Modifications of Epitaxial Graphene on SiC for the Electrochemical Detection and Identification of Heavy Metal Salts in Seawater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Epitaxial Graphene Synthesis, Oxygen Plasma Modification, and Surface Characterization
2.3. Electrochemistry
2.4. Machine Learning
3. Results and Discussion
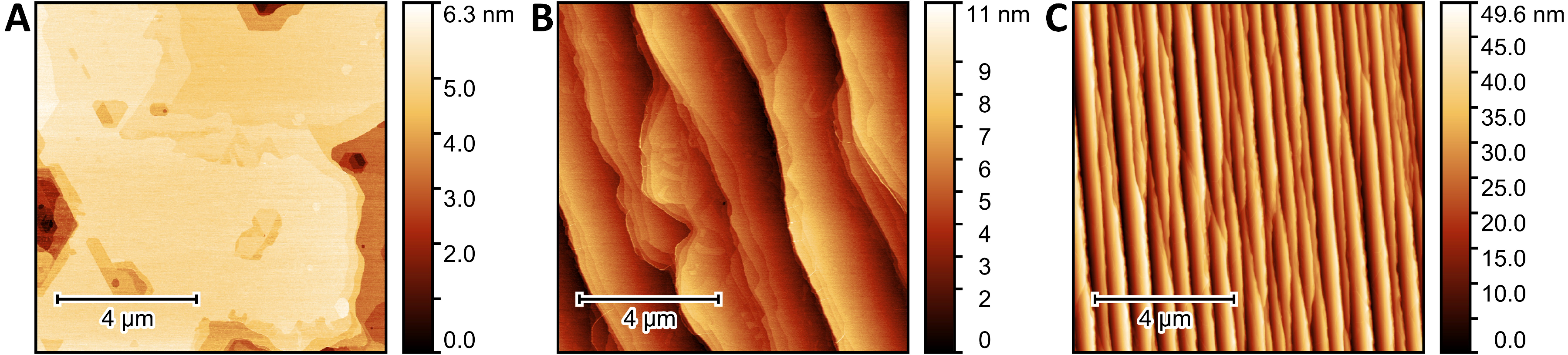
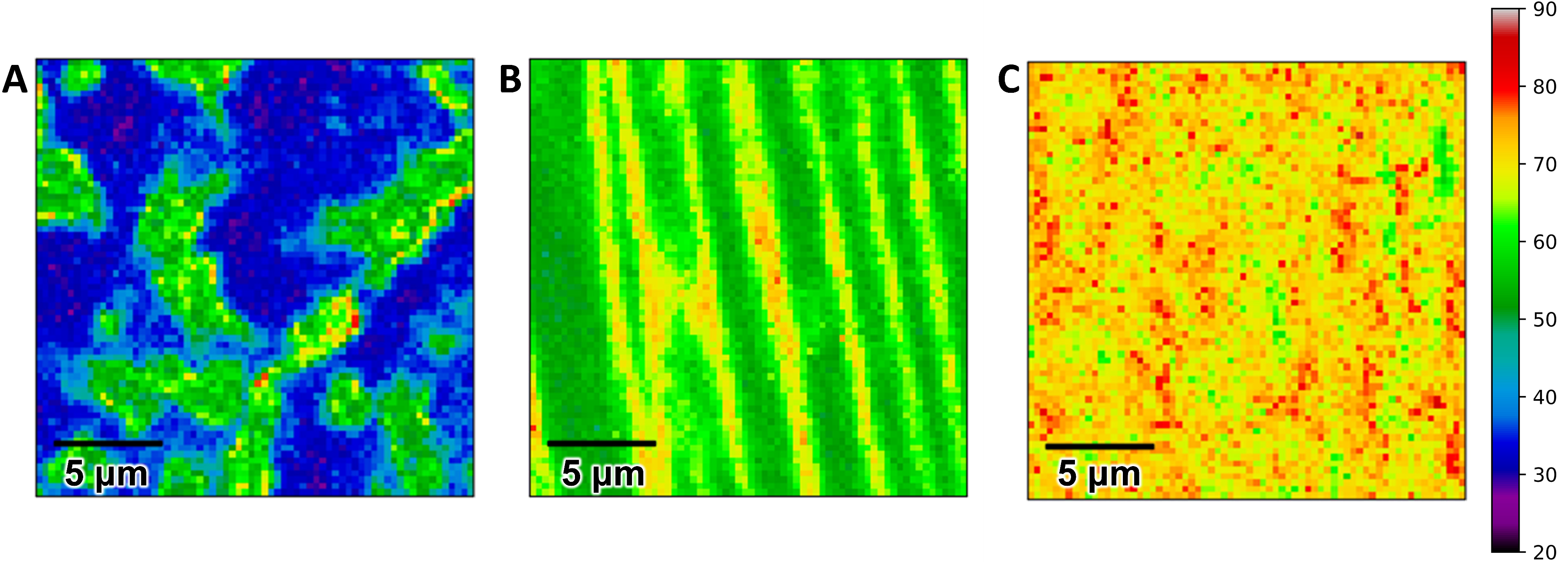
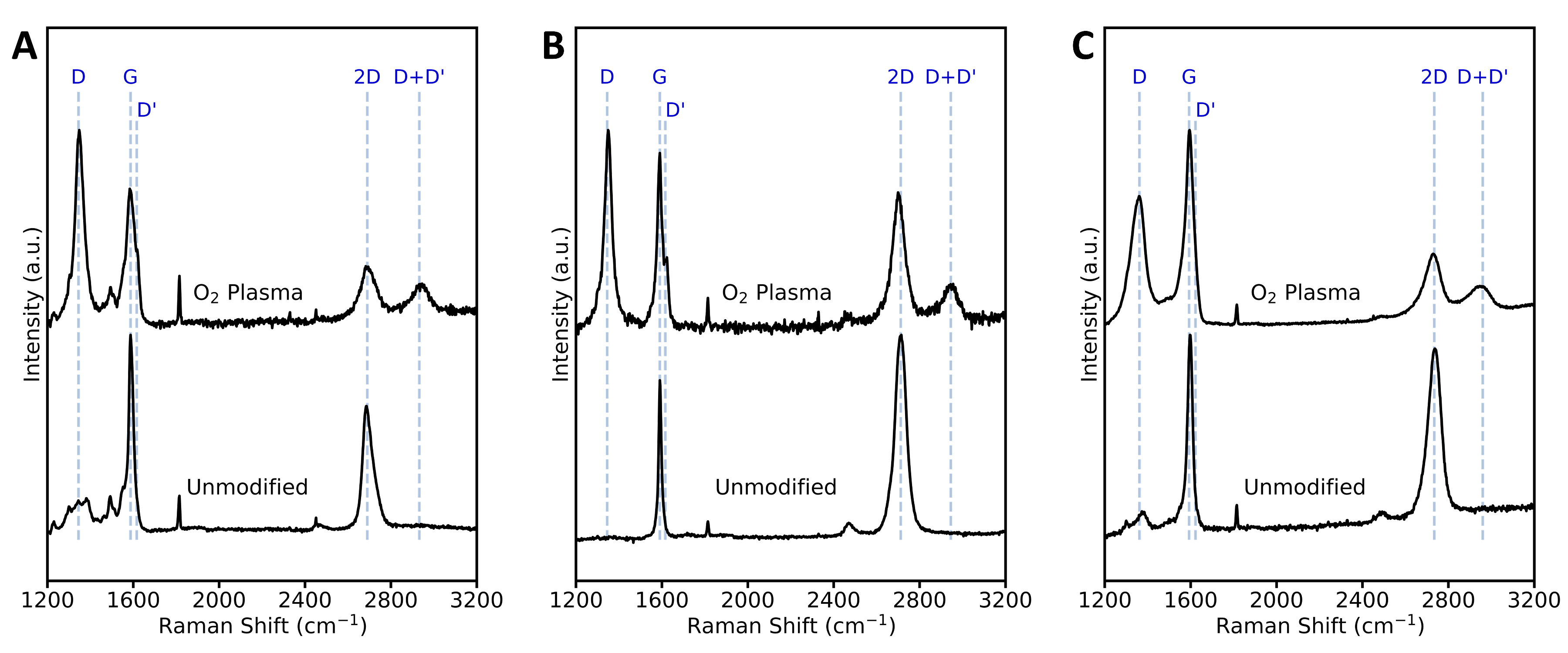
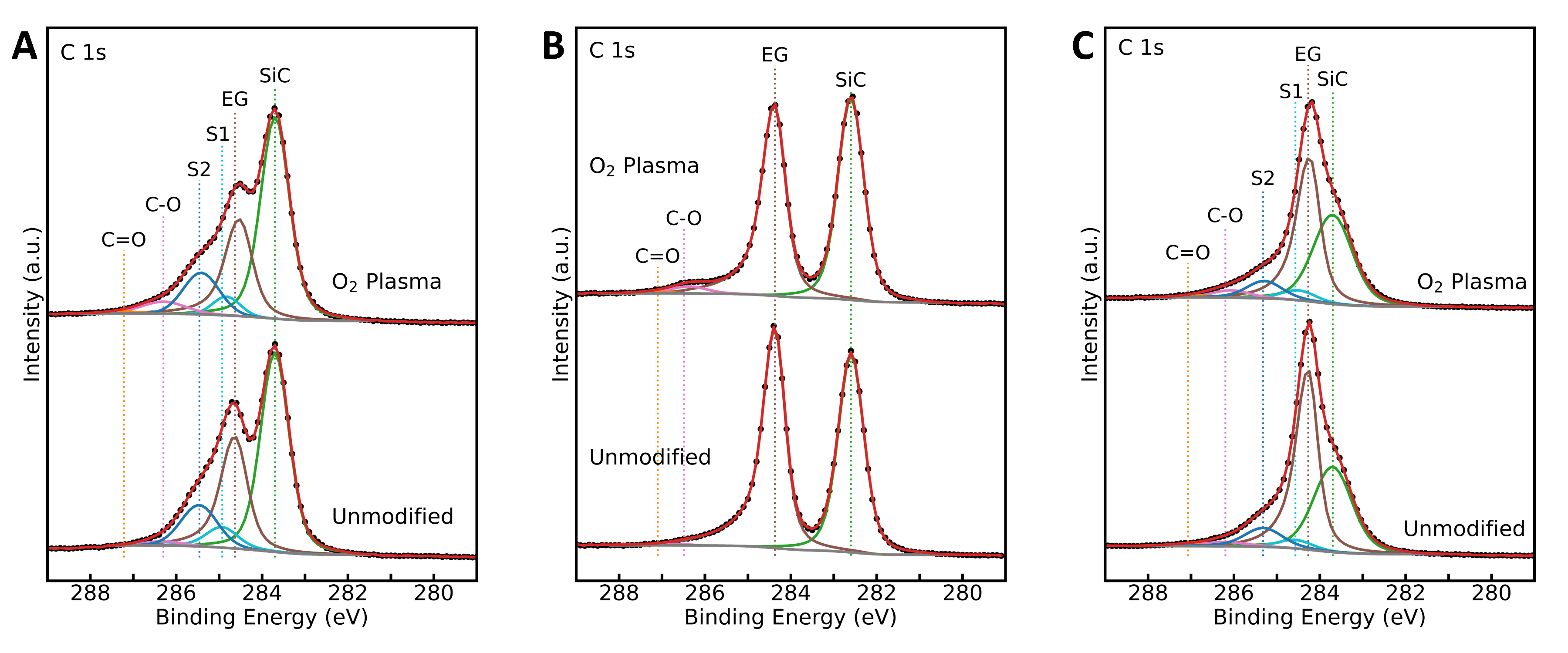
3.1. Epitaxial Graphene Characterization and Oxygen Plasma Modification
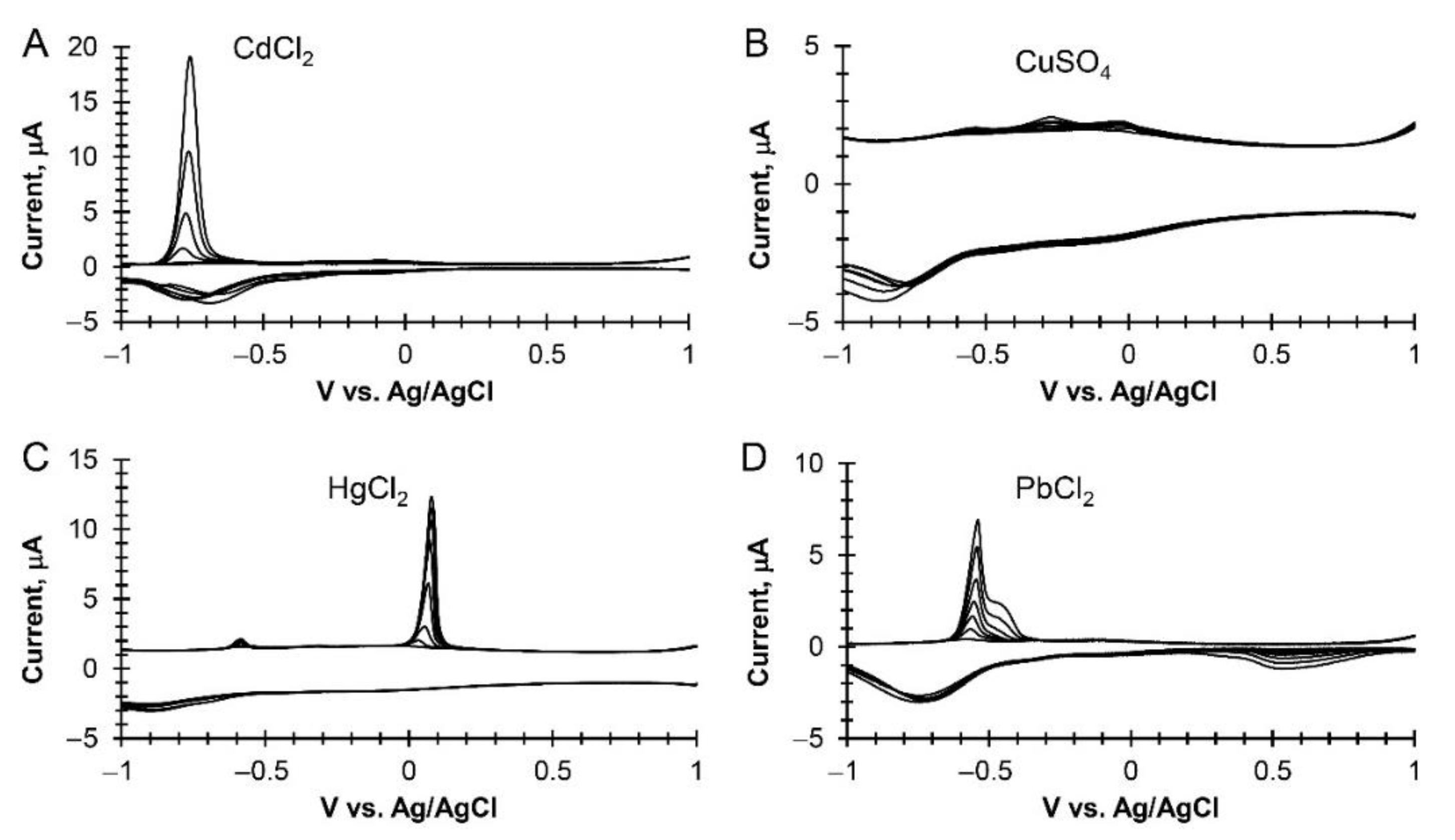
3.2. Heavy Metal Electrochemical Detection at Modified Epitaxial Graphene
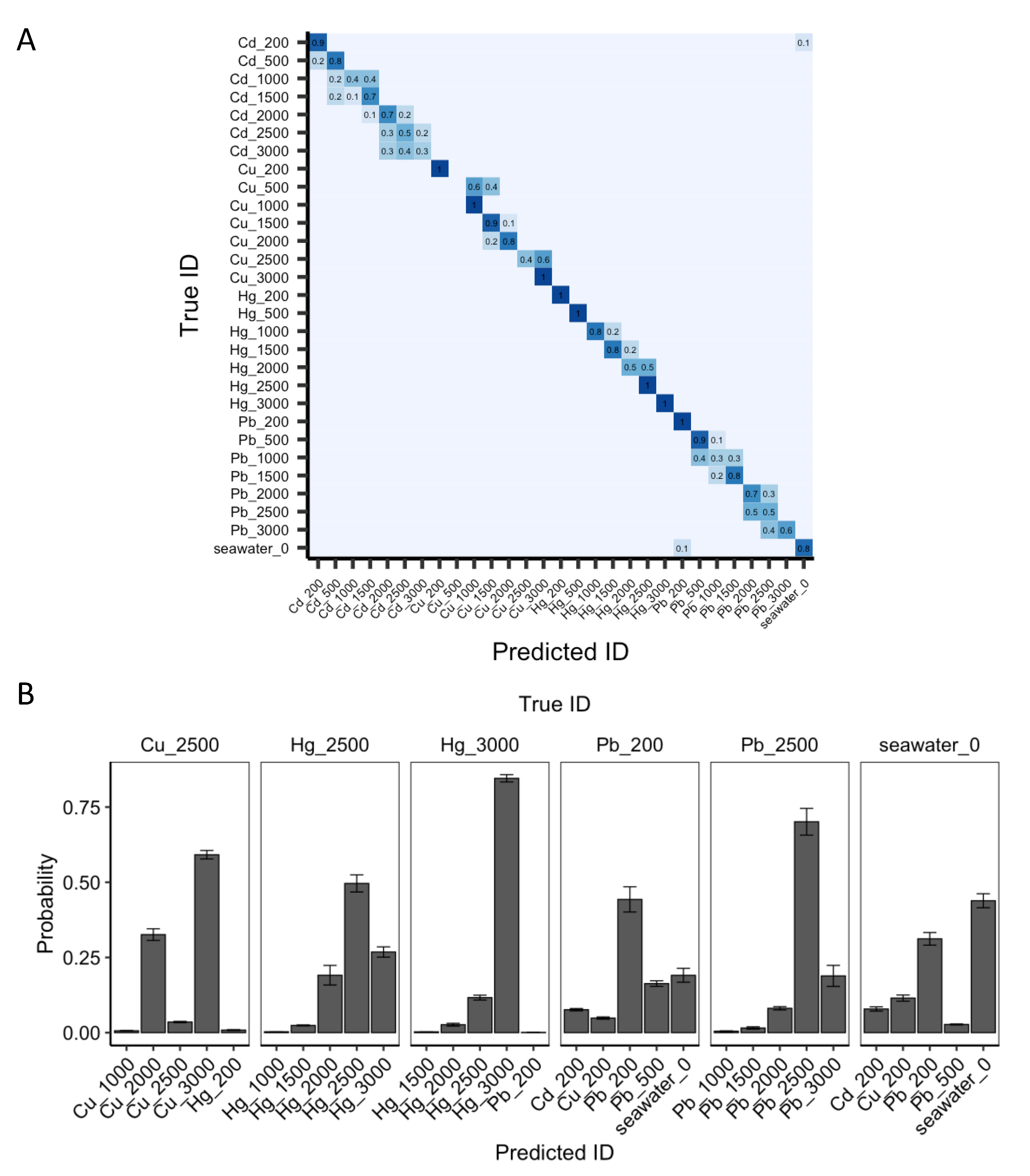
3.3. Heavy Metal Identifiction at Modified Epitaxial Graphene with Machine Learning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denuault, G. Electrochemical Techniques and Sensors for Ocean Research. Ocean Sci. 2009, 5, 697–710. [Google Scholar] [CrossRef] [Green Version]
- Mills, G.; Fones, G. A Review of in Situ Methods and Sensors for Monitoring the Marine Environment. Sens. Rev. 2012, 32, 17–28. [Google Scholar] [CrossRef]
- Taillefert, M.; Luther, G.W.; Nuzzio, D.B. The Application of Electrochemical Tools for in Situ Measurements in Aquatic Systems. Electroanalysis 2000, 12, 401–412. [Google Scholar] [CrossRef]
- Johnson, K.S.; Needoba, J.A.; Riser, S.C.; Showers, W.J. Chemical Sensor Networks for the Aquatic Environment. Chem. Rev. 2007, 107, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Freitas, A.C.; Duarte, A.C.; Santos, T.A.P.R. Sensors and Biosensors for Monitoring Marine Contaminants. Trends Environ. Anal. Chem. 2015, 6–7, 21–30. [Google Scholar] [CrossRef]
- Malzahn, K.; Windmiller, J.R.; Valdes-Ramirez, G.; Schoning, M.J.; Wang, J. Wearable Electrochemical Sensors for in Situ Analysis in Marine Environments. Analyst 2011, 136, 2912–2917. [Google Scholar] [CrossRef] [PubMed]
