NaCl Ionization-Based Moisture Sensor Prepared by Aerosol Deposition for Monitoring Respiratory Patterns
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of NaCl/BaTiO3 Composite Films via AD Process
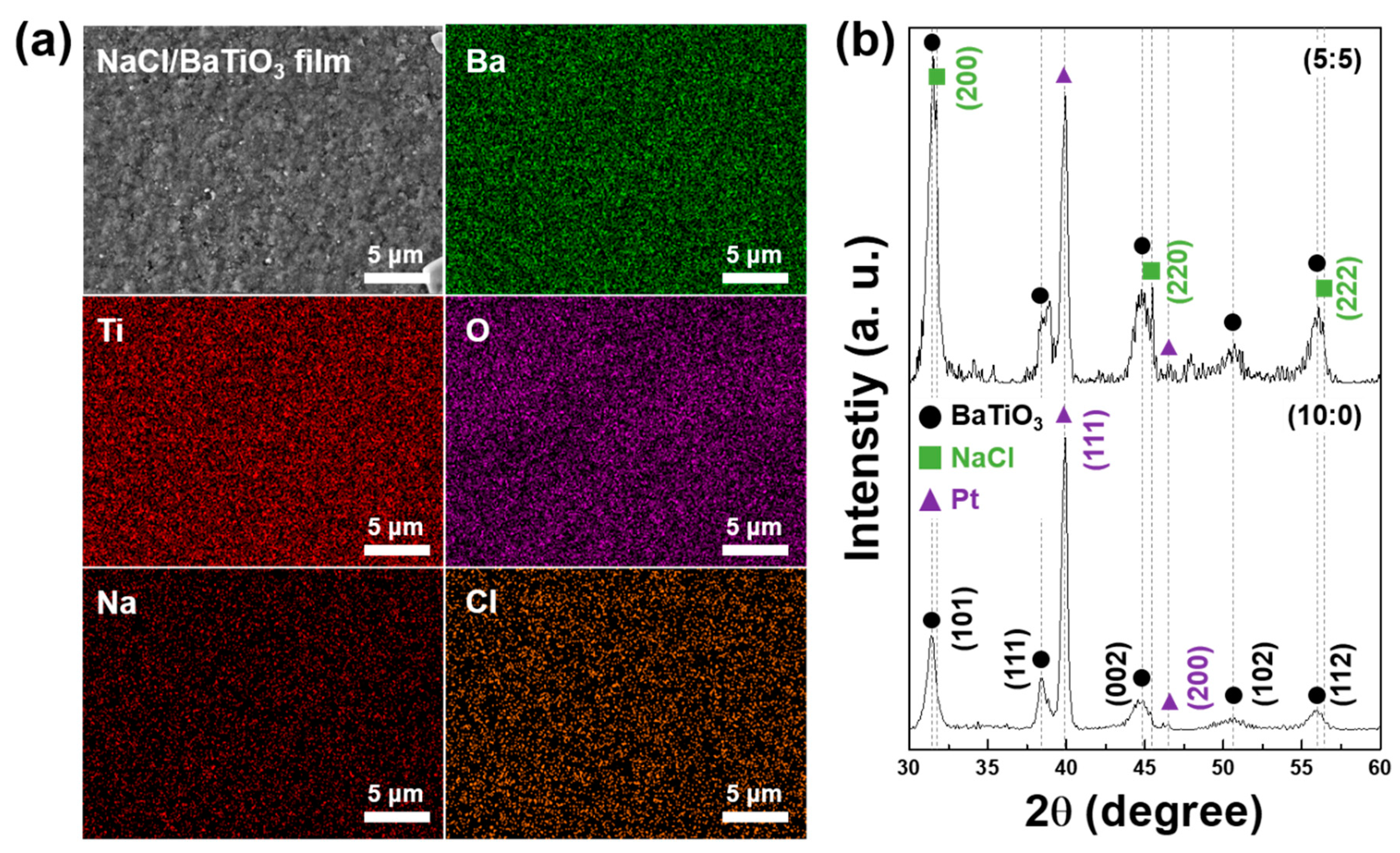
2.2. Characterizations
3. Results and Discussion
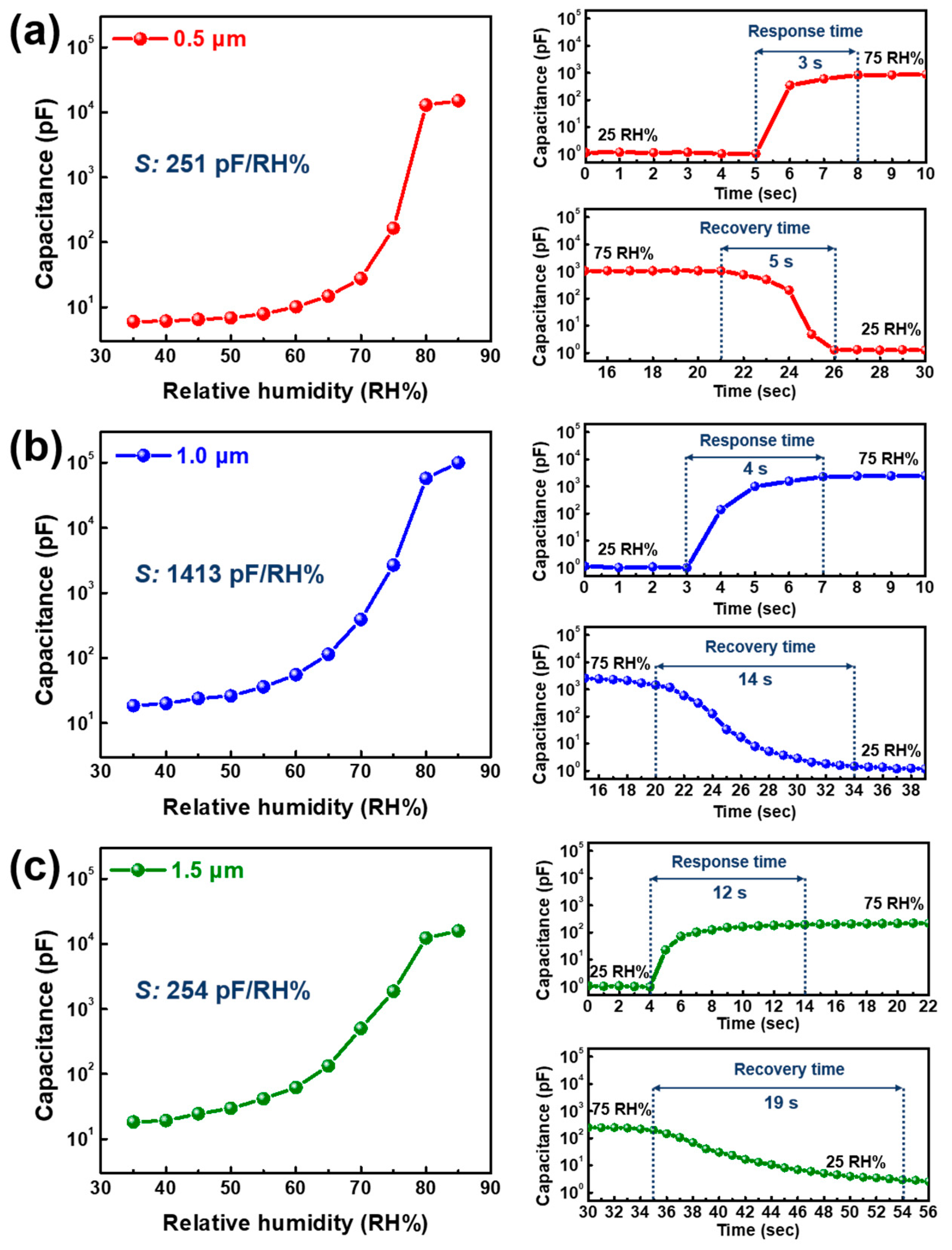
3.1. Sensing Properties via Layer Thickness Control
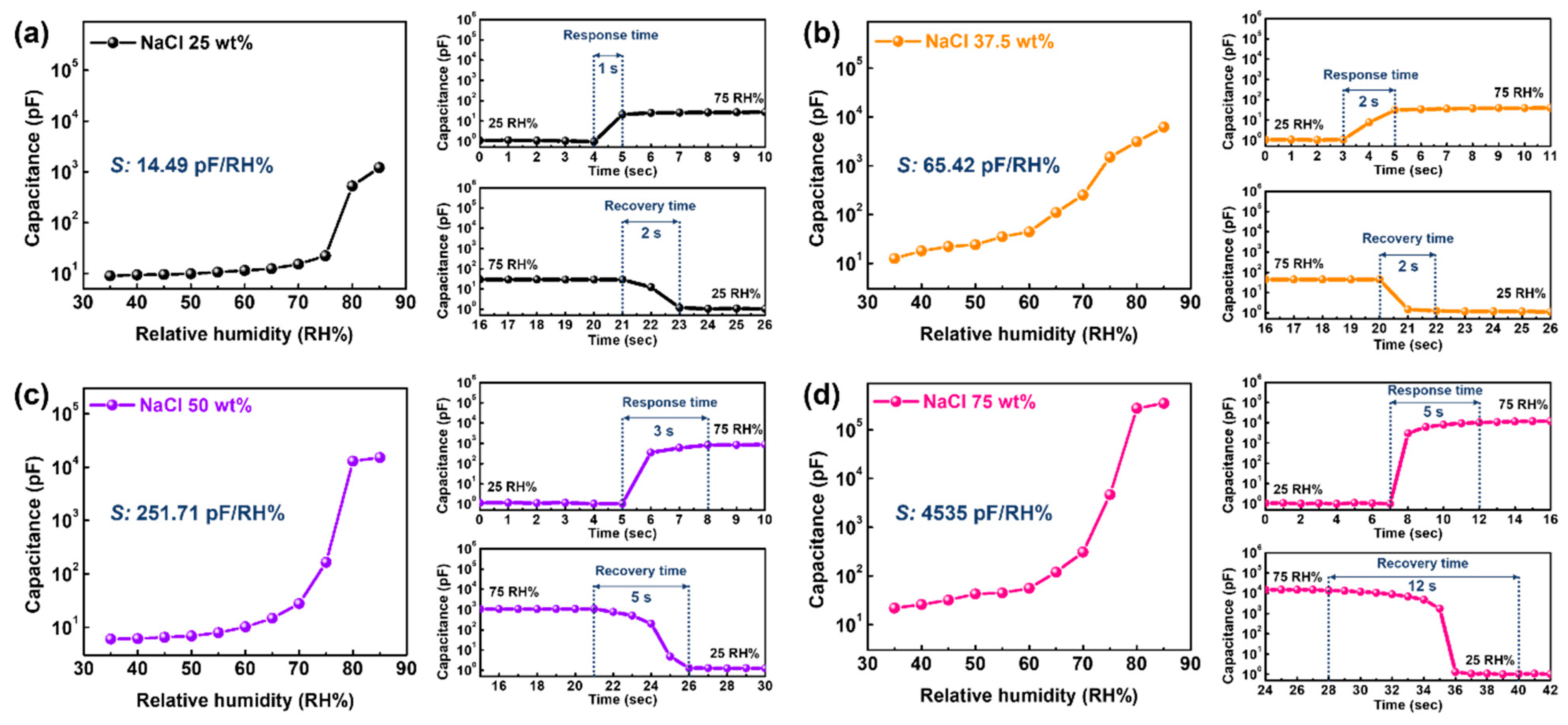
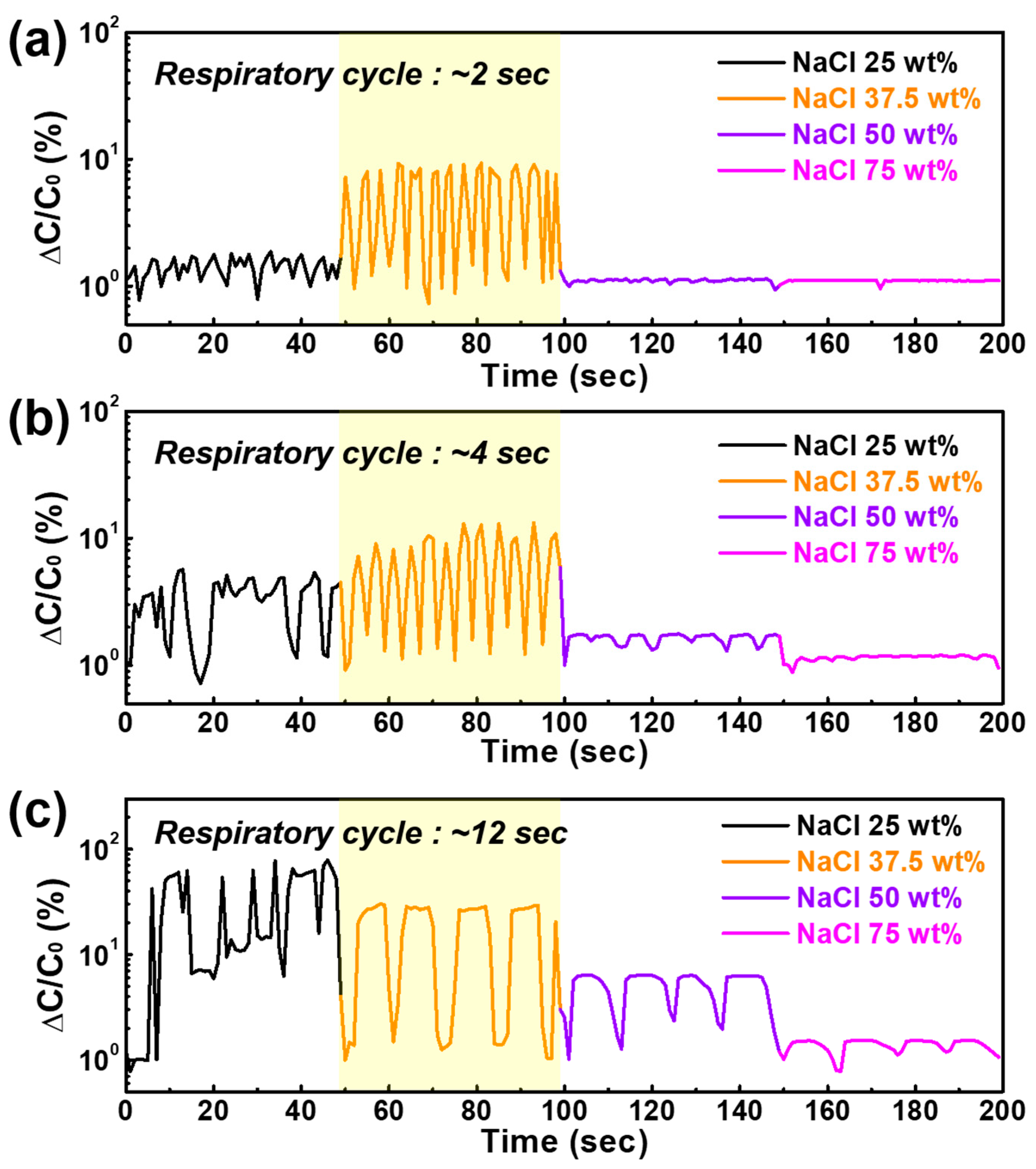
3.2. Optimization of NaCl Ratio for Real-Time Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eckberg, D.L.; Kifle, Y.T.; Roberts, V.L. Phase relationship between normal human respiration and baroreflex responsiveness. J. Physiol. 1980, 304, 489–502. [Google Scholar] [CrossRef]
- Peng, C.-K.; Mietus, J.E.; Liu, Y.; Lee, C.; Hausdorff, J.M.; Stanley, H.E.; Goldberger, A.L.; Lipsitz, L.A. Quantifying fractal dynamics of human respiration: Age and gender effects. Ann. Biomed. Eng. 2002, 30, 683–692. [Google Scholar] [CrossRef]
- Yumino, D.; Bradley, T.D. Central sleep apnea and Cheyne-Stokes respiration. Proc. Am. Thorac. Soc. 2008, 5, 226–236. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Sekitani, T.; Someya, T. Human-friendly organic integrated circuits. Mater. Today 2011, 14, 398–407. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoringand personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuator. B 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Ganong, W.F. Review of Medical Physiology; McGraw-Hill Medical: New York, NY, USA, 2005. [Google Scholar]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008, 188, 657–659. [Google Scholar] [CrossRef]
- Karlen, W.; Gan, H.; Chiu, M.; Dunsmuir, D.; Zhou, G.; Dumont, G.A.; Ansermino, J.M. Improving the accuracy and efficiency of respiratory rate measurements in children using mobile devices. PLoS ONE 2014, 9, e99266. [Google Scholar] [CrossRef]
- Storck, K.; Karlsson, M.; Ask, P.; Loyd, D. Heat transfer evaluation of the nasal thermistor technique. IEEE Trans. Biomed. Eng. 1996, 43, 1187–1191. [Google Scholar] [CrossRef]
- Norman, R.G.; Ahmed, M.M.; Walsleben, J.A.; Rapoport, D.M. Detection of respiratory events during NPSG: Nasal cannula/pressure sensor versus thermistor. Sleep 1997, 20, 1175–1184. [Google Scholar]
