Effects of a Long-Term Wearable Activity Tracker-Based Exercise Intervention on Cardiac Morphology and Function of Patients with Cystic Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
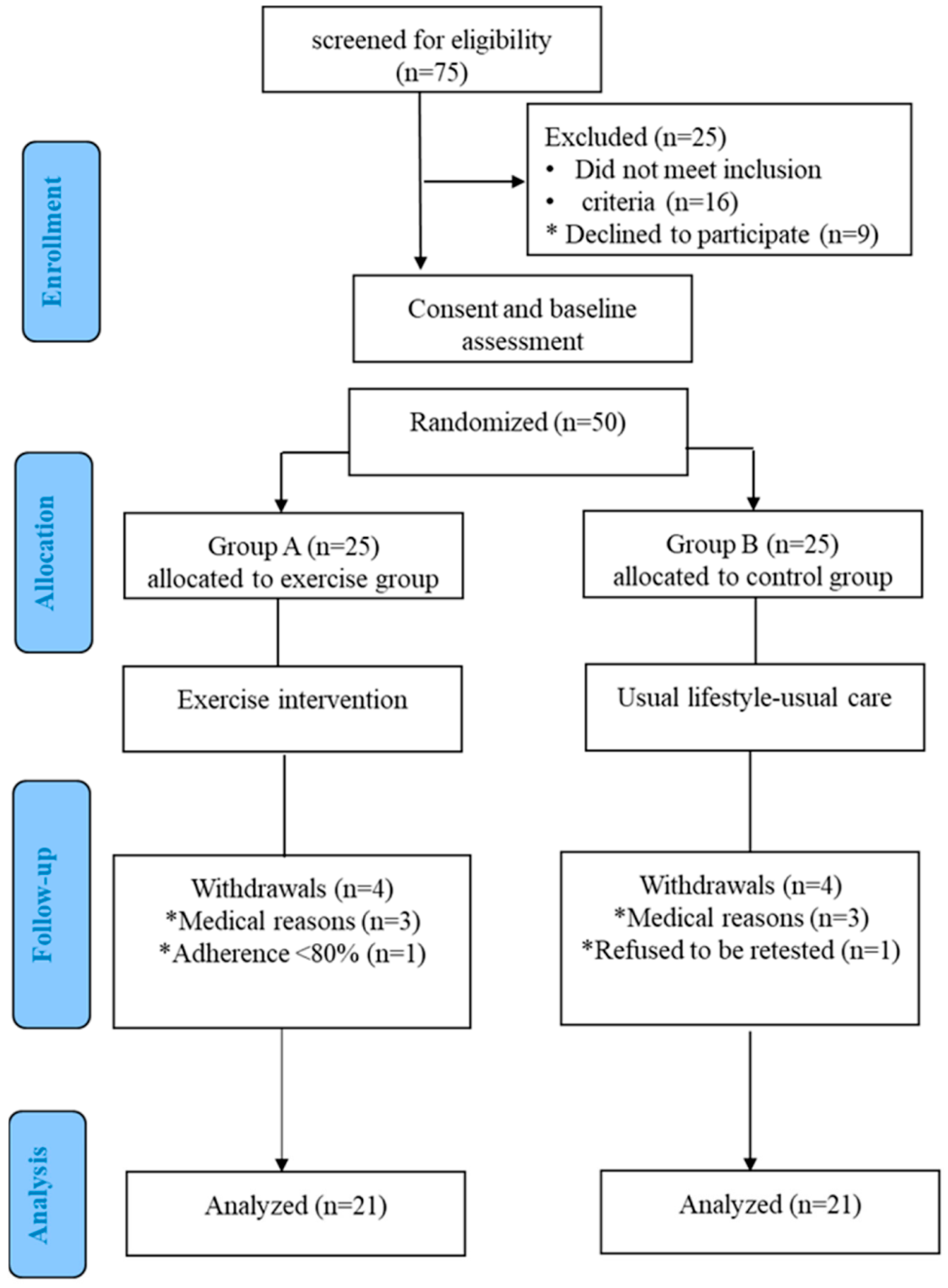
2.1. Study Population
2.2. Study Protocol
2.3. Measurements
2.3.1. Functional Capacity Assessment
2.3.2. Echocardiographic Study
2.3.3. WAT-Based ET Program
- Did you accept wearing the wearable activity tracker sensor during your exercise training? (Yes/No).