- Naval Sea Systems Command. Guidance for Diving in Contaminated Waters. Available online: https://www.navsea.navy.mil/Resources/Strategic-Documents/ (accessed on 3 August 2021).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A Review on Detection of Heavy Metal Ions in Water-An Electrochemical Approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Gao, W.; Nyein, H.Y.Y.; Shahpar, Z.; Fahad, H.M.; Chen, K.; Emaminejad, S.; Gao, Y.; Tai, L.C.; Ota, H.; Wu, E.; et al. Wearable Microsensor Array for Multiplexed Heavy Metal Monitoring of Body Fluids. ACS Sens. 2016, 1, 866–874. [Google Scholar] [CrossRef] [Green Version]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the Practicalities of Anodic Stripping Voltammetry for Heavy Metal Detection: A Tutorial Review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Goh, M.S.; Pumera, M. Graphene-Based Electrochemical Sensor for Detection of 2,4,6-Trinitrotoluene (TNT) in Seawater: The Comparison of Single-, Few-, and Multilayer Graphene Nanoribbons and Graphite Microparticles. Anal. Bioanal. Chem. 2011, 399, 127–131. [Google Scholar] [CrossRef]
- Chang, J.; Zhou, G.; Christensen, E.R.; Heideman, R.; Chen, J. Graphene-Based Sensors for Detection of Heavy Metals in Water: A Review. Anal. Bioanal. Chem. 2014, 406, 3957–3975. [Google Scholar] [CrossRef]
- Ma, S.; Wei, H.; Pan, D.W.; Pan, F.; Wang, C.C.; Kang, Q. Voltammetric Determination of Trace Zn(II) in Seawater on a Poly (Sodium 4-Styrenesulfonate)/Wrinkled Reduced Graphene Oxide Composite Modified Electrode. J. Electrochem. Soc. 2020, 167, 46519. [Google Scholar] [CrossRef]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-Based Electrochemical Detection of Heavy Metals in Water: Current Status, Challenges and Future Direction. TrAC Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Wanekaya, A.K. Applications of Nanoscale Carbon-Based Materials in Heavy Metal Sensing and Detection. Analyst 2011, 136, 4383–4391. [Google Scholar] [CrossRef]
- Bo, X.; Zhou, M.; Guo, L. Electrochemical Sensors and Biosensors Based on Less Aggregated Graphene. Biosens. Bioelectron. 2017, 89, 167–186. [Google Scholar] [CrossRef]
- Riedl, C.; Coletti, C.; Iwasaki, T.; Zakharov, A.A.; Starke, U. Quasi-Free-Standing Epitaxial Graphene on SiC Obtained by Hydrogen Intercalation. Phys. Rev. Lett. 2009, 103, 246804. [Google Scholar] [CrossRef] [Green Version]