- Lee-Chiong, T.L. Sleep: A Comprehensive Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hök, B.; Blückert, A.; Sandberg, G. A non-contacting sensor system for respiratory air flow detection. Sens. Actuators A Phys. 1996, 52, 81–85. [Google Scholar] [CrossRef]
- Jin, H.; Lee, L.-A.; Song, L.; Li, Y.; Peng, J.; Zhong, N.; Li, H.-Y.; Zhang, X. Acoustic analysis of snoring in the diagnosis of obstructive sleep apnea syndrome: A call for more rigorous studies. J. Clin. Sleep Med. 2015, 11, 765–771. [Google Scholar] [CrossRef]
- Autet, L.; Frasca, D.; Pinsard, M.; Cancel, A.; Rousseau, L.; Debaene, B.; Mimoz, O. Evaluation of acoustic respiration rate monitoring after extubation in intensive care unit patients. Br. J. Anaesth. 2014, 113, 195–197. [Google Scholar] [CrossRef][Green Version]
- Jung, D.; Kim, J.; Lee, G.S. Enhanced humidity-sensing response of metal oxide coated carbon nanotube. Sens. Actuators A Phys. 2015, 223, 11–17. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Sun, H.; Xu, Y.; Wang, X.; Luo, H.; Liu, Z.; Zhang, T. Porous ionic membrane based flexible humidity sensor and its multifunctional applications. Adv. Sci. 2017, 4, 1600404. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Chan, Y.; Zhang, K. Fast response resistive humidity sensitivity of polyimide/multiwall carbon nanotube composite films. Sens. Actuators B Chem. 2011, 152, 99–106. [Google Scholar] [CrossRef]
- Agmon, N. The grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, L.; Yu, P.; Mao, L. Sensitive and fast humidity sensor based on a redox conducting supramolecular ionic material for respiration monitoring. Anal. Chem. 2017, 89, 996–1001. [Google Scholar] [CrossRef]
- Mogera, U.; Sagade, A.A.; George, S.J.; Kulkarni, G.U. Ultrafast response humidity sensor using supramolecular nanofibre and its application in monitoring breath humidity and flow. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef]
- Pan, X.; Xue, Q.; Zhang, J.; Guo, Q.; Jin, Y.; Lu, W.; Li, X.; Ling, C. Effective enhancement of humidity sensing characteristics of novel thermally treated MWCNTs/Polyvinylpyrrolidone film caused by interfacial effect. Adv. Mater. Interfaces 2016, 3, 1600153. [Google Scholar] [CrossRef]