- Do you think that the wearable activity tracker provided you support and motivation to increase your level of participation in the exercise training program? (1 = not at all/10 = very much).
- Do you think the wearable activity tracker increased your adherence to the exercise training program? (1 = not at all/10 = very much).
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ward, N.; Stiller, K.; Holland, A.E. Exercise as a therapeutic intervention for people with cystic fibrosis. Expert Rev. Respir. Med. 2019, 13, 449–458. [Google Scholar] [CrossRef]
- Ding, S.; Zhong, C. Exercise and cystic fibrosis. In Physical Exercise for Human Health. Advances in Experimental Medicine and Biology; Xiao, J., Ed.; Springer: Singapore, 2020; Volume 1228. [Google Scholar] [CrossRef]
- Hebestreit, H.; Kriemler, S.; Radtke, T. Exercise for all cystic fibrosis patients: Is the evidence strengthening? Curr. Opin. Pulm. Med. 2015, 21, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Nevitt, S.J.; Hebestreit, H.; Kriemler, S. Physical exercise training for cystic fibrosis. Cochrane Database Syst. Rev. 2017, 11, CD002768. [Google Scholar] [CrossRef] [PubMed]
- Casado-Robles, C.; Viciana, J.; Guijarro-Romero, S.; Mayorga-Vega, D. Effects of Consumer-Wearable Activity Tracker-Based Programs on Objectively Measured Daily Physical Activity and Sedentary Behavior among School-Aged Children: A Systematic Review and Meta-analysis. Sports Med. Open 2022, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Franssen, W.M.A.; Franssen, G.H.L.M.; Spaas, J.; Solmi, F.; Eijnde, B.O. Can consumer wearable activity tracker-based interventions improve physical activity and cardiometabolic health in patients with chronic diseases? A systematic review and meta-analysis of randomised controlled trials. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 57. [Google Scholar] [CrossRef]
- Bradley, J.; O’Neill, B.; Kent, L.; Hulzebos, E.H.; Arets, B.; Hebestreit, H.; Exercise Working Group European CF Society, for publication in Journal of CF; Exercise Working Group European CF Society. Physical activity assessment in cystic fibrosis: A position statement. J. Cyst. Fibros. 2015, 14, e25–e32. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, T.J.; Alison, J.A.; McKeough, Z.J.; Elkins, M.R.; Bye, P.T. Evaluation of the SenseWear activity monitor during exercise in cystic fibrosis and in health. Respir. Med. 2009, 103, 1511–1517. [Google Scholar] [CrossRef] [Green Version]
- Quon, B.S.; Patrick, D.L.; Edwards, T.C.; Aitken, M.L.; Gibson, R.L.; Genatossio, A.; McNamara, S.; Goss, C.H. Feasibility of using pedometers to measure daily step counts in cystic fibrosis and an assessment of its responsiveness to changes in health state. J. Cyst. Fibros. 2012, 11, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Pastré, J.; Prévotat, A.; Tardif, C.; Langlois, C.; Duhamel, A.; Wallaert, B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. BMC Pulm. Med. 2014, 14, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troosters, T.; Langer, D.; Vrijsen, B.; Segers, J.; Wouters, K.; Janssens, W.; Gosselink, R.; Decramer, M.; Dupont, L. