BrainGAN: Brain MRI Image Generation and Classification Framework Using GAN Architectures and CNN Models
Abstract
:1. Introduction
- An expanded Brain MRI dataset that involves around 1400 images using two GAN architectures: Vanilla GAN (original GAN) and Deep Conditional GAN (DCGAN). The expanded dataset will enable us to develop more general and accurate deep learning models for diagnosing brain MRI images for tumors.
- A framework, denoted as BrainGAN, for generating brain MRI images using multiple GAN architectures. This framework can be considered a guide for future experiments in terms of GAN architecture and parameters’ configurations. To the best of our knowledge. Generating two MRI dataset samples allows comparisons between the different GAN architectures in generating brain MRI images that are more similar to the real images.
- A novel approach to automatically validate the images generated by GANs. Although manual validation may be more accurate, however, it is time-consuming and may not be practical due to the limited availability of MRI radiologists. Thus, this study proposes an automatic validation of generated images using deep transfer learning models, i.e., three models. The validation is performed by training the deep transfer models with the generated images by the two GAN architectures, i.e., Vanilla GAN and DCGAN, and then evaluating their performance on a test set composed of real brain MRI images.
2. Literature Review
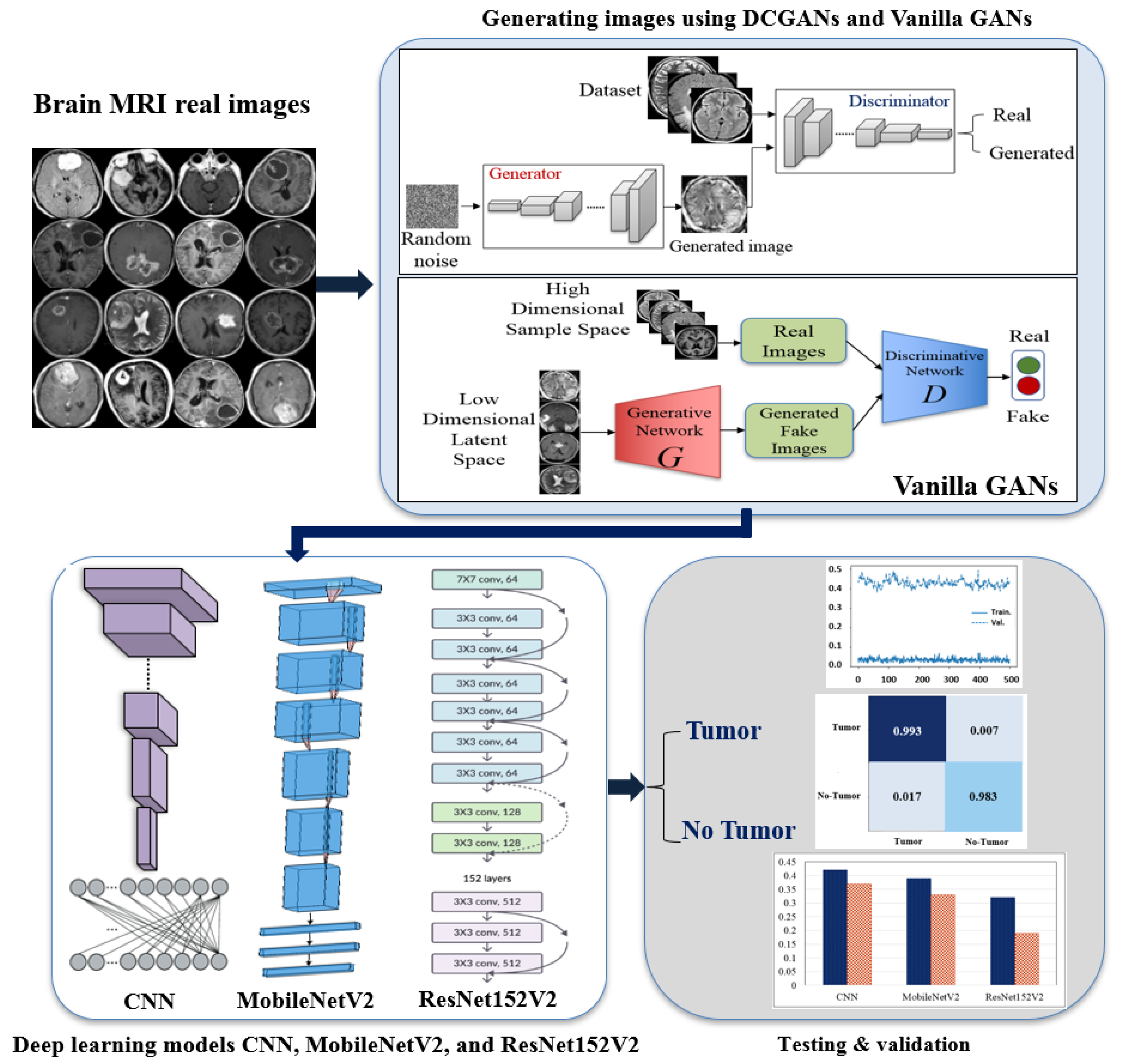
3. BrainGAN: The Proposed Framework
4. Experiment
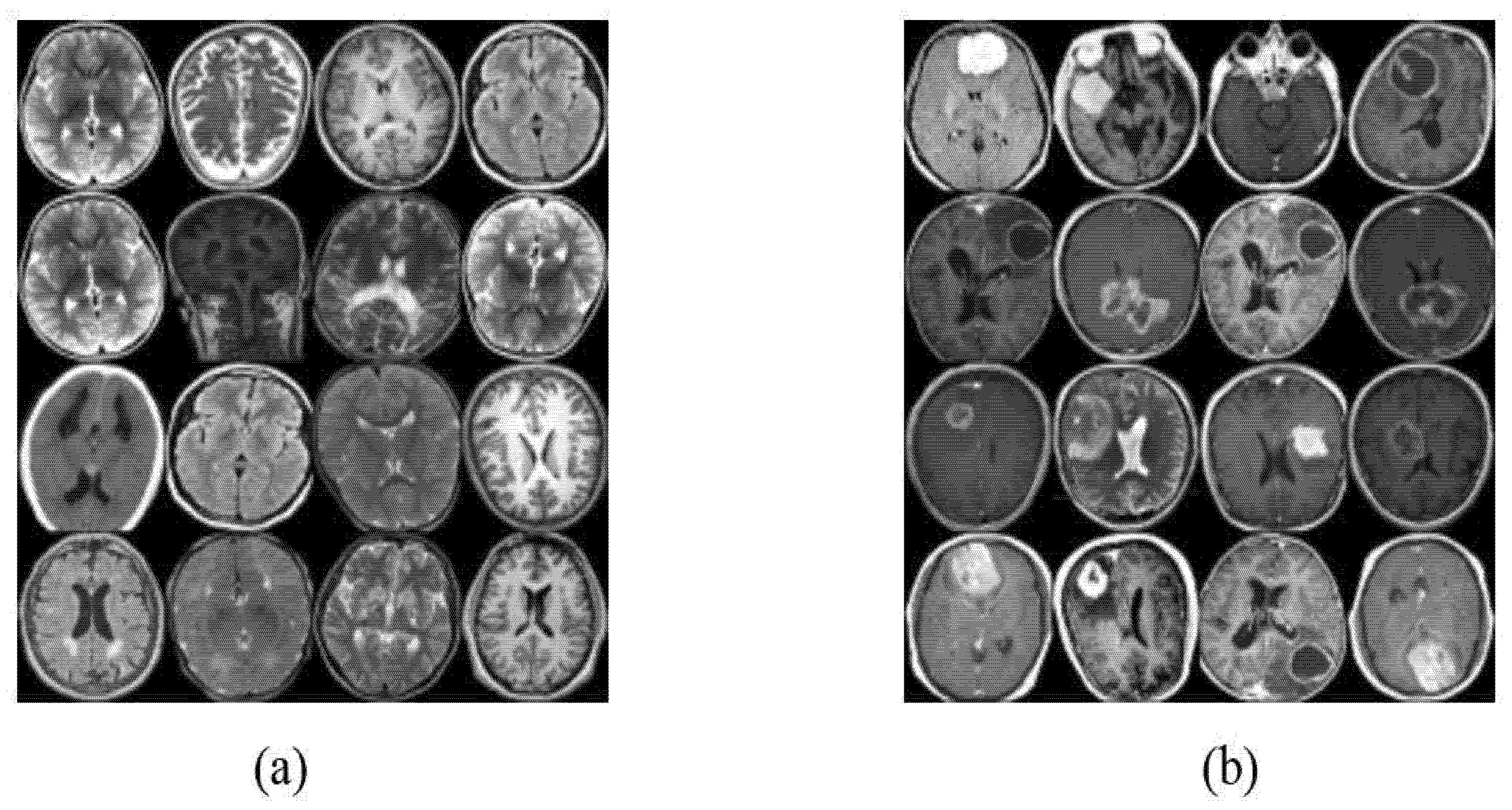
4.1. Datasets of the Study
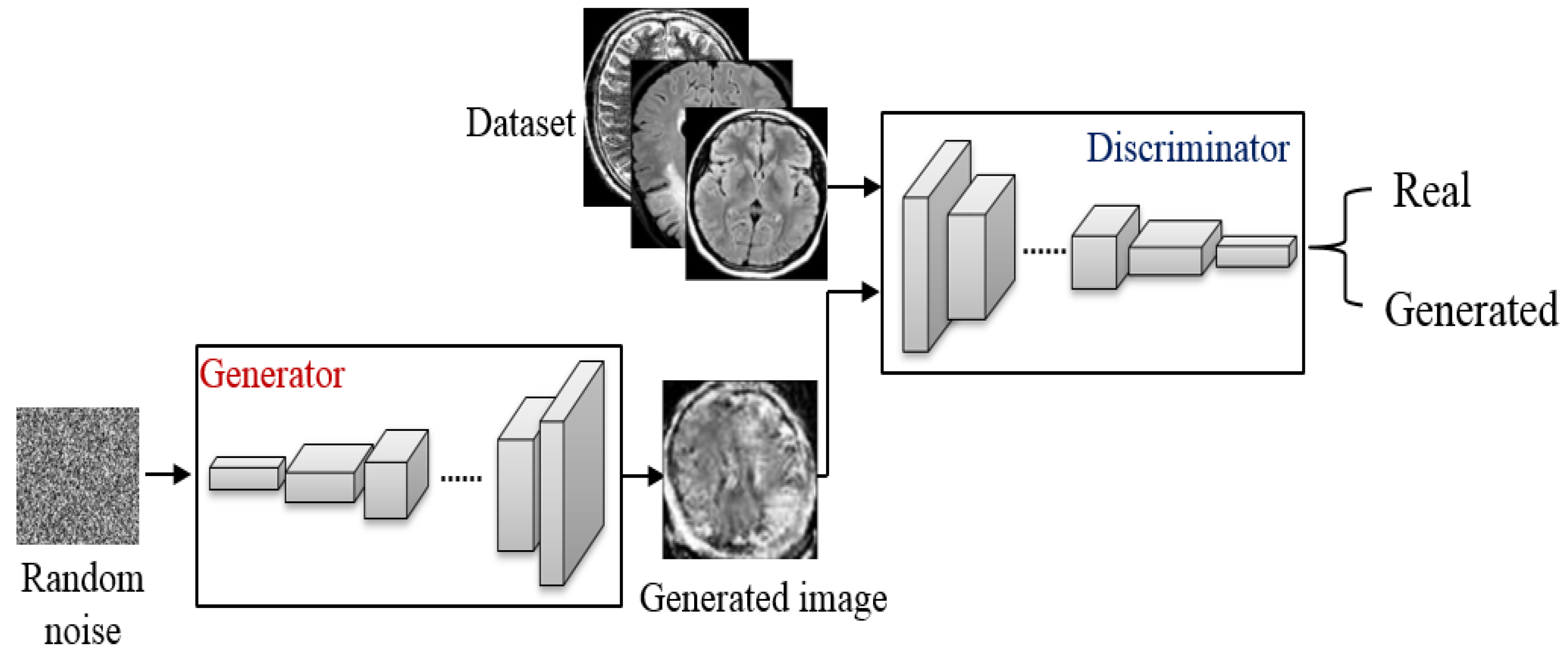
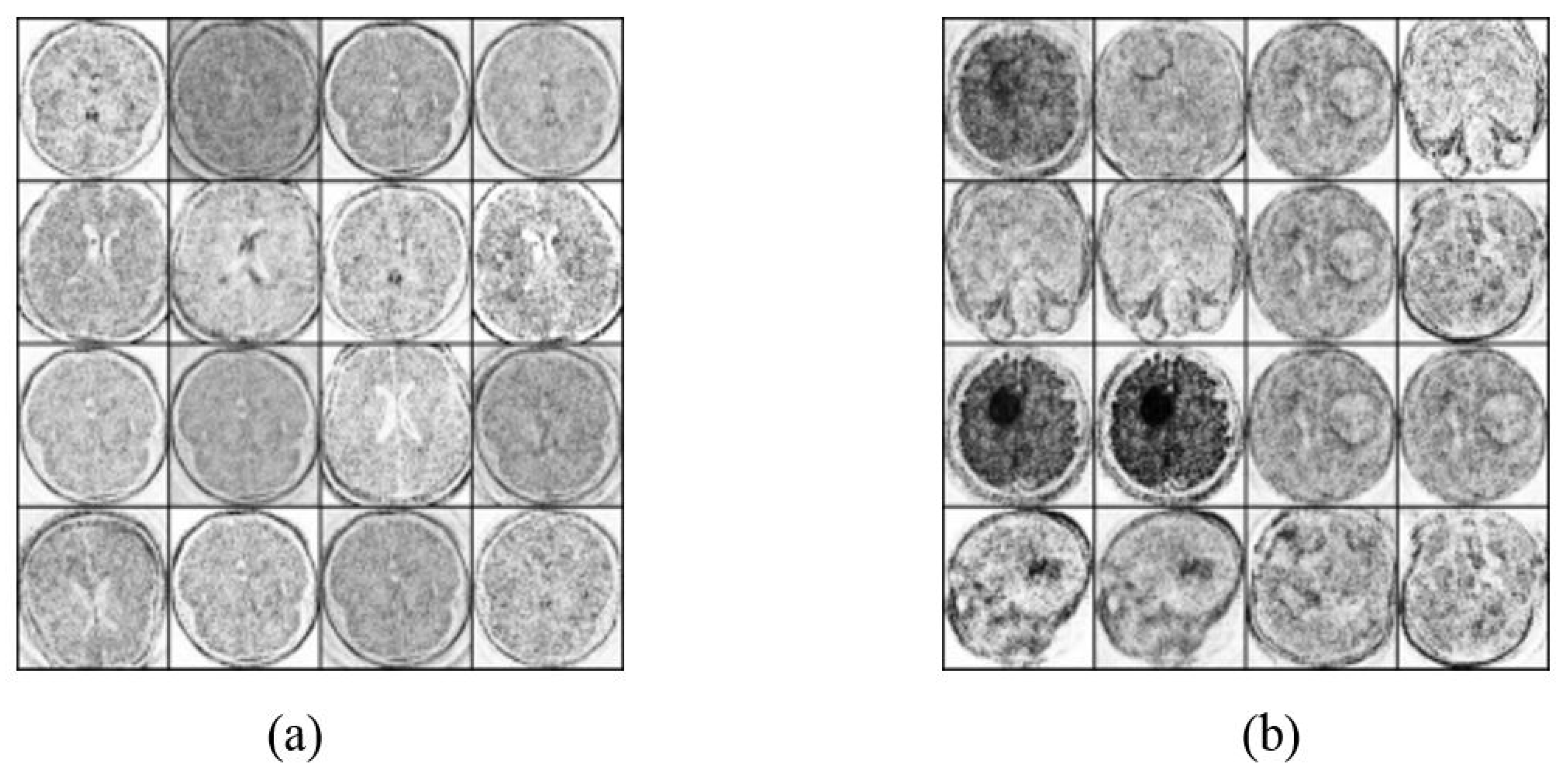
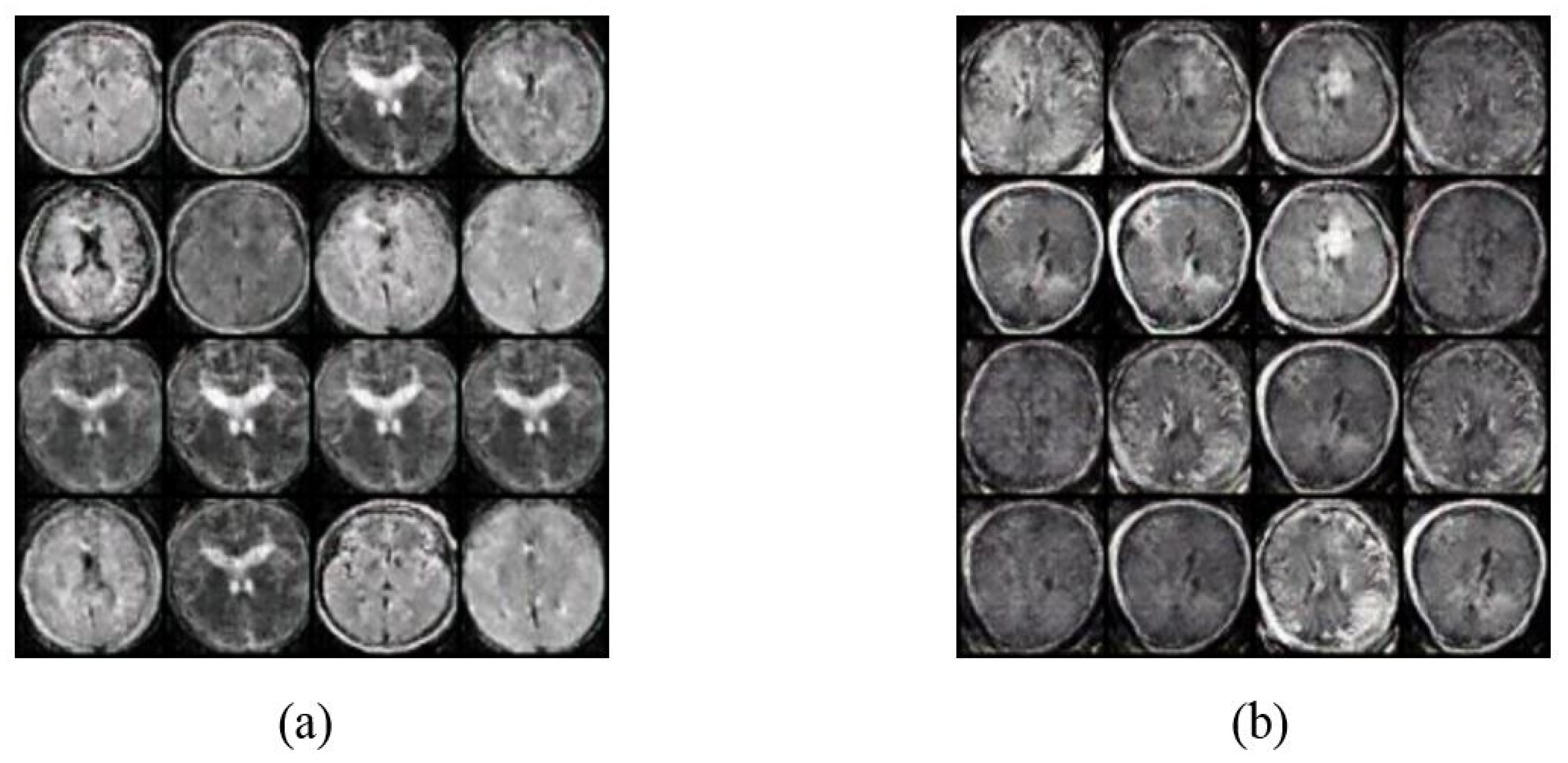
4.2. Image Augmentation Using Vanilla GAN and DCGAN
4.3. Deep Learning Proposed Classification Models
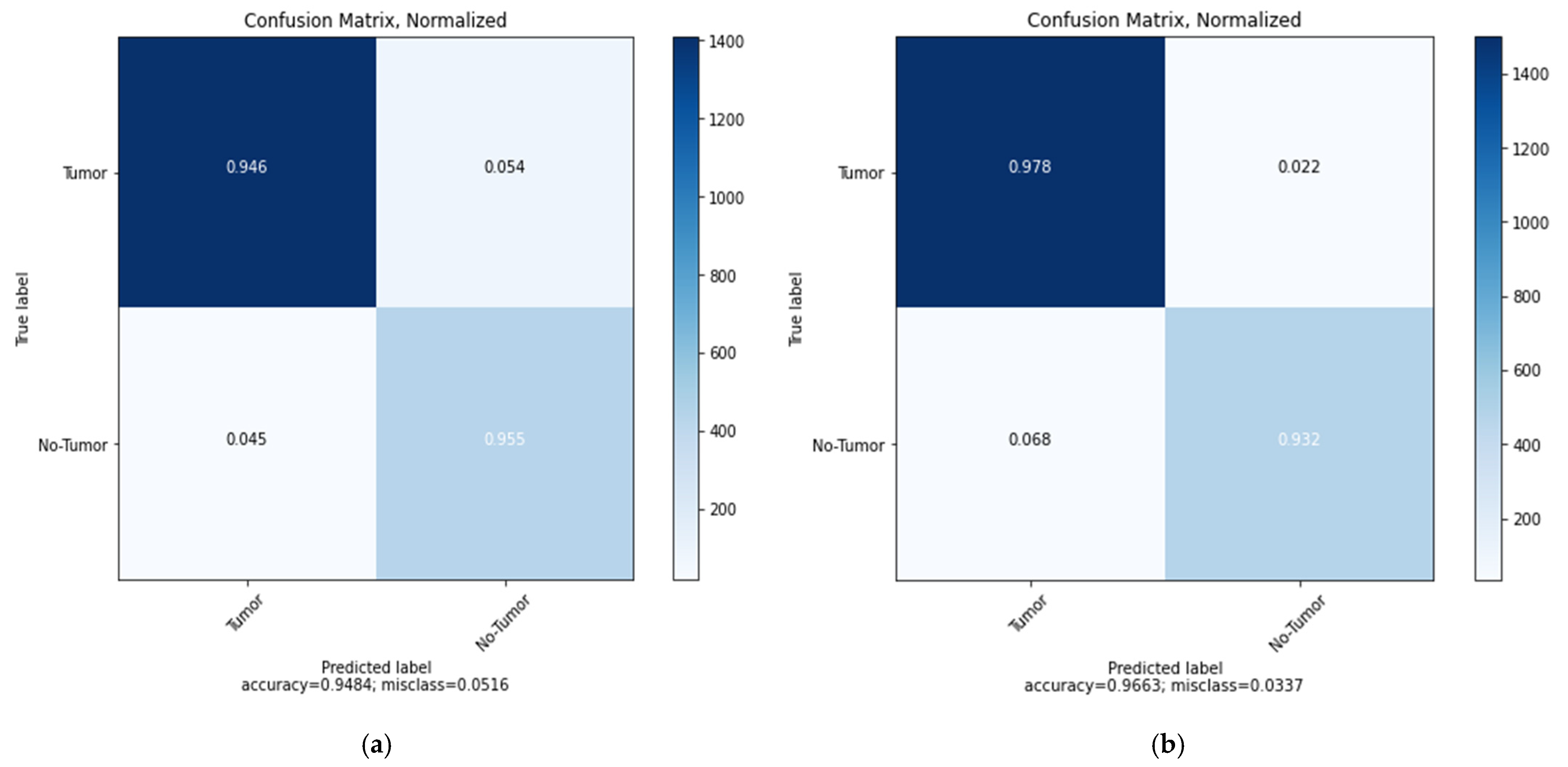
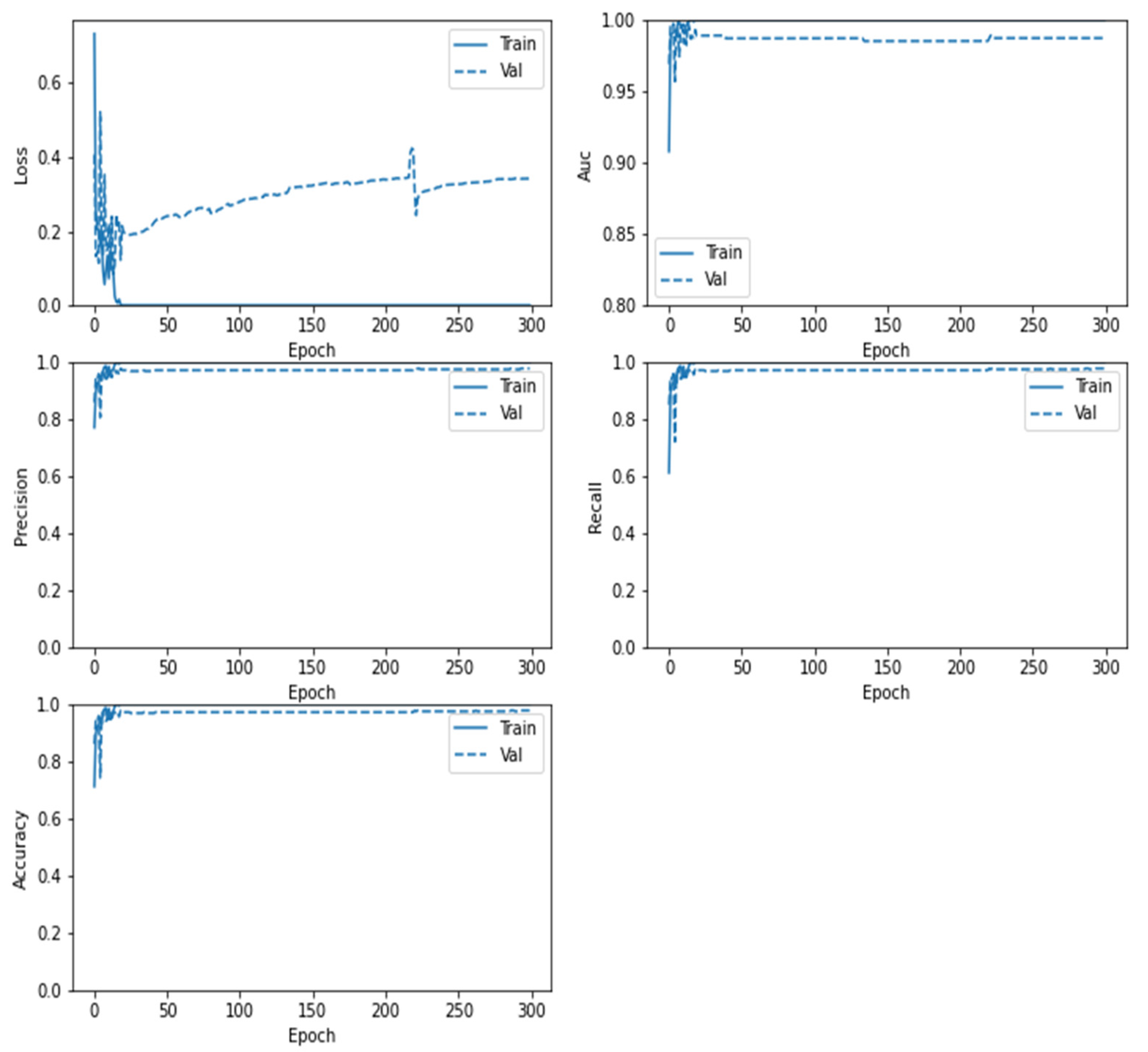
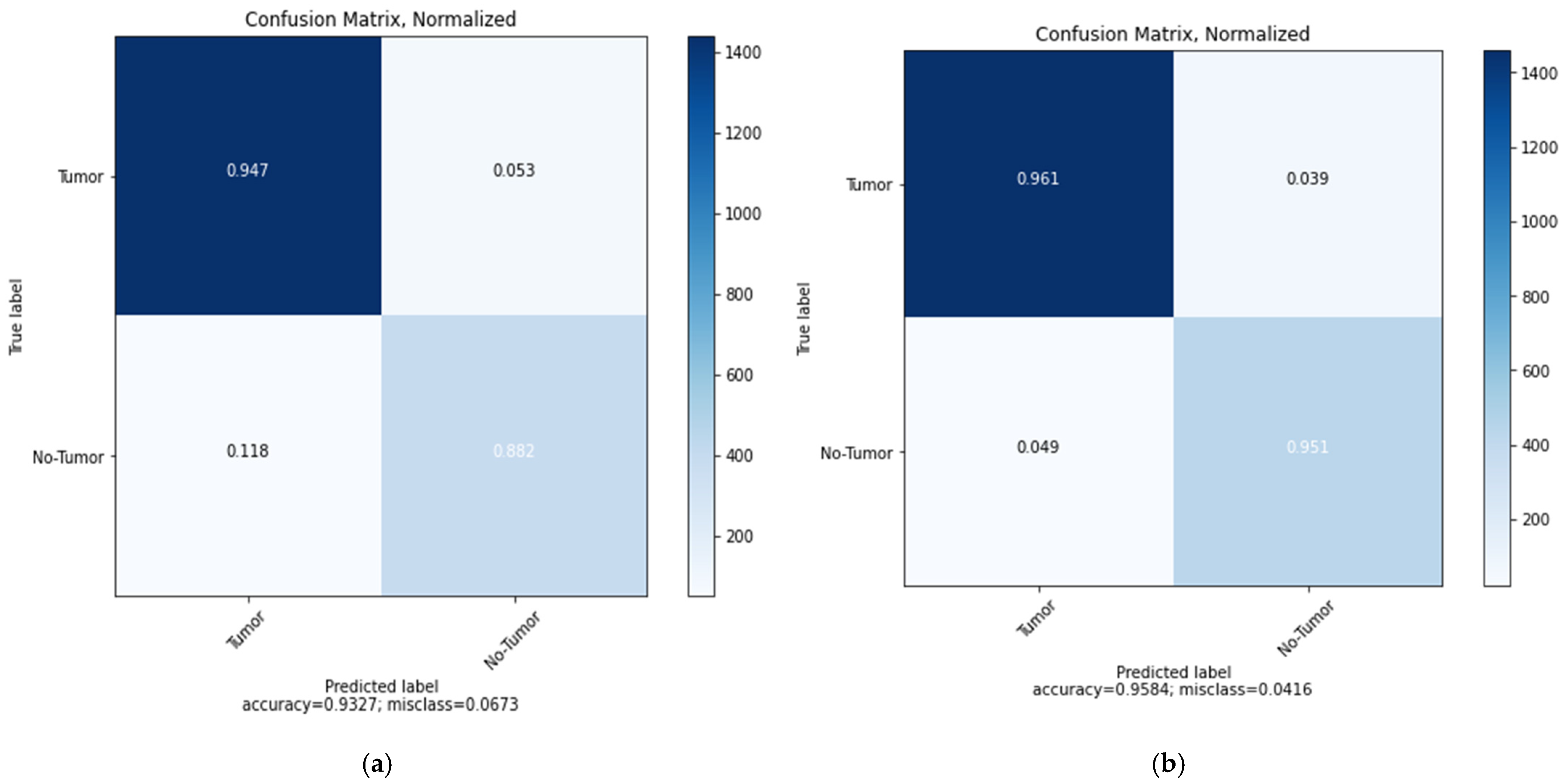
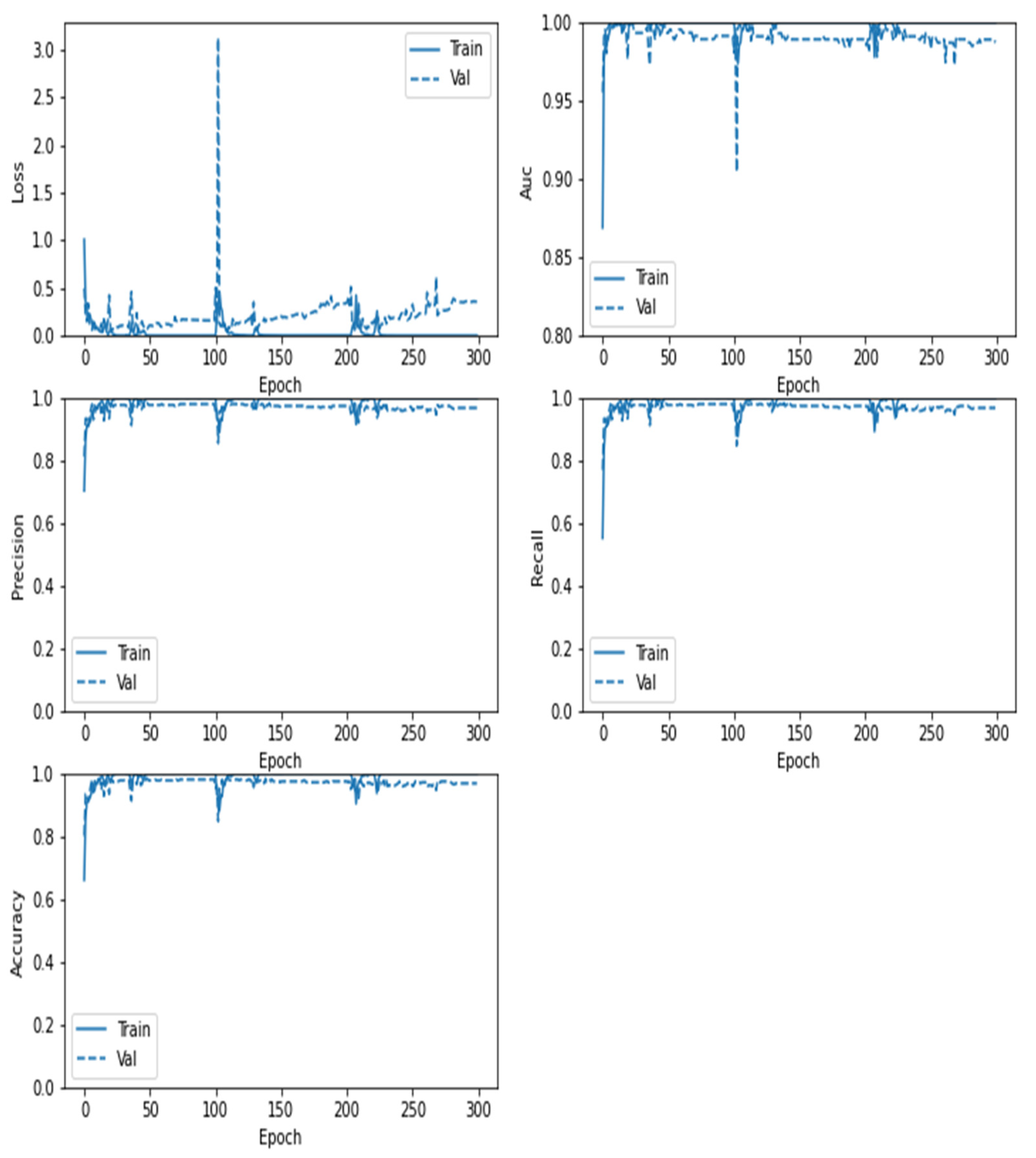
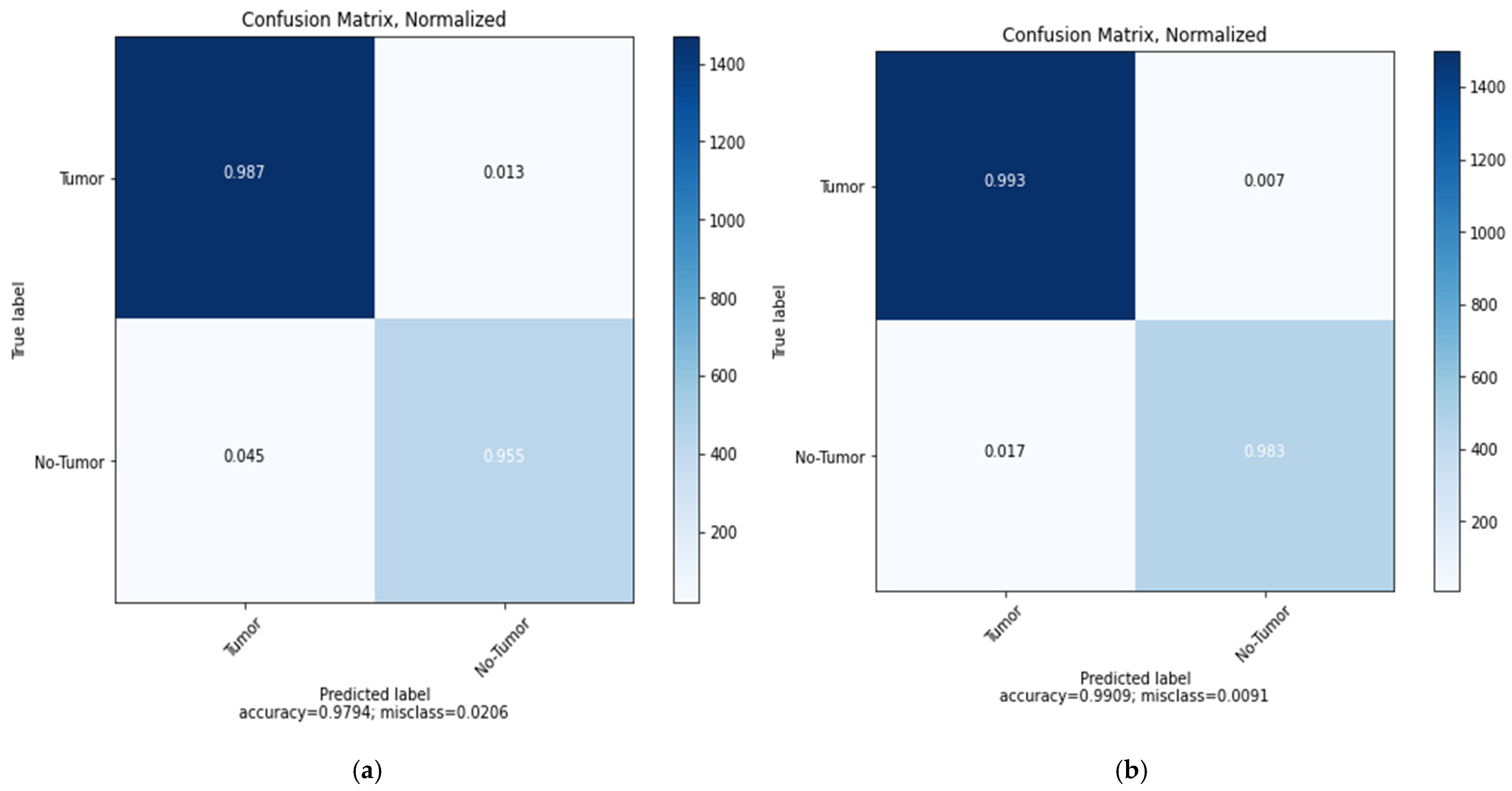
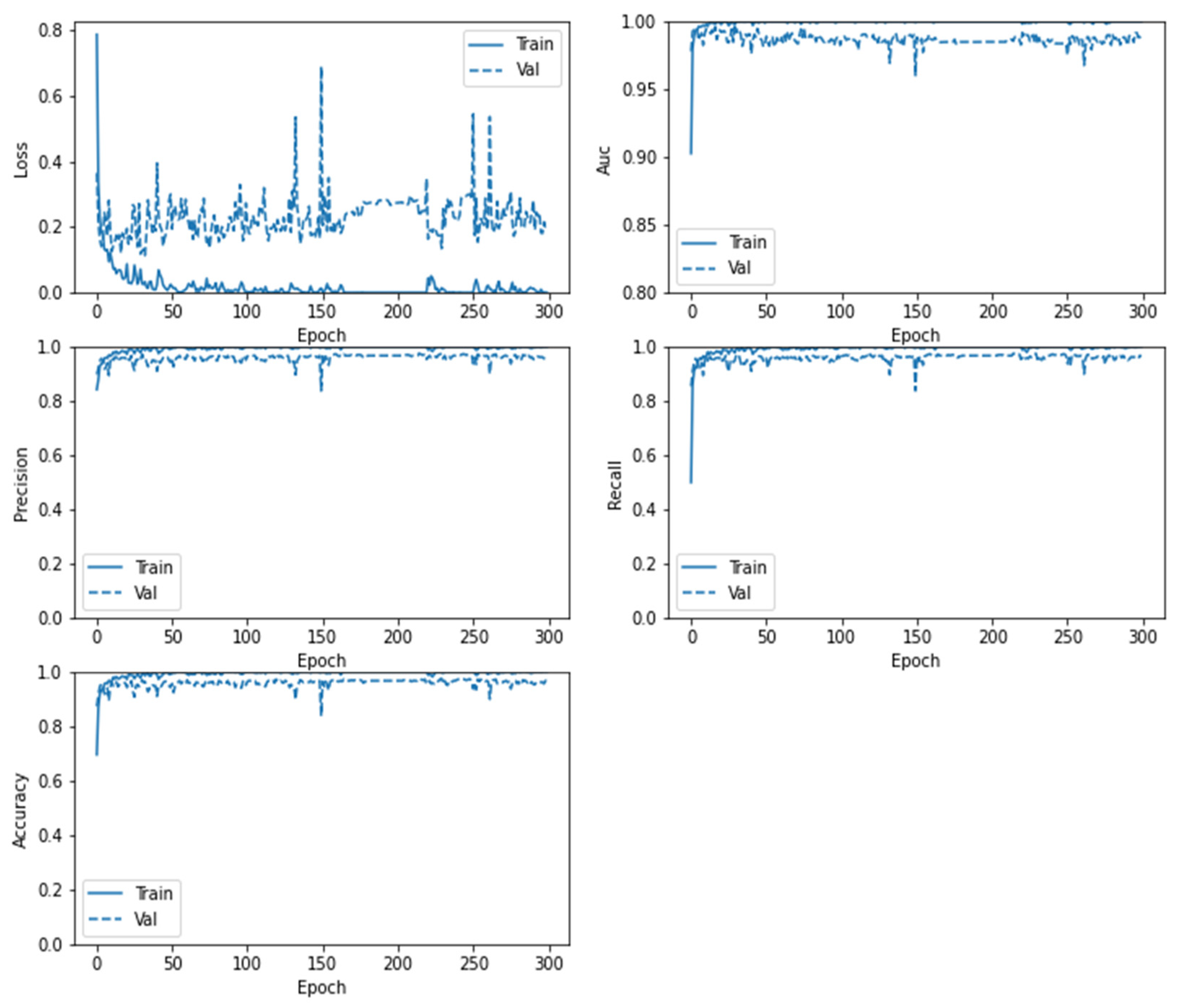
5. Results and Discussions
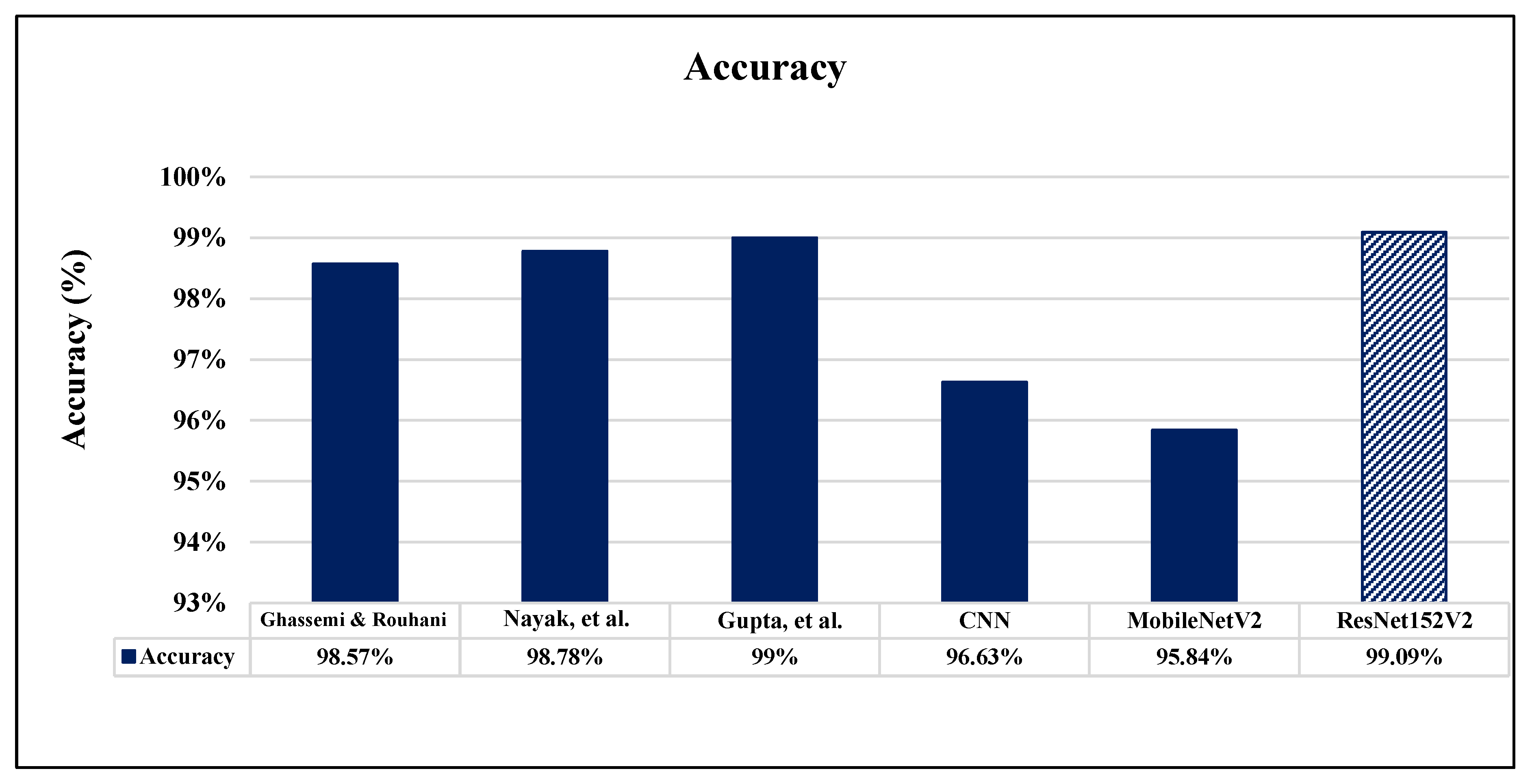
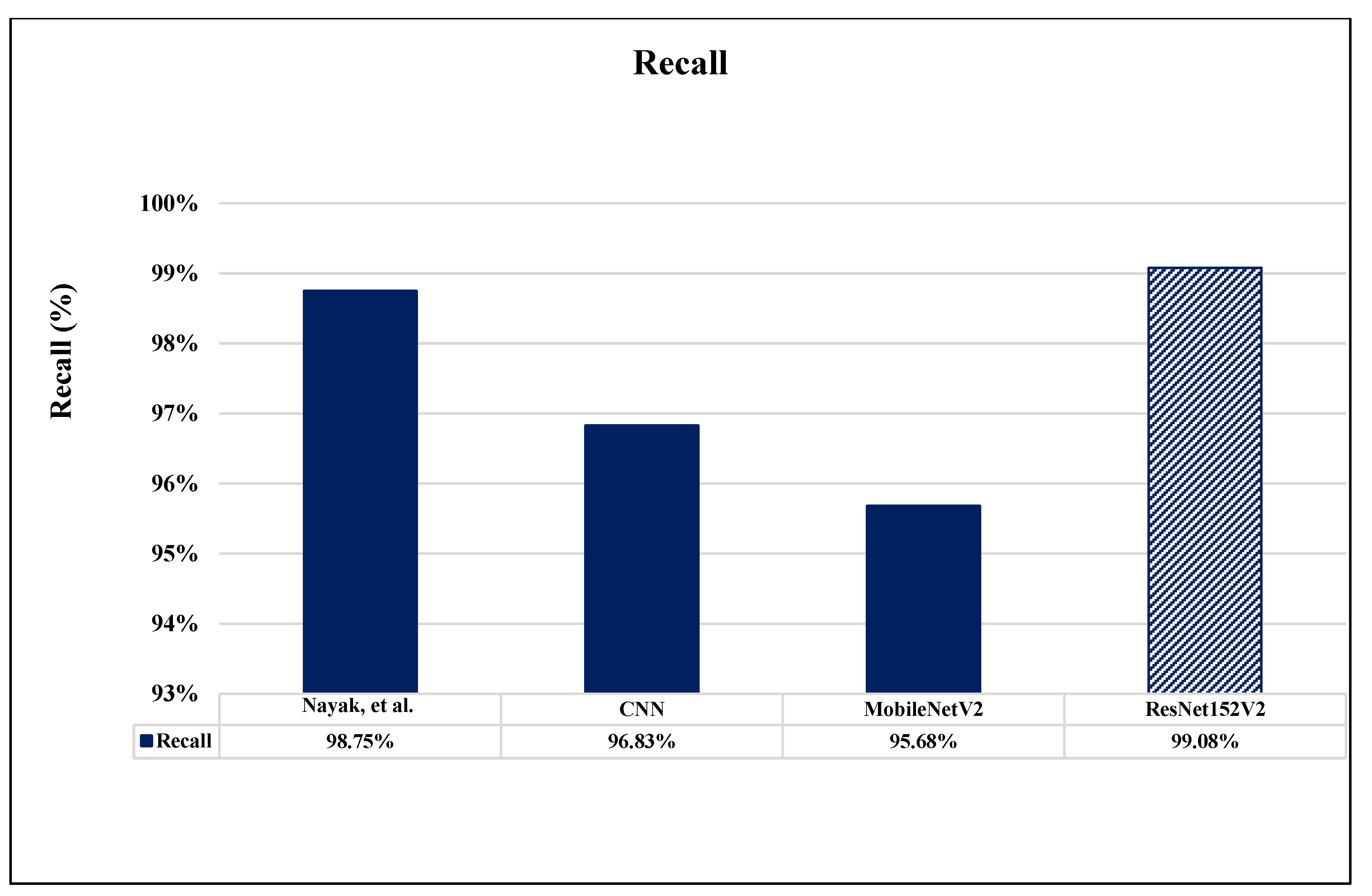
6. Comparative Analysis and Discussion
7. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Courville, A.; Bengio, Y. Generative adversarial nets. Adv. Neural Inf. Process. Syst. 2014, 3, 2672–2680. [Google Scholar]