- Shtepliuk, I.; Vagin, M.; Iakimov, T.; Yakimova, R. Fundamentals of Environmental Monitoring of Heavy Metals Using Graphene. Chem. Eng. Trans. 2019, 73, 7–12. [Google Scholar]
- Shtepliuk, I.; Vagin, M.; Ivanov, I.G.; Iakimov, T.; Yazdi, G.R.; Yakimova, R. Lead (Pb) Interfacing with Epitaxial Graphene. Phys. Chem. Chem. Phys. 2018, 20, 17105–17116. [Google Scholar] [CrossRef] [Green Version]
- Shtepliuk, I.; Santangelo, M.F.; Vagin, M.; Ivanov, I.G.; Khranovskyy, V.; Iakimov, T.; Eriksson, J.; Yakimova, R. Understanding Graphene Response to Neutral and Charged Lead Species: Theory and Experiment. Materials 2018, 11, 2059. [Google Scholar] [CrossRef] [Green Version]
- Shtepliuk, I.; Vagin, M.; Yakimova, R. Insights into the Electrochemical Behavior of Mercury on Graphene/SiC Electrodes. C J. Carbon Res. 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Shtepliuk, I.; Yakimova, R. Interaction of Epitaxial Graphene with Heavy Metals: Towards Novel Sensing Platform. Nanotechnology 2019, 30, 294002. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Vagin, M.; Yakimova, R. Electrochemical Deposition of Copper on Epitaxial Graphene. Appl. Sci. 2020, 10, 1405. [Google Scholar] [CrossRef] [Green Version]
- Elgengehi, S.M.; El-Taher, S.; Ibrahim, M.A.A.; Desmarais, J.K.; El-Kelany, K.E. Graphene and Graphene Oxide as Adsorbents for Cadmium and Lead Heavy Metals: A Theoretical Investigation. Appl. Surf. Sci. 2020, 507, 145038. [Google Scholar] [CrossRef]
- Trammell, S.A.; Myers-Ward, R.L.; Hangarter, S.C.; Gaskill, D.K.; Zabetakis, D.; Stenger, D.A.; Walton, S.G. Plasma Modified Epitaxial Fabricated Graphene on SiC for Electrochemical Trace Detection of Explosives. U.S. Patent #10,928,351 B2, 23 February 2021. [Google Scholar]
- Trammell, S.A.; Hernández, S.C.; Myers-Ward, R.L.; Zabetakis, D.; Stenger, D.A.; Gaskill, D.K.; Walton, S.G. Plasma-Modified, Epitaxial Fabricated Graphene on SiC for the Electrochemical Detection of TNT. Sensors 2016, 16, 1281. [Google Scholar] [CrossRef] [Green Version]