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. Eur. Respir. J. 2009, 33, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruet, M.; Troosters, T.; Verges, S. Peripheral muscle abnormalities in cystic fibrosis: Etiology, clinical implications and response to therapeutic interventions. J. Cyst. Fibros. 2017, 16, 538–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labombarda, F.; Saloux, E.; Brouard, J.; Bergot, E.; Milliez, P. Heart involvement in cystic fibrosis: A specific cystic fibrosis-related myocardial changes? Respir. Med. 2016, 118, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendrusculo, F.M.; Heinzmann-Filho, J.P.; da Silva, J.S.; Perez Ruiz, M.; Donadio, M.V.F. Peak Oxygen Uptake and Mortality in Cystic Fibrosis: Systematic Review and Meta-Analysis. Respir. Care 2019, 64, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Turcios, N.L. Cystic Fibrosis Lung Disease: An Overview. Respir. Care 2020, 65, 233–251. [Google Scholar] [CrossRef]
- Sellers, Z.M.; McGlocklin, L.; Brasch, A. Strain rate echocardiography uncovers subclinical left ventricular dysfunction in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 654–660. [Google Scholar] [CrossRef]
- Eising, J.B.; van der Ent, C.K.; Teske, A.J.; Vanderschuren, M.M.; Uiterwaal, C.S.P.M.; Meijboom, F.J. Young patients with cystic fibrosis demonstrate subtle alterations of the cardiovascular system. J. Cyst. Fibros. 2018, 17, 643–649. [Google Scholar] [CrossRef]
- Sciatti, E.; Vizzardi, E.; Bonadei, I.; Valentini, F.; Menotti, E.; Prati, F.; Dallapellegrina, L.; Berlendis, M.; Poli, P.; Padoan, R.; et al. Focus on echocardiographic right ventricular strain analysis in cystic fibrosis adults without cardiovascular risk factors: A case–control study. Intern. Emerg. Med. 2019, 14, 1279–1285. [Google Scholar] [CrossRef]
- Tonelli, A.R. Pulmonary hypertension survival effects and treatment options in cystic fibrosis. Curr. Opin. Pulm Med. 2013, 19, 652–661. [Google Scholar] [CrossRef] [Green Version]
- Dordevic, A.; Genger, M.; Schwarz, C.; Cuspidi, C.; Tahirovic, E.; Pieske, B.; Düngen, H.D.; Tadic, M. Biatrial Remodeling in Patients with Cystic Fibrosis Running Title: Atrial Function in Cystic Fibrosis. J. Clin. Med. 2019, 8, 1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellers, Z.; Kovacs, A.; Weinheimer, C.; Best, P. Left ventricular and aortic dysfunction in cystic fibrosis mice. J. Cyst. Fibros. 2013, 12, 517–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Miguelez, P.; Seigler, N.; Ishii, H.; Crandall, R.; McKie, K.T.; Foreseen, C.; Harris, R.A. Exercise Intolerance in Cystic Fibrosis: Importance of Skeletal Muscle. Med. Sci. Sports Exerc. 2021, 53, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Estévez-González, A.J.; Donadio, M.V.F.; Cobo-Vicente, F.; Fernández-Luna, A.; Sanz-Santiago, V.; Asensi, J.R.V.; Ramirez, T.I.; Fernández-del-Valle, M.; Diez-Vega, I.; Larumbe-Zabala, E.; et al. Effects of a Short-Term Resistance-Training Program on Heart Rate Variability in Children with Cystic Fibrosis—A Randomized Controlled Trial. Front. Physiol. 2021, 12, 652029. [Google Scholar] [CrossRef]