- Kalkan, A.K.; Li, H.; O’Brien, C.J.; Fonash, S.J. A rapid-response, high-sensitivity nanophase humidity sensor for respiratory monitoring. IEEE Electron Device Lett. 2004, 25, 526–528. [Google Scholar] [CrossRef]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhanen, T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef]
- Choi, S.J.; Yu, H.; Jang, J.S.; Kim, M.H.; Kim, S.J.; Jeong, H.S.; Kim, I.D. Nitrogen-doped single graphene fiber with platinum water dissociation catalyst for wearable humidity sensor. Small 2018, 14, 1703934. [Google Scholar] [CrossRef]
- Kundu, S.K.; Kumagai, S.; Sasaki, M. A wearable capacitive sensor for monitoring human respiratory rate. Jpn. J. Appl. Phys. 2013, 52, 04CL05. [Google Scholar] [CrossRef]
- Park, S.W.; Das, P.S.; Chhetry, A.; Park, J.Y. A flexible capacitive pressure sensor for wearable respiration monitoring system. IEEE Sens. J. 2017, 17, 6558–6564. [Google Scholar] [CrossRef]
- Casalbore-Miceli, G.; Yang, M.; Li, Y.; Zanelli, A.; Martelli, A.; Chen, S.; She, Y.; Camaioni, N. A polyelectrolyte as humidity sensing material: Influence of the preparation parameters on its sensing property. Sens. Actuators B Chem. 2006, 114, 584–590. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Li, P.; Yang, M. A resistive-type humidity sensor based on crosslinked polyelectrolyte prepared by UV irradiation. Sens. Actuators B Chem. 2009, 135, 581–586. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.-D.; Lim, H.; Kim, S.Y.; Ko, H. Giant tunneling piezoresistance of composite elastomers with interlocked microdome arrays for ultrasensitive and multimodal electronic skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef]
- Bondarenka, V.; Grebinskij, S.; Mickevičius, S.; Volkov, V.; Zacharova, G. Thin films of poly-vanadium-molybdenum acid as starting materials for humidity sensors. Sens. Actuators B Chem. 1995, 28, 227–231. [Google Scholar] [CrossRef]
- Zhen, Z.; Li, Z.; Zhao, X.; Zhong, Y.; Zhang, L.; Chen, Q.; Yang, T.; Zhu, H. Formation of uniform water microdroplets on wrinkled graphene for ultrafast humidity sensing. Small 2018, 14, 1703848. [Google Scholar] [CrossRef] [PubMed]
