 83.3 ±8.7 | 82.8 ±8.7 | |
| θ | 80.6 ±7.5 | 80.7 ±7.2 | 81.3 ±7.3 | 80.6 ±7.6 | 80.6 ±7.4 | 80.6 ±7.8 | 80.7 ±7.5 | 80.8 ±6.9 | 80.9 ±6.8 | 80.9 ±7.3 | |
| α | 84.8 ±8.4 | 85.0 ±7.5 | 85.2 ±8.4 | 85.1 ±7.6 | 85.3 ±7.5 | 85.8 ±7.0 | 85.5 ±7.0 | 86.0 ±8.1 | 85.8 ±7.2 | 85.8 ±7.3 | |
| β | 89.0 ±6.8 | 89.4 ±5.9 | 89.0 ±6.8 | 89.5 ±6.6 | 89.5 ±6.0 | 89.7 ±6.3 | 89.8 ±5.8 | 89.7 ±6.3 | 90.0 ±6.0 | 90.0 ±5.3 | |
| γ | 84.6 ±7.4 | 84.9 ±7.4 | 84.7 ±7.3 | 83.8 ±7.7 | 84.1 ±7.3 | 84.6 ±7.2 | 84.3 ±7.3 | 83.9 ±7.3 | 83.3 ±7.1 | 84.2 ±6.9 | |
| β γ | 91.9 ±4.9 | 91.0 ±5.8 | 91.4 ±5.4 | 91.2 ±5.5 | 91.4 ±5.0 | 91.4 ±5.5 | 91.5 ±5.2 | 91.8 ±5.2 | 91.4 ±5.6 | 91.2 ±5.7 | |
| α β | 90.3 ±7.7 | 90.7 ±7.0 | 90.8 ±7.1 | 91.0 ±6.6 | 91.4 ±6.5 | 91.5 ±5.7 | 91.7 ±6.2 | 91.4 ±6.3 | 91.4 ±6.3 | 91.6 ±6.7 | |
| α β γ | 92.7 ±5.1 | 92.4 ±5.4 | 92.4 ±6.1 | 93.0 ±5.5 | 92.6 ±5.7 | 92.2 ±6.1 | 92.1 ±6.4 | 92.6 ±6.0 | 92.9 ±4.5 | 92.0 ±6.0 | |
| δ α β γ | 93.0 ±5.8 | 93.0 ±5.8 | 93.3 ±5.8 | 93.1 ±5.4 | 92.9 ±5.1 | 93.0 ±5.6 | 93.0 ±5.6 | 92.8 ±5.8 | 93.0 ±5.9 | 93.0 ±5.1 | |
| All | 93.1 ±5.6 | 93.1 ±5.8 | 93.4 ±5.2 | 93.3 ±5.6 | 92.9 ±5.8 | 93.0 ±6.1 | 92.6 ±5.8 | 93.2 ±5.4 | 92.9 ±6.0 | 92.9 ±5.2 | |
| EEG Bands | ACC (%) | SEN (%) | SPEC (%) | PREC (%) |
|---|---|---|---|---|
| δ | 0.03 | 7.0 × 10−3 | 0.36 | 0.13 |
| θ | 0.07 | 2.1 × 10−3 | 0.41 | 0.81 |
| α | 0.17 | 0.03 | 0.46 | 0.80 |
| β | 0.17 | 0.07 | 0.95 | 0.65 |
| γ | 0.04 | 1.1 × 10−3 | 0.66 | 0.67 |
| β γ | 0.10 | 0.03 | 0.74 | 0.56 |
| α β | 0.05 | 0.03 | 0.39 | 0.18 |
| α β γ | 0.05 | 0.02 | 0.61 | 0.41 |
| δ α β γ | 0.03 | 0.02 | 0.23 | 0.15 |
| All | 0.02 | 0.01 | 0.44 | 0.26 |
| EEG Bands | ACC (%) | SEN (%) | SPEC (%) | PREC (%) |
|---|---|---|---|---|
| δ | 87.3 ± 6.0 | 88.6 ± 6.5 | 85.9 ± 6.6 | 86.4 ± 6.0 |
| θ | 83.8 ± 5.3 | 85.9 ± 5.6 | 81.6 ± 6.6 | 82.5 ± 5.8 |
| α | 87.3 ± 5.0 | 88.7 ± 5.4 | 86.0 ± 6.1 | 86.5 ± 5.5 |
| β | 91.0 ± 4.1 | 92.1 ± 4.6 | 90.0 ± 4.6 | 90.3 ± 4.4 |
| γ | 87.9 ± 4.5 | 89.5 ± 4.0 | 86.3 ± 6.4 | 86.9 ± 5.6 |
| β γ | 93.7 ± 3.3 | 94.4 ± 4.1 | 93.1 ± 3.3 | 93.2 ± 3.2 |
| α β | 93.5 ± 3.5 | 94.2 ± 3.6 | 92.8 ± 4.1 | 92.9 ± 3.9 |
| α β γ | 94.9 ± 3.1 | 95.6 ± 3.2 | 94.2 ± 3.7 | 94.4 ± 3.6 |
| δ α β γ | 95.7 ± 2.7 | 95.7 ± 3.3 | 95.6 ± 2.7 | 95.6 ± 2.7 |
| All | 95.7 ± 2.3 | 96.2 ± 2.9 | 95.2 ± 2.8 | 95.3 ± 2.6 |
| EEG Bands | ACC (%) | SEN (%) | SPEC (%) | PREC (%) |
|---|---|---|---|---|
| δ | 82.6 ± 9.2 | 81.0 ± 13.1 | 84.1 ± 8.3 | 83.5 ± 8.5 |