- Kumar, S.; Dabas, C.; Godara, S. Classification of brain MRI tumor images: A hybrid approach. Procedia Comput. Sci. 2017, 122, 510–517. [Google Scholar] [CrossRef]
- Gab Allah, A.M.; Sarhan, A.M.; Elshennawy, N.M. Classification of Brain MRI Tumor Images Based on Deep Learning PGGAN Augmentation. Diagnostics 2021, 11, 2343. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Rice, C.M.; Wang, X. Cancer bioinformatics: A new approach to systems clinical medicine. BMC Bioinform. 2012, 13, S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brain MRI Segmentation. 2019. Available online: https://www.kaggle.com/mateuszbuda/lgg-mri-segmentation (accessed on 5 March 2022).
- Ahmad, R.; Bouman, C.A.; Buzzard, G.T.; Chan, S.; Liu, S.; Reehorst, E.T.; Schniter, P. Plug-and-play methods for magnetic resonance imaging: Using denoisers for image recovery. IEEE Signal Process. Mag. 2020, 37, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Rundo, L.; Araki, R.; Nagano, Y.; Furukawa, Y.; Mauri, G.; Nakayama, H.; Hayashi, H. Combining noise-to-image and image-to-image GANs: Brain MR image augmentation for tumor detection. IEEE Access 2019, 7, 156966–156977. [Google Scholar] [CrossRef]