- Ryu, G.H.; Lee, J.; Kang, D.; Jo, H.J.; Shin, H.S.; Lee, Z. Effects of Dry Oxidation Treatments on Monolayer Graphene. 2D Mater. 2017, 4, 024011. [Google Scholar] [CrossRef]
- Felten, A.; Eckmann, A.; Pireaux, J.J.; Krupke, R.; Casiraghi, C. Controlled Modification of Mono- and Bilayer Graphene in O2, H2 and CF4 Plasmas. Nanotechnology 2013, 24, 355705. [Google Scholar] [CrossRef] [Green Version]
- Eelbo, T.; Waåniowska, M.; Gyamfi, M.; Forti, S.; Starke, U.; Wiesendanger, R. Influence of the Degree of Decoupling of Graphene on the Properties of Transition Metal Adatoms. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 87, 205443. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.S.; Shriver-Lake, L.C.; Zabetakis, D.; Stenger, D.A.; Trammell, S.A. A Simple and Inexpensive Electrochemical Assay for the Identification of Nitrogen Containing Explosives in the Field. Sensors 2017, 17, 1769. [Google Scholar] [CrossRef] [Green Version]
- Shriver-Lake, L.C.; Myers-Ward, R.L.; Dean, S.N.; Erickson, J.S.; Stenger, D.A.; Trammell, S.A. Multilayer Epitaxial Graphene on Silicon Carbide: A Stable Working Electrode for Seawater Samples Spiked with Environmental Contaminants. Sensors 2020, 20, 4006. [Google Scholar] [CrossRef]
- Dean, S.N.; Shriver-Lake, L.C.; Stenger, D.A.; Erickson, J.S.; Golden, J.P.; Trammell, S.A. Machine Learning Techniques for Chemical Identification Using Cyclic Square Wave Voltammetry. Sensors 2019, 19, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyakiti, L.O.; Wheeler, V.D.; Garces, N.Y.; Myers-Ward, R.L.; Eddy, C.R.; Gaskill, D.K. Enabling Graphene-Based Technologies: Toward Wafer-Scale Production of Epitaxial Graphene. MRS Bull. 2012, 37, 1149–1157. [Google Scholar] [CrossRef]
- Emery, J.D.; Wheeler, V.H.; Johns, J.E.; McBriarty, M.E.; Detlefs, B.; Hersam, M.C.; Gaskill, D.K.; Bedzyk, M.J. Structural Consequences of Hydrogen Intercalation of Epitaxial Graphene on SiC(0001). Appl. Phys. Lett. 2014, 105, 161602. [Google Scholar] [CrossRef] [Green Version]