- Ionescu, A.A.; Ionescu, A.A.; Payne, N.; Obieta-Fresnedo, I.; Fraser, A.G.; Shale, D.J. Subclinical right ventricular dysfunction in cystic fibrosis. A study using tissue Doppler echocardiography. Am. J. Respir. Crit. Care Med. 2001, 163, 1212–1218. [Google Scholar] [CrossRef]
- Solway, S.; Brooks, D.; Lacasse, Y.; Thomas, S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest 2001, 119, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, L.H. Utility of the six-minute walk test in patients with idiopathic pulmonary fibrosis. Multidiscip. Respir. Med. 2018, 13, 45. [Google Scholar] [CrossRef] [Green Version]
- Wheatley, C.M.; Wilkins, B.W.; Snyder, E.M. Exercise is medicine in cystic fibrosis. Exerc. Sport Sci. Rev. 2011, 39, 155–160. [Google Scholar] [CrossRef]
- Li, X.; Yu, R.; Wang, P.; Wang, A.; Huang, H. Effects of Exercise Training on Cardiopulmonary Function and Quality of Life in Elderly Patients with Pulmonary Fibrosis: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7643. [Google Scholar] [CrossRef]
- Wheatley, C.M.; Baker, S.E.; Morgan, M.A.; Martinez, M.G.; Morgan, W.J.; Wong, E.C.; Karpen, S.R.; Snyder, E.M. Effects of exercise intensity compared to albuterol in individuals with cystic fibrosis. Respir. Med. 2015, 109, 463–474. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.A.; Crandall, R.; Seigler, N.; Rodriguez-Miguelez, P.; McKie, K.T.; Forseen, C.; Thomas, J.; Harris, R.A. A single bout of maximal exercise improves lung function in patients with cystic fibrosis. J. Cyst. Fibros. 2017, 16, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K.D.; Walsh, A.; McCormack, P.; Southern, K.W. Exercise versus airway clearance techniques for people with cystic fibrosis. Cochrane Database Syst. Rev. 2019, 3, CD013285. [Google Scholar] [CrossRef]
- Slimani, M.; Ramirez-Campillo, R.; Paravlic, A.; Hayes, L.D.; Bragazzi, N.L.; Sellami, M. The Effects of Physical Training on Quality of Life, Aerobic Capacity, and Cardiac Function in Older Patients with Heart Failure: A Meta-Analysis. Front. Physiol. 2018, 9, 1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanar, B.G.; Ozmen, I.; Yildirim, E.O.; Ozturk, M.; Sunbul, M. Right Ventricular Functional Improvement after Pulmonary Rehabilitation Program in Patients with COPD Determined by Speckle Tracking Echocardiography. Arq. Bras. Cardiol. 2018, 111, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Alhumaid, W.; Small, S.D.; Kirkham, A.A.; Becher, H.; Pituskin, E.; Prado, C.M.; Thompson, R.B.; Haykowsky, M.J.; Paterson, D.I. A Contemporary Review of the Effects of Exercise Training on Cardiac Structure and Function and Cardiovascular Risk Profile: Insights from Imaging. Front. Cardiovasc. Med. 2022, 9, 753652. [Google Scholar] [CrossRef]
- Hambrecht, R.; Gielen, S.; Linke, A.; Fiehn, E.; Yu, J.; Walther, C.; Schoene, N.; Schuler, G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA 2000, 283, 3095–3101. [Google Scholar] [CrossRef]
- Belardinelli, R.; Georgiou, D.; Cianci, G.; Purcaro, A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999, 99, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Haykowsky, M.J.; Liang, Y.; Pechter, D.; Jones, L.W.; McAlister, F.A.; Clark, A.M. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: The benefit depends on the type of training performed. J. Am. Coll. Cardiol. 2007, 49, 2329–2336. [Google Scholar] [CrossRef] [Green Version]