- Viviani, M.; Buscaglia, M.; Buscaglia, V.; Leoni, M.; Nanni, P. Barium perovskites as humidity sensing materials. J. Eur. Ceram. Soc. 2001, 21, 1981–1984. [Google Scholar] [CrossRef]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Liu, Y.; Liu, X.; Fei, T.; Zhang, T. Ultrafast response polyelectrolyte humidity sensor for respiration monitoring. ACS Appl. Mater. Interfaces 2019, 11, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Zhou, L.; Duan, L.; Gu, J.; Zhang, Q.; Yuan, Q.; Jiang, X. Humidity sensing property of NaCl-added mesoporous Silica synthesized by a facile way with low energy cost. Int. J. Appl. Ceram. Technol. 2015, 12, 169–175. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Schubert, M.; Stöcker, T.; Fuierer, P.; Moos, R. An overview of the aerosol deposition method: Process fundamentals and new trends in materials applications. J. Ceram. Sci. Technol. 2015, 6, 147–182. [Google Scholar]
- Akedo, J. Aerosol deposition of ceramic thick films at room temperature: Densification mechanism of ceramic layers. J. Am. Ceram. Soc. 2006, 89, 1834–1839. [Google Scholar] [CrossRef]
- Akedo, J.; Lebedev, M. Microstructure and electrical properties of lead zirconate titanate (Pb(Zr52/Ti48)O3) thick films deposited by aerosol deposition method. Jpn. J. Appl. Phys. 1999, 38, 5397. [Google Scholar] [CrossRef]
- Cho, M.-Y.; Lee, D.-W.; Kim, W.-J.; Kim, Y.-N.; Koo, S.-M.; Lee, D.; Moon, K.-S.; Oh, J.-M. Fabrication of TiO2/Cu hybrid composite films with near zero TCR and high adhesive strength via aerosol deposition. Ceram. Int. 2018, 44, 18736–18742. [Google Scholar] [CrossRef]
- Lee, C.; Cho, M.-Y.; Kim, M.; Jang, J.; Oh, Y.; Oh, K.; Kim, S.; Park, B.; Kim, B.; Koo, S.-M. Applicability of aerosol deposition process for flexible electronic device and determining the film formation mechanism with cushioning effects. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Kwon, O.-Y.; Na, H.-J.; Kim, H.-J.; Lee, D.-W.; Nam, S.-M. Effects of mechanical properties of polymer on ceramic-polymer composite thick films fabricated by aerosol deposition. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef]
- Liang, J.-G.; Kim, E.-S.; Wang, C.; Cho, M.-Y.; Oh, J.-M.; Kim, N.-Y. Thickness effects of aerosol deposited hygroscopic films on ultra-sensitive humidity sensors. Sens. Actuators B Chem. 2018, 265, 632–643. [Google Scholar] [CrossRef]