- Daniels, K.M.; Jadidi, M.M.; Sushkov, A.B.; Nath, A.; Boyd, A.K.; Sridhara, K.; Drew, H.D.; Murphy, T.E.; Myers-Ward, R.L.; Gaskill, D.K. Narrow Plasmon Resonances Enabled by Quasi-Freestanding Bilayer Epitaxial Graphene. 2D Mater. 2017, 4, 025034. [Google Scholar] [CrossRef]
- Sherwood, P.M.A. Appendix 3. In Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; Briggs, D., Seah, M.P., Eds.; Wiley: Hoboken, NJ, USA, 1990; pp. 460–464. [Google Scholar]
- Kotsakidis, J.C.; Grubisic-Cabo, A.; Yin, Y.F.; Tadich, A.; Myers-Ward, R.L.; DeJarld, M.; Pavunny, S.P.; Currie, M.; Daniels, K.M.; Liu, C.; et al. Freestanding N-Doped Graphene via Intercalation of Calcium and Magnesium into the Buffer Layer-SiC(0001) Interface. Chem. Mater. 2020, 32, 6464–6482. [Google Scholar] [CrossRef]
- Speck, F.; Ostler, M.; Besendorfer, S.; Krone, J.; Wanke, M.; Seyller, T. Growth and Intercalation of Graphene on Silicon Carbide Studied by Low-Energy Electron Microscopy. Ann. Phys. 2017, 529, 1700046. [Google Scholar] [CrossRef] [Green Version]
- Mammadov, S.; Ristein, J.; Koch, R.J.; Ostler, M.; Raidel, C.; Wanke, M.; Vasiliauskas, R.; Yakimova, R.; Seyller, T. Polarization Doping of Graphene on Silicon Carbide. 2D Mater. 2014, 1, 35003. [Google Scholar] [CrossRef]
- Oliveira, M.H.; Schumann, T.; Fromm, F.; Koch, R.; Ostler, M.; Ramsteiner, M.; Seyller, T.; Lopes, J.M.J.; Riechert, H. Formation of High-Quality Quasi-Free-Standing Bilayer Graphene on SiC(0 0 0 1) by Oxygen Intercalation upon Annealing in Air. Carbon 2013, 52, 83–89. [Google Scholar] [CrossRef]
- Briggs, N.; Gebeyehu, Z.M.; Vera, A.; Zhao, T.; Wang, K.; De La Fuente Duran, A.; Bersch, B.; Bowen, T.; Knappenberger, K.L., Jr.; Robinson, J.A. Epitaxial Graphene/Silicon Carbide Intercalation: A Minireview on Graphene Modulation and Unique 2D Materials. Nanoscale 2019, 11, 15440–15447. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.; Tai, S.; Pedowitz, M.; Rzasa, J.R.; Pennachio, D.J.; Hajzus, J.R.; Milton, D.K.; Myers-Ward, R.; Daniels, K.M. Real-Time Ultra-Sensitive Detection of SARS-CoV-2 by Quasi-Freestanding Epitaxial Graphene-Based Biosensor. Biosens Bioelectron 2022, 197, 113803. [Google Scholar] [CrossRef]