- Deligiannis, A.; Kouidi, E.; Tassoulas, E.; Gigis, P.; Tourkantonis, A.; Coats, A.S. Cardiac effects of exercise rehabilitation in hemodialysis patients. Int. J. Cardiol. 1999, 70, 253–266. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Kouidi, E.; Efthimiadis, G.K.; Deligiannis, A.; Geleris, P.; Vassilikos, V. Right atrial and ventricular adaptations to training in male Caucasian athletes: An echocardiographic study. J. Am. Soc. Echocardiogr. 2013, 26, 1344–1352. [Google Scholar] [CrossRef]
- La Gerche, A.; Claessen, G. Is exercise good for the right ventricle? Concepts for health and disease. Can. J. Cardiol. 2015, 31, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, M.; Ogawa, A.; Matsubara, H. High Right Ventricular Afterload during Exercise in Patients with Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Craig, C.; Beets, M.; Belton, S.; Cardon, G.; Duncan, S.; Hatano, Y.; Lubans, D.; Olds, T.; Raustorp, A.; et al. How Many Steps/Day are Enough? For Children and Adolescents. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 78–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudor-Locke, C.; Bassett, D.R., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004, 34, 1–8. [Google Scholar] [CrossRef]
- Evenson, K.; Spade, C. Review of Validity and Reliability of Garmin Activity Trackers. J. Meas. Phys. Behav. 2020, 3, 170–185. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Frommelt, P.; Ensing, G.; Kendall, K.; Younoszai, A.; Lai, W.; Geva, T.M. Recommendations for Quantification Methods during the Performance of a Pediatric Echocardiogram: A Report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef]
- Caminati, A.; Bianchi, A.; Cassandro, R.; Mirenda, M.R.; Harari, S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir. Med. 2009, 103, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Bois, R.M.; Weycker, D.; Albera, C.; Bradford, W.Z.; Costabel, U.; Kartashov, A.; Lancaster, L.; Noble, P.W.; Sahn, S.A.; Szwarcberg, J.; et al. Six-minute-walk test in idiopathic pulmonary fibrosis: Test validation and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011, 183, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Ghofraniha, L.; Dalir Sani, Z.; Vakilian, F.; Khajedalooyi, M.; Arabshahi, Z. The Six-minute Walk Test (6MWT) for the Evaluation of Pulmonary Diseases. J. Cardio-Thoracic. Med. 2015, 3, 284–287. [Google Scholar] [CrossRef]


| Variables | Group A Baseline | Group A After 1 Year | Group B Baseline | Group B After 1 Year |
|---|---|---|---|---|
| Age (years) | 17.0 ± 3.3 | 18.0 ± 3.3 | 16.1 ± 3.3 | 17.1 ± 3.3 |
| BMI (kg/m2) | 20.0 ± 3.3 | 20.8 ± 3.5 | 20.1 ± 2.6 | 19.7 ± 3.8 |
| BSA (m2) | 1.50 ± 0.2 | 1.56 ± 0.1 | 1.55 ± 0.1 | 1.56 ± 0.1 |
| SBP (mmHg) | 110.8 ± 0.4 | 118.6 ± 0.9 | 108.5 ± 0.8 | 110.04 ± 0.6 |
| DBP (mmHg) | 82.2 ± 0.3 | 81.4 ± 0.4 | 81.4 ± 0.5 | 82.1 ± 0.5 |
| HR (bpm) | 74.5 ± 5.2 | 72.4 ± 6.2 | 76.5 ± 7.1 | 78.2 ± 6.4 |
| LV Indices | Group A Baseline | Group A After 1 Year | Group B Baseline | Group B After 1 Year |