- Cho, M.-Y.; Kim, I.-S.; Kim, S.-h.; Park, C.; Kim, N.-Y.; Kim, S.-W.; Kim, S.; Oh, J.-M. Unique Noncontact Monitoring of Human Respiration and Sweat Evaporation Using a CsPb2Br5-Based Sensor. ACS Appl. Mater. Interfaces 2021, 13, 5602–5613. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Liang, J.-G.; Wang, C.; Cho, M.-Y.; Oh, J.-M.; Kim, N.-Y. Inter-digital capacitors with aerosol-deposited high-K dielectric layer for highest capacitance value in capacitive super-sensing applications. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.-Y.; Lee, D.-W.; Kim, I.-S.; Kim, W.-J.; Koo, S.-M.; Lee, D.; Kim, Y.-H.; Oh, J.-M. Evaluation of structural and mechanical properties of aerosol-deposited bioceramic films for orthodontic brackets. Ceram. Int. 2019, 45, 6702–6711. [Google Scholar] [CrossRef]
- Akedo, J. Room temperature impact consolidation and application to ceramic coatings: Aerosol deposition method. J. Ceram. Soc. Jpn. 2020, 128, 101–116. [Google Scholar] [CrossRef]
- Hong, W.B.; Li, L.; Cao, M.; Chen, X.M. Plastic deformation and effects of water in room-temperature cold sintering of NaCl microwave dielectric ceramics. J. Am. Ceram. Soc. 2018, 101, 4038–4043. [Google Scholar] [CrossRef]
- Li, H.; Liu, R.; Chen, J.; Wang, Z.; Xiong, X. Preparation of nickel porous materials by sintering nickel oxalate and sodium chloride after blending and reduction. J. Mater. Res. Technol. 2020, 9, 3149–3157. [Google Scholar] [CrossRef]
- He, X.; Geng, W.; Zhang, B.; Jia, L.; Duan, L.; Zhang, Q. Ultrahigh humidity sensitivity of NaCl-added 3D mesoporous silica KIT-6 and its sensing mechanism. RSC Adv. 2016, 6, 38391–38398. [Google Scholar] [CrossRef]
- Liang, J.-G.; Wang, C.; Yao, Z.; Liu, M.-Q.; Kim, H.-K.; Oh, J.-M.; Kim, N.-Y. Preparation of ultrasensitive humidity-sensing films by aerosol deposition. ACS Appl. Mater. Interfaces 2018, 10, 851–863. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, S.; Kim, I.S.; Kim, E.S.; Wang, Z.J.; Kim, N.Y.; Kim, S.W.; Oh, J.M. Perovskite-induced ultrasensitive and highly stable humidity sensor systems prepared by aerosol deposition at room temperature. Adv. Funct. Mater. 2020, 30, 1907449. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Yuan, Z.; Li, S.; Liang, J.; Jiang, Y.; Tai, H. High performance humidity sensor based on 3D mesoporous Co3O4 hollow polyhedron for multifunctional applications. Appl. Surf. Sci. 2022, 585, 152698. [Google Scholar] [CrossRef]