- Han, C.; Rundo, L.; Araki, R.; Furukawa, Y.; Mauri, G.; Nakayama, H.; Hayashi, H. Infinite brain MR images: PGGAN-based data augmentation for tumor detection. In Neural Approaches to Dynamics of Signal Exchanges; Springer: Singapore, 2020; pp. 291–303. [Google Scholar]
- Han, C.; Murao, K.; Noguchi, T.; Kawata, Y.; Uchiyama, F.; Rundo, L.; Nakayama, H.; Satoh, S.I. Learning more with less: Conditional PGGAN-based data augmentation for brain metastases detection using highly-rough annotation on MR images. In Proceedings of the 28th ACM International Conference on Information and Knowledge Management, Beijing, China, 3–7 November 2019; pp. 119–127. [Google Scholar]
- Ge, C.; Gu IY, H.; Jakola, A.S.; Yang, J. Enlarged training dataset by pairwise gans for molecular-based brain tumor classification. IEEE Access 2020, 8, 22560–22570. [Google Scholar] [CrossRef]
- Ghassemi, N.; Shoeibi, A.; Rouhani, M. Deep neural network with generative adversarial networks pre-training for brain tumor classification based on MR images. Biomed. Signal Process. Control 2020, 57, 101678. [Google Scholar] [CrossRef]