- Zabetakis, D.; Shriver-Lake, L.C.; Erickson, J.S.; Trammell, S.A.; Hajzus, J.R.; Pennachio, D.J.; Myers-Ward, R.L. Demonstration of a Teflon Electrochemical Test Cell for Epitaxial Graphene; NRL/6930/MR—2021/1; U.S. Naval Research Laboratory: Defense Technical Information Center: Washington, DC, USA, 2021. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lee, D.S.; Riedl, C.; Krauss, B.; von Klitzing, K.; Starke, U.; Smet, J.H. Raman Spectra of Epitaxial Graphene on SiC and of Epitaxial Graphene Transferred to SiO2. Nano Lett. 2008, 8, 4320–4325. [Google Scholar] [CrossRef] [Green Version]
- van Bommel, A.J.; Crombeen, J.E.; van Tooren, A. LEED and Auger Electron Observations of the SiC (0001) Surface. Surf. Sci. 1975, 48, 463–472. [Google Scholar] [CrossRef]
- Emtsev, K.V.; Speck, F.; Seyller, T.; Ley, L.; Riley, J.D. Interaction, Growth, and Ordering of Epitaxial Graphene on SiC{0001} Surfaces: A Comparative Photoelectron Spectroscopy Study. Phys. Rev. B 2008, 77, 155303. [Google Scholar] [CrossRef] [Green Version]
- Kochat, V.; Pal, A.N.; Sneha, E.S.; Sampathkumar, A.; Gairola, A.; Shivashankar, S.A.; Raghavan, S.; Ghosh, A. High Contrast Imaging and Thickness Determination of Graphene with In-Column Secondary Electron Microscopy. J. Appl. Phys. 2011, 110, 14315. [Google Scholar] [CrossRef] [Green Version]
- Fromm, F.; Oliveira, M.H.; Molina-Sánchez, A.; Hundhausen, M.; Lopes, J.M.J.; Riechert, H.; Wirtz, L.; Seyller, T. Contribution of the Buffer Layer to the Raman Spectrum of Epitaxial Graphene on SiC(0001). New J. Phys. 2013, 15, 43031. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Zandiatashbar, A.; Lee, G.H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of Defects on the Intrinsic Strength and Stiffness of Graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying Ion-Induced Defects and Raman Relaxation Length in Graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Tian, J.; Chen, Y.P. Effect of Oxygen Plasma Etching on Graphene Studied Using Raman Spectroscopy and Electronic Transport Measurements. New J. Phys. 2011, 13, 025008. [Google Scholar] [CrossRef]