| θ | 80.6 ± 7.5 | 78.2 ± 11.7 | 83.0 ± 6.6 | 82.1 ± 6.7 |
| α | 84.8 ± 8.4 | 82.6 ± 14.2 | 87.1 ± 5.6 | 86.1 ± 7.1 |
| β | 89.0 ± 6.8 | 88.0 ± 10.7 | 89.9 ± 5.5 | 89.7 ± 5.8 |
| γ | 84.6 ± 7.4 | 82.1 ± 10.7 | 87.0 ± 6.0 | 86.2 ± 6.6 |
| β γ | 91.9 ± 4.9 | 91.0 ± 7.1 | 92.8 ± 4.0 | 92.6 ± 4.2 |
| α β | 90.3 ± 7.7 | 88.8 ± 12.5 | 91.8 ± 4.6 | 91.3 ± 5.3 |
| α β γ | 92.7 ± 5.1 | 91.6 ± 8.0 | 93.7 ± 3.9 | 93.5 ± 4.1 |
| δ α β γ | 93.0 ± 5.8 | 91.4 ± 9.3 | 94.5 ± 3.8 | 94.3 ± 4.1 |
| All | 93.1 ± 5.6 | 91.6 ± 9.0 | 94.6 ± 3.6 | 94.3 ± 3.9 |
| EEG Bands | δ | θ | A | β | γ | β γ | α β | α β γ | δ α β γ | All |
|---|---|---|---|---|---|---|---|---|---|---|
| δ | 0.04 | 1.00 | 0.02 | 1.00 | 2.5 × 10−7 | 6.1 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | |
| θ | 0.03 | 1.3 × 10−7 | 0.01 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | ||
| α | 0.02 | 1.00 | 3.1 × 10−7 | 8.4 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | |||
| β | 0.10 | 0.28 | 0.42 | 0.01 | 9.0 × 10−4 | 6.7 × 10−4 | ||||
| γ | 3.4 × 10−6 | 1.1 × 10−5 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | |||||
| β γ | 1.00 | 0.99 | 0.76 | 0.71 | ||||||
| α β | 0.95 | 0.60 | 0.56 | |||||||
| α β γ | 1.00 | 1.00 | ||||||||
| δ α β γ | 1.00 | |||||||||
| All |
| EEG Bands | δ | θ | α | β | γ | β γ | α β | α β γ | δ α β γ | All |
|---|---|---|---|---|---|---|---|---|---|---|
| δ | 0.99 | 0.97 | 0.02 | 0.99 | 1.7 × 10−5 | 1.0 × 10−3 | 1.8 × 10−6 | 7.4 × 10−7 | 5.5 × 10−7 | |
| θ | 0.39 | 2.2 × 10−4 | 0.49 | 1.6 × 10−7 | 5.6 × 10−6 | 1.3 × 10−7 | 1.3 × 10−7 | 1.3 × 10−7 | ||
| α | 0.42 | 1.00 | 4.8 × 10−3 | 0.08 | 8.5 × 10−4 | 3.9 × 10−4 | 2.9 × 10−4 | |||
| β | 0.32 | 0.86 | 1.00 | 0.59 | 0.47 | 0.43 | ||||
| γ | 2.6 × 10−3 | 0.05 | 4.2 × 10−4 | 1.9 × 10−4 | 1.4 × 10−4 | |||||
| β γ | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| α β | 0.96 | 0.91 | 0.89 | |||||||
| α β γ | 1.00 | 1.00 | ||||||||
| δ α β γ | 1.00 | |||||||||
| All |
| hn | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| fb | |||||||||||
| δ | 14.96 | 15.18 | 14.51 | 15.24 | 16.66 | 15.26 | 14.99 | 14.74 | 14.86 | 15.27 | |
| θ | 22.08 | 21.08 | 21.18 | 22.16 | 21.90 | 22.29 | 20.72 | 22.79 | 22.50 | 22.74 | |
| α | 17.83 | 17.95 | 18.04 | 16.82 | 18.07 | 17.49 | 17.45 | 18.95 | 18.87 | 19.18 | |
| β | 14.06 | 13.59 | 13.24 | 13.13 | 13.27 | 14.38 | 13.64 | 13.29 | 13.32 | 13.85 | |
| γ | 18.06 | 16.60 | 17.63 | 17.84 | 17.57 | 17.22 | 17.99 | 18.56 | 17.66 | 19.31 | |