|---|---|---|---|---|
| LVIVSd (mm) | 7.1 ± 0.7 | 7.5 ± 0.7 | 7.5 ± 0.8 | 7.9 ± 0.8 |
| LVEDD (mm) | 43.7 ± 3.5 | 45.3 ± 4.0 | 45.0 ± 2.7 | 46.7 ± 3.8 |
| LVPWd (mm) | 6.5 ± 0.6 | 6.9 ± 0.4 | 7.0 ± 0.8 | 7.6 ± 0.9 |
| LVEDV (mL) | 91.3 ± 16.3 | 96.9 ± 17.0 | 97.5 ± 10.0 | 93.0 ± 11.5 |
| LVEF (%) | 64.4 ± 4.1 | 66.3 ± 5.2 | 64.2 ± 4.1 | 65.3 ± 3.2 |
| MVE (m/s) | 0.92 ± 0.1 | 0.88 ± 0.1 | 0.90 ± 0.1 | 0.99 ± 0.1 |
| MVA (m/s) | 0.56 ± 0.1 | 0.54 ± 0.1 | 0.57 ± 0.1 | 0.63 ± 0.1 |
| MVE/A | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.2 |
| LA (cm) | 3.1 ± 0.3 | 3.1 ± 0.2 | 3.09 ± 0.3 | 3.13 ± 0.3 |
| RV Indices | Group A Baseline | Group A After 1 Year | Group B Baseline | Group B After 1 Year |
|---|---|---|---|---|
| RV bas (mm) | 37.0 ± 3.0 | 37.3 ± 4.1 | 35.3 ± 2.6 | 35.3 ± 3.3 |
| RV bas/BSA (mm) | 25.5 ± 3.7 | 24.3 ± 3.3 | 23.8 ± 2.2 | 22.7 ± 2.5 |
| RVOT prox (mm) | 27.9 ± 3.5 | 27.8 ± 4.6 | 26.9 ± 2.9 | 27.4 ± 1.7 |
| RVOTprox/BSA (mm) | 19.1 ± 2.7 | 18.0 ± 2.3 | 17.6 ± 1.7 | 17.6 ± 1.6 |
| RAVol/BSA (mL/m2) | 16.0 ± 2.9 | 17.4 ± 4.1 | 15.8 ± 1.6 | 15.7 ± 1.9 |
| TVE (m/s) | 0.75 ± 0.1 | 0.70 ± 0.10 | 0.70 ± 0.1 | 0.69 ± 0.06 |
| TVA (m/s) | 0.53 ± 0.1 | 0.44 ± 0.09 * | 0.48 ± 0.1 | 0.46 ± 0.04 |
| TVE/A | 1.4 ± 0.2 | 1.6 ± 0.2 * | 1.5 ± 0.2 | 1.5 ± 0.1 |
| TVS’ (m/s) | 0.11 ± 0.01 | 0.12 ± 0.01 * | 0.11 ± 0.01 | 0.11 ± 0.01 |
| TVE’ (m/s) | 0.13 ± 0.03 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.01 |
| TVA’ (m/s) | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.00 |
| TAPSE (mm) | 20.4 ± 2.7 | 22.1 ± 1.5 * | 20.2 ± 1.4 | 20.8 ± 1.3 # |
| PASP (mmHg) | 24.8 ± 3.5 | 22.9 ± 3.3 * | 24.0 ± 4.0 | 24.5 ± 3.4 |
| RVFWSL (%) | −22.6 ± 1.4 | −25.7 ± 2.5 * | −23.6 ± 2.0 | −23.1 ± 1.5 # |
| RV4CSL (%) | −18.8 ± 1.2 | −23.7 ± 2.0 * | −19.1 ± 1.4 | −19.7 ± 1.4 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anifanti, M.; Giannakoulakos, S.; Hatziagorou, E.; Kampouras, A.; Tsanakas, J.; Deligiannis, A.; Kouidi, E. Effects of a Long-Term Wearable Activity Tracker-Based Exercise Intervention on Cardiac Morphology and Function of Patients with Cystic Fibrosis. Sensors 2022, 22, 4884. https://doi.org/10.3390/s22134884
Anifanti M, Giannakoulakos S, Hatziagorou E, Kampouras A, Tsanakas J, Deligiannis A, Kouidi E. Effects of a Long-Term Wearable Activity Tracker-Based Exercise Intervention on Cardiac Morphology and Function of Patients with Cystic Fibrosis. Sensors. 2022; 22(13):4884. https://doi.org/10.3390/s22134884
Chicago/Turabian StyleAnifanti, Maria, Stavros Giannakoulakos, Elpis Hatziagorou, Asterios Kampouras, John Tsanakas, Asterios Deligiannis, and Evangelia Kouidi. 2022. "Effects of a Long-Term Wearable Activity Tracker-Based Exercise Intervention on Cardiac Morphology and Function of Patients with Cystic Fibrosis" Sensors 22, no. 13: 4884. https://doi.org/10.3390/s22134884
APA StyleAnifanti, M., Giannakoulakos, S., Hatziagorou, E., Kampouras, A., Tsanakas, J., Deligiannis, A., & Kouidi, E. (2022). Effects of a Long-Term Wearable Activity Tracker-Based Exercise Intervention on Cardiac Morphology and Function of Patients with Cystic Fibrosis. Sensors, 22(13), 4884. https://doi.org/10.3390/s22134884