- Deepak, S.; Ameer, P.M. MSG-GAN Based Synthesis of Brain MRI with Meningioma for Data Augmentation. In Proceedings of the 2020 IEEE International Conference on Electronics, Computing and Communication Technologies (CONECCT), Bangalore, India, 2–4 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–6. [Google Scholar]
- Han, C.; Rundo, L.; Murao, K.; Noguchi, T.; Shimahara, Y.; Milacski, Z.Á.; Koshino, S.; Sala, E.; Nakayama, H.; Satoh, S.I. MADGAN: Unsupervised medical anomaly detection GAN using multiple adjacent brain MRI slice reconstruction. BMC Bioinform. 2021, 22, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, B.; Priyatharshini, R.; Ramya, B.; Monish, S.; Raja, G.R.S. Reconstruction, Identification and Classification of Brain Tumor Using Gan and Faster Regional-CNN. In Proceedings of the 2021 3rd International Conference on Signal Processing and Communication (ICPSC), Coimbatore, India, 13–14 May 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 238–242. [Google Scholar]
- Mondal, M.; Faruk, M.F.; Raihan, N.; Ahammed, P. Deep Transfer Learning Based Multi-Class Brain Tumors Classification Using MRI Images. In Proceedings of the 2021 3rd International Conference on Electrical & Electronic Engineering (ICEEE), Rajshahi, Bangladesh, 22–24 December 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 73–76. [Google Scholar]