- Martins Ferreira, E.H.; Moutinho, M.V.O.; Stavale, F.; Lucchese, M.M.; Capaz, R.B.; Achete, C.A.; Jorio, A. Evolution of the Raman Spectra from Single-, Few-, and Many-Layer Graphene with Increasing Disorder. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 125429. [Google Scholar] [CrossRef] [Green Version]
- Jorio, A.; Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A. Raman Study of Ion-Induced Defects in N -Layer Graphene. J. Phys. Condens. Matter 2010, 22, 334204. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfo-Liated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Jernigan, G.G.; Nolde, J.A.; Mahadik, N.A.; Cleveland, E.R.; Boercker, J.E.; Katz, M.B.; Robinson, J.T.; Aifer, E.H. Physical Properties of Nanometer Graphene Oxide Films Partially and Fully Reduced by Annealing in Ultra-High Vacuum. J. Appl. Phys. 2017, 122, 75301. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and Structural Studies of High Quality Graphene Oxide and Reduced Graphene Oxide Prepared by Different Chemical Oxidation Methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Gokus, T.; Nair, R.R.; Bonetti, A.; Böhmler, M.; Lombardo, A.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C.; Hartschuh, A. Making Graphene Luminescent by Oxygen Plasma Treatment. ACS Nano 2009, 3, 3963–3968. [Google Scholar] [CrossRef] [Green Version]
- Ristein, J.; Mammadov, S.; Seyller, T. Origin of Doping in Quasi-Free-Standing Graphene on Silicon Carbide. Phys. Rev. Lett. 2012, 108, 246104. [Google Scholar] [CrossRef] [Green Version]


| Analyte | Graphene Type and Modification | Response 1 | MSE 2 | Fitting Parameters | ||
|---|---|---|---|---|---|---|
| a | b | xo | ||||
| CdCl2 | multilayer EG | sigmoidal | 0.022 | 7.454 | 590.19 | 1749.080 |
| CdCl2 | multilayer EG + O2 | sigmoidal | 0.433 | 22.820 | 676.20 | 2088.70 |
| CdCl2 | QFS bilayer EG | sigmoidal | 0.222 | 15.190 | 559.59 | 1834.78 |
| CdCl2 | QFS bilayer EG + O2 | sigmoidal | 1.260 | 6.851 | 1461.96 | 557.92 |
| CuSO4 | multilayer EG | hyperbolic | 0.018 | 0.024 | 0.027 | - |
| CuSO4 | multilayer EG + O2 | hyperbolic | 0.005 | 0.014 | 0.015 | - |
| CuSO4 | QFS bilayer EG | hyperbolic | 0.014 | 0.051 | 0.075 | - |