- Abid, H.N.; Nayef, U.M.; Mutlak, F.A. Preparation and characterization Co3O4 nanoparticles on porous silicon for humidity sensor by photoluminescence. Optik 2019, 178, 379–383. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Wang, X.; Cheng, Z.; Xu, J. Facile preparation of N-rich functional polymer with porous framework as QCM sensing material for rapid humidity detection. Sens. Actuators B Chem. 2019, 288, 289–297. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, B.; Lee, H.; Kim, H.; Lee, K.; Park, H. Capacitive humidity sensor design based on anodic aluminum oxide. Sens. Actuators B Chem. 2009, 141, 441–446. [Google Scholar] [CrossRef]
- Kotnala, R.; Shah, J.; Singh, B.; Singh, S.; Dhawan, S.; Sengupta, A. Humidity response of Li-substituted magnesium ferrite. Sens. Actuators B Chem. 2008, 129, 909–914. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, H.J.; Kim, Y.H.; Yun, Y.H.; Nam, S.M. Growth process of α-Al2O3 ceramic films on metal substrates fabricated at room temperature by aerosol deposition. J. Am. Ceram. Soc. 2011, 94, 3131–3138. [Google Scholar] [CrossRef]
- Kim, I.-S.; Ko, P.-J.; Cho, M.-Y.; Lee, Y.-S.; Sohn, H.; Park, C.; Shin, W.H.; Koo, S.-M.; Lee, D.-W.; Oh, J.-M. Fabrication of high-quality alumina coating through novel, dual-particle aerosol deposition. Ceram. Int. 2020, 46, 23686–23694. [Google Scholar] [CrossRef]
- Kim, H.-K.; Lee, S.-H.; Lee, S.-G.; Lee, Y.-H. Densification mechanism of BaTiO3 films on Cu substrates fabricated by aerosol deposition. Electron. Mater. Lett. 2015, 11, 388–397. [Google Scholar] [CrossRef]
- Lee, D.-W.; Kwon, O.-Y.; Cho, W.-J.; Song, J.-K.; Kim, Y.-N. Characteristics and mechanism of Cu films fabricated at room temperature by aerosol deposition. Nanoscale Res. Lett. 2016, 11, 1–8. [Google Scholar] [CrossRef]