- Dixit, A.; Nanda, A. An improved whale optimization algorithm-based radial neural network for multi-grade brain tumor classification. Vis. Comput. 2021, 1–16. [Google Scholar] [CrossRef]
- Devanathan, B.; Kamarasan, M. Automated Brain Tumor Diagnosis using Residual Network with Optimal Kernel Extreme Learning Machine. In Proceedings of the 2022 4th International Conference on Smart Systems and Inventive Technology (ICSSIT), Tirunelveli, India, 20–22 January 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 860–865. [Google Scholar]
- Nayak, D.R.; Padhy, N.; Mallick, P.K.; Zymbler, M.; Kumar, S. Brain Tumor Classification Using Dense Efficient-Net. Axioms 2022, 11, 34. [Google Scholar] [CrossRef]
- Dhaniya, R.D.; Umamaheswari, K.M. Brain tumor identification and classification of MRI images using data augmented support vector machine. Cogn. Neurodyn. 2022, 1–11. [Google Scholar] [CrossRef]
- Gupta, R.K.; Bharti, S.; Kunhare, N.; Sahu, Y.; Pathik, N. Brain Tumor Detection and Classification Using Cycle Generative Adversarial Networks. Interdiscip. Sci. Comput. Life Sci. 2022, 14, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Kaggle. Available online: https://www.kaggle.com/navoneel/brainmri-images-for-brain-tumor-detection (accessed on 26 May 2022).
- Kaggle. Available online: https://www.kaggle.com/simeondee/brain-tumor-images-dataset (accessed on 26 May 2022).
- BRATS. Available online: https://www.smir.ch/BRATS/Start2015 (accessed on 26 May 2022).
- Cherian, A.; Sullivan, A. Sem-GAN: Semantically-consistent image-to-image translation. In Proceedings of the 2019 IEEE Winter Conference on Applications of Computer Vision (WACV), Waikoloa, HI, USA, 7–11 January 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Chen, Z.; Li, W. Dual-stream interactive networks for no-reference stereoscopic image quality assessment. IEEE Trans. Image Process. 2019, 28, 3946–3958. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, Z.; Lin, J.; Chen, Z.; Zhou, W. Unsupervised single image deraining with self-supervised constraints. In Proceedings of the 2019 IEEE International Conference on Image Processing (ICIP), Taipei, Taiwan, 22–25 September 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2761–2765. [Google Scholar] [CrossRef] [Green Version]
- Brain Tumor Classification (MRI). 2019. Available online: https://www.kaggle.com/sartajbhuvaji/brain-tumor-classification-mri (accessed on 3 May 2022).