| β γ | 11.94 | 12.45 | 12.08 | 11.63 | 12.18 | 11.76 | 13.12 | 11.31 | 12.66 | 12.27 | |
| α β | 11.83 | 12.41 | 11.77 | 11.38 | 12.18 | 12.34 | 12.77 | 12.52 | 12.20 | 11.41 | |
| α β γ | 10.31 | 10.59 | 10.44 | 11.03 | 11.34 | 11.03 | 10.40 | 11.44 | 11.11 | 11.42 | |
| δ α β γ | 9.00 | 9.16 | 9.69 | 10.19 | 9.82 | 8.85 | 9.41 | 10.57 | 9.80 | 10.63 | |
| All | 8.41 | 8.48 | 9.09 | 9.76 | 8.88 | 9.05 | 9.41 | 9.41 | 10.59 | 10.43 | |
| hn | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| fb | |||||||||||
| δ | 14.99 | 15.39 | 16.02 | 15.83 | 16.05 | 15.11 | 15.31 | 15.95 | 16.24 | 15.74 | |
| θ | 21.82 | 22.22 | 21.35 | 21.61 | 22.75 | 20.62 | 21.21 | 20.96 | 21.85 | 21.55 | |
| α | 16.32 | 16.35 | 16.62 | 18.51 | 17.36 | 16.95 | 17.70 | 18.40 | 19.28 | 18.36 | |
| β | 14.61 | 13.62 | 12.94 | 13.87 | 13.52 | 14.07 | 13.54 | 14.22 | 14.27 | 13.61 | |
| γ | 15.87 | 16.49 | 16.87 | 16.76 | 17.49 | 18.59 | 17.60 | 17.56 | 17.60 | 18.82 | |
| β γ | 11.98 | 12.55 | 12.52 | 11.79 | 11.24 | 12.28 | 11.88 | 12.39 | 12.64 | 11.78 | |
| α β | 10.96 | 11.22 | 11.88 | 11.29 | 11.65 | 12.52 | 11.67 | 11.73 | 11.36 | 11.52 | |
| α β γ | 10.48 | 10.14 | 10.11 | 9.83 | 11.13 | 10.97 | 11.36 | 11.69 | 10.56 | 10.93 | |
| δ α β γ | 9.24 | 9.35 | 8.56 | 8.50 | 9.65 | 9.79 | 9.12 | 8.95 | 9.57 | 10.41 | |
| All | 8.46 | 8.24 | 8.32 | 9.25 | 8.52 | 9.39 | 9.25 | 9.11 | 10.10 | 10.48 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plucińska, R.; Jędrzejewski, K.; Waligóra, M.; Malinowska, U.; Rogala, J. Impact of EEG Frequency Bands and Data Separation on the Performance of Person Verification Employing Neural Networks. Sensors 2022, 22, 5529. https://doi.org/10.3390/s22155529
Plucińska R, Jędrzejewski K, Waligóra M, Malinowska U, Rogala J. Impact of EEG Frequency Bands and Data Separation on the Performance of Person Verification Employing Neural Networks. Sensors. 2022; 22(15):5529. https://doi.org/10.3390/s22155529
Chicago/Turabian StylePlucińska, Renata, Konrad Jędrzejewski, Marek Waligóra, Urszula Malinowska, and Jacek Rogala. 2022. "Impact of EEG Frequency Bands and Data Separation on the Performance of Person Verification Employing Neural Networks" Sensors 22, no. 15: 5529. https://doi.org/10.3390/s22155529
APA StylePlucińska, R., Jędrzejewski, K., Waligóra, M., Malinowska, U., & Rogala, J. (2022). Impact of EEG Frequency Bands and Data Separation on the Performance of Person Verification Employing Neural Networks. Sensors, 22(15), 5529. https://doi.org/10.3390/s22155529