- Cho, M.-Y.; Park, S.-J.; Kim, S.-M.; Lee, D.-W.; Kim, H.-K.; Koo, S.-M.; Moon, K.-S.; Oh, J.-M. Hydrophobicity and transparency of Al2O3-based poly-tetra-fluoro-ethylene composite thin films using aerosol deposition. Ceram. Int. 2018, 44, 16548–16555. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, H.-J.; Koh, J.-H.; Ha, J.-G.; Yun, Y.-H.; Nam, S.-M. Fabrication of BaTiO3-PTFE composite film for embedded capacitor employing aerosol deposition. Ceram. Int. 2011, 37, 1859–1864. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.-H.; Nam, S.-M.; Yoon, Y.J.; Kim, J.-H. Calculation of Al2O3 Contents in Al2O3-PTFE composite thick films fabricated by using the aerosol deposition. J. Korean Phys. Soc. 2010, 57, 1086–1091. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.-H.; Lee, J.-W.; Nam, S.-M.; Yoon, Y.J.; Kim, J.-H. Residual stress relief in Al2O3–Poly-tetra-fluoro-ethylene hybrid thick films for integrated substrates using aerosol deposition. J. Nanoelectron. 2012, 7, 287–291. [Google Scholar] [CrossRef]
- Kim, H.-K.; Oh, J.-M.; In Kim, S.; Kim, H.-J.; Lee, C.W.; Nam, S.-M. Relation between electrical properties of aerosol-deposited BaTiO3 thin films and their mechanical hardness measured by nano-indentation. Nanoscale Res. Lett. 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, I.-S.; Ko, P.-J.; Cho, M.-Y.; Kim, H.-K.; Lee, D.-W.; Koo, S.-M.; Lee, D.; Park, C.; Oh, J.-M. High-density BaTiO3–Cu composite films with optimized BaTiO3 matrix for embedded capacitors. Ceram. Int. 2019, 45, 20634–20641. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Tang, N.; Fang, Y.; Zhang, H.; Duan, X. Rapid response flexible humidity sensor for respiration monitoring using nano-confined strategy. Nanotechnology 2020, 31, 125302. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yao, Y.; Duan, X.; Liu, T. Force and humidity dual sensors fabricated by laser writing on polyimide/paper bilayer structure for pulse and respiration monitoring. J. Mater. Chem. C 2018, 6, 4727–4736. [Google Scholar] [CrossRef]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.; Whitesides, G.M. Paper-based electrical respiration sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Fang, Z.; Kuang, Y.; Zhang, Y.; Peng, C.; Chen, G. Flexible and highly sensitive humidity sensor based on cellulose nanofibers and carbon nanotube composite film. Langmuir 2019, 35, 4834–4842. [Google Scholar] [CrossRef]