| CuSO4 | QFS bilayer EG + O2 | hyperbolic | 0.233 | 0.013 | 0.005 | - |
| HgCl2 | multilayer EG | linear | 0.493 | 0.004 | −0.296 | - |
| HgCl2 | multilayer EG + O2 | linear | 0.127 | 0.0003 | 0.394 | - |
| HgCl2 | QFS bilayer EG | sigmoidal | 2.737 | 9.141 | 408.33 | 1184.821 |
| HgCl2 | QFS bilayer EG + O2 | sigmoidal | 0.044 | 9.990 | 535.98 | 1164.120 |
| PbCl2 | multilayer EG | sigmoidal | 0.386 | 6.825 | 1008.97 | 2441.725 |
| PbCl2 | multilayer EG + O2 | sigmoidal | 0.010 | 2.347 | 1104.56 | 2449.485 |
| PbCl2 | QFS bilayer EG | hyperbolic | 0.717 | 0.001 | 0.0003 | - |
| PbCl2 | QFS bilayer EG + O2 | sigmoidal | 0.042 | 10.040 | 699.70 | 2277.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajzus, J.R.; Shriver-Lake, L.C.; Dean, S.N.; Erickson, J.S.; Zabetakis, D.; Golden, J.; Pennachio, D.J.; Myers-Ward, R.L.; Trammell, S.A. Modifications of Epitaxial Graphene on SiC for the Electrochemical Detection and Identification of Heavy Metal Salts in Seawater. Sensors 2022, 22, 5367. https://doi.org/10.3390/s22145367
Hajzus JR, Shriver-Lake LC, Dean SN, Erickson JS, Zabetakis D, Golden J, Pennachio DJ, Myers-Ward RL, Trammell SA. Modifications of Epitaxial Graphene on SiC for the Electrochemical Detection and Identification of Heavy Metal Salts in Seawater. Sensors. 2022; 22(14):5367. https://doi.org/10.3390/s22145367
Chicago/Turabian StyleHajzus, Jenifer R., Lisa C. Shriver-Lake, Scott N. Dean, Jeffrey S. Erickson, Daniel Zabetakis, Joel Golden, Daniel J. Pennachio, Rachael L. Myers-Ward, and Scott A. Trammell. 2022. "Modifications of Epitaxial Graphene on SiC for the Electrochemical Detection and Identification of Heavy Metal Salts in Seawater" Sensors 22, no. 14: 5367. https://doi.org/10.3390/s22145367
APA StyleHajzus, J. R., Shriver-Lake, L. C., Dean, S. N., Erickson, J. S., Zabetakis, D., Golden, J., Pennachio, D. J., Myers-Ward, R. L., & Trammell, S. A. (2022). Modifications of Epitaxial Graphene on SiC for the Electrochemical Detection and Identification of Heavy Metal Salts in Seawater. Sensors, 22(14), 5367. https://doi.org/10.3390/s22145367