- Kano, S.; Kim, K.; Fujii, M. Fast-response and flexible nanocrystal-based humidity sensor for monitoring human respiration and water evaporation on skin. ACS Sens. 2017, 2, 828–833. [Google Scholar] [CrossRef]
- Kanaparthi, S. Pencil-drawn paper-based non-invasive and wearable capacitive respiration sensor. Electroanalysis 2017, 29, 2680–2684. [Google Scholar] [CrossRef]



| No. | Material | Process | Type of Signal | Signal Variation Rate [%] |
|---|---|---|---|---|
| 1 | GO [33] | Chemical vapor deposition | Resistance | 3 |
| 2 | PEDOT-PSS [68] | Spin coating | Resistance | 6 |
| 3 | PMDA-ODA PAA [69] | Laser writing | Resistance | 1 |
| 4 | Graphite [70] | Solution | Current | 2 |
| 5 | Cellulose and CNT [71] | Solution | Current | 65 |
| 6 | Si nanocrystals [72] | Co-sputtering | Current | 2.2 |
| 7 | Cellulose [73] | Pencil drawing | Capacitance | 30 |
| 8 | NaCl/BaTiO3(This work) | AD process | Capacitance | 54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, M.-Y.; Kim, I.-S.; Kim, M.-J.; Hyun, D.-E.; Koo, S.-M.; Sohn, H.; Kim, N.-Y.; Kim, S.; Ko, S.; Oh, J.-M. NaCl Ionization-Based Moisture Sensor Prepared by Aerosol Deposition for Monitoring Respiratory Patterns. Sensors 2022, 22, 5178. https://doi.org/10.3390/s22145178
Cho M-Y, Kim I-S, Kim M-J, Hyun D-E, Koo S-M, Sohn H, Kim N-Y, Kim S, Ko S, Oh J-M. NaCl Ionization-Based Moisture Sensor Prepared by Aerosol Deposition for Monitoring Respiratory Patterns. Sensors. 2022; 22(14):5178. https://doi.org/10.3390/s22145178
Chicago/Turabian StyleCho, Myung-Yeon, Ik-Soo Kim, Min-Ji Kim, Da-Eun Hyun, Sang-Mo Koo, Hiesang Sohn, Nam-Young Kim, Sunghoon Kim, Seunghoon Ko, and Jong-Min Oh. 2022. "NaCl Ionization-Based Moisture Sensor Prepared by Aerosol Deposition for Monitoring Respiratory Patterns" Sensors 22, no. 14: 5178. https://doi.org/10.3390/s22145178
APA StyleCho, M.-Y., Kim, I.-S., Kim, M.-J., Hyun, D.-E., Koo, S.-M., Sohn, H., Kim, N.-Y., Kim, S., Ko, S., & Oh, J.-M. (2022). NaCl Ionization-Based Moisture Sensor Prepared by Aerosol Deposition for Monitoring Respiratory Patterns. Sensors, 22(14), 5178. https://doi.org/10.3390/s22145178