- MRI Based Brain Tumor Images. 2021. Available online: https://www.kaggle.com/mhantor/mri-based-brain-tumor-images (accessed on 3 May 2022).
- Cai, L.; Chen, Y.; Cai, N.; Cheng, W.; Wang, H. Utilizing amari-alpha divergence to stabilize the training of generative adversarial networks. Entropy 2020, 22, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, A.; Metz, L.; Chintala, S. Unsupervised representation learning with deep convolutional generative adversarial networks. arXiv 2016, arXiv:1511.06434. [Google Scholar]
- Huang, H.; Yu, P.S.; Wang, C. An Introduction to Image Synthesis with Generative Adversarial Nets. arXiv 2018, arXiv:1803.04469. [Google Scholar]
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative Adversarial Networks: An Overview. IEEE Signal Process. Mag. 2018, 35, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Kotsiantis, S.; Zaharakis, I.; Pintelas, P. Machine learning: A review of classification and combining techniques. Artif. Intell. Rev. 2006, 26, 159–190. [Google Scholar] [CrossRef]
- Image ClassifierUsing Cnn Image Classifier Using Convolutional Neural Networks 2021. Available online: https://www.geeksforgeeks.org/image-classifier-using-cnn/ (accessed on 3 May 2022).
- Goutte, C.; Gaussier, E. A Probabilistic Interpretation of Precision, Recall and F-Score, with Implication for Evaluation. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3408, pp. 345–359. [Google Scholar] [CrossRef]
- Gulli, A.; Sujit, P. Deep Learning with Keras; Packt Publishing Ltd.: Birmingham, UK, 2017. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A method for stochastic optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Bisong, E. Google Colaboratory in Building Machine Learning and Deep Learning Models on Google Cloud Platform; Springer: Berlin/Heidelberg, Germany, 2019; pp. 59–64. [Google Scholar]




| Ref. | Year | Classification Method | Image Type | Dataset | Performance | GAN |
|---|---|---|---|---|---|---|
| [7] | 2019 | Two-Step GANs | MRI | BRATS | Sensitivity 93.67–97.48% | √ |
| [8] | 2019 | CPGGANs | MRI | BRATS | Accuracy 0.64% Specificity 6.84% | √ |
| [9] | 2019 | CPGGANs | MRI | Contrast-Enhanced T1-Weighted (T1c) Brain Axial MR Images | Sensitivity 10% | √ |
| [10] | 2020 | Pairwise GANs | MRI | 3D Brain Volume Images from TCGA-GBM and TCGA-LGG | Average Accuracy 88.82% | √ |
| [11] | 2020 | Pre-Trained GAN | MRI | Nanfang Hospital General Hospital MRI Brain Images Tianjin Medical University in China [2005–2010] | Accuracy 98.57% | √ |
| [12] | 2020 | MSG-GAN | MRI | Figshare BRATS (220 Patient Images) | Accuracy 88.7% | √ |
| [13] | 2021 | MADGAN | MRI | 1133 Healthy T1-Weighted (T1) 135 Healthy Contrast-Enhanced T1 (T1c) | AUC 0.921 | √ |
| [14] | 2021 | Faster Regional CNNs | MRI | 3064 T1-Weighted and Contrast-Enhanced Images Glioma 1426, Pituitary 930 and Meningioma 708 Images From 233 Patients | Accuracy 93% Sensitivity 89.23% | - |
| [15] | 2021 | VGG-19 | MRI | Figshare BRATS (220 Patient Images) | Accuracy 94% F1-score 94% | - |
| [16] | 2021 | FCM-IWOA- Based RBNN Classification | MRI | Dataset 1: Kaggle [21] Dataset 2: Kaggle [22] Dataset 3: BRATS [23] | Max. Specificity of 0.945 Max. Sensitivity of 0.96 Max. Accuracy of 0.951 Max. F1-Score of 0.961 Max. Precision of 0.96 | - |
| [17] | 2022 | RN-OKELM | MRI | BT (98/155 Images) Abnormal/Tumor Class. | Accuracy 97.93 Sensitivity 97.92 Specificity 97.98 | - |
| [18] | 2022 | Dense EfficientNet | MRI | T1 Contrast Brain Tumors Kaggle.com. 3260 Different Types of Brain MRI Images | Accuracy 98.78% Precision 98.75% Recall 98.75% | - |
| [19] | 2022 | DA-SVM | MRI | Publicly Datasets for Tumor (Bakas et al. 2017a, b; Tobon-gomez et al. 2015). | Accuracy 89.93 Sensitivity 88.96 Specificity 88.96 | - |
| [20] | 2022 | C-GAN | MRI | Publicly datasets for Tumor Detection and Classification. | Detection (Acc) 99% Classification (Acc) 98% | √ |
| Parameter | Vanilla GAN | DCGAN |
|---|---|---|
| Mini Batch Size | 128 | 64 |
| Number of Epochs | 1000 | 2000 |
| Discriminator Learning rate | 0.0001 | 0.0001 |
| Generator Learning rate | 0.0002 | 0.0002 |
| Optimizer | Adam | Adam |
| Layer (Type) | Output Shape | Parameters |
|---|---|---|
| conv2d_1 (Conv2D) | (None, 224, 224, 16) | 438 |
| activation_1 (Activation) | (None, 224, 224, 16) | 0 |
| batch_normalization_1 (Batch) | (None, 224, 224, 16) | 64 |
| conv2d_2 (Conv2D) | (None, 224, 224, 32) | 4630 |
| activation_2 (Activation) | (None, 224, 224, 32) | 0 |
| max_pooling2d_1 (MaxPooling2d) | (None, 74, 74, 32) | 0 |
| dropout_1 (Dropout) | (None, 74, 74, 32) | 0 |
| conv2d_3 (Conv2D) | (None, 72, 72, 64) | 18,486 |
| activation_3 (Activation) | (None, 72, 72, 64) | 0 |
| batch_normalization_2 (Batch) | (None, 72, 72, 64) | 256 |
| conv2d_4 (Conv2D) | (None, 71, 71, 128) | 32,896 |
| max_pooling2d_2 (MaxPooling2d) | (None, 24, 24, 128) | 0 |
| dropout_2 (Dropout) | (None, 24, 24, 128) | 0 |
| flatten_1 (Flatten) | (None, 73728) | 0 |
| dense_1 (Dense) | (None, 512) | 27,649,248 |
| dropout_1 (Dropout) | (None, 512) | 0 |
| dense_2 (Dense) | (None, 1000) | 413,000 |
| dropout_2 (Dropout) | (None, 1000) | 0 |
| dense_0 (Dense) | (None, 1) | 1001 |
| activation_4 (Activation) | (None, 1) | 0 |
| Layer (Type) | Output Shape | Parameters |
|---|---|---|
| resnet152v2 (Model) | (None, 4, 4, 2048) | 54,331,648 |
| reshape_2 (Reshape) | (None, 4, 4, 2048) | 0 |
| flatten_2 (Flatten) | (None, 100352) | 0 |
| dense_3 (Dense) | (None, 256) | 25,690,368 |
| dropout_2 (Dropout) | (None, 256) | 0 |
| dense_4 (Dense) | (None, 1) | 257 |
| Layer (Type) | Output Shape | Parameters |
|---|---|---|
| mobilenetv2_1.00_224 (Model) | (None, 7, 7, 1280) | 2,257,984 |
| reshape_2 (Reshape) | (None, 7, 7, 1280) | 0 |
| flatten_2 (Flatten) | (None, 62720) | 0 |
| dense_3 (Dense) | (None, 512) | 33,113,152 |
| dropout_2 (Dropout) | (None, 512) | 0 |
| dense_4 (Dense) | (None, 1) | 513 |
| Models | Optimizer | LR | Total Number of Parameters |
|---|---|---|---|
| ResNet152V2 | SGD | 0.0001 | 54,382,533 |
| MobileNetV2 | SGD | 0.0001 | 34,371,649 |
| CNN | Adamax | 0.00003 | 27,429,828 |
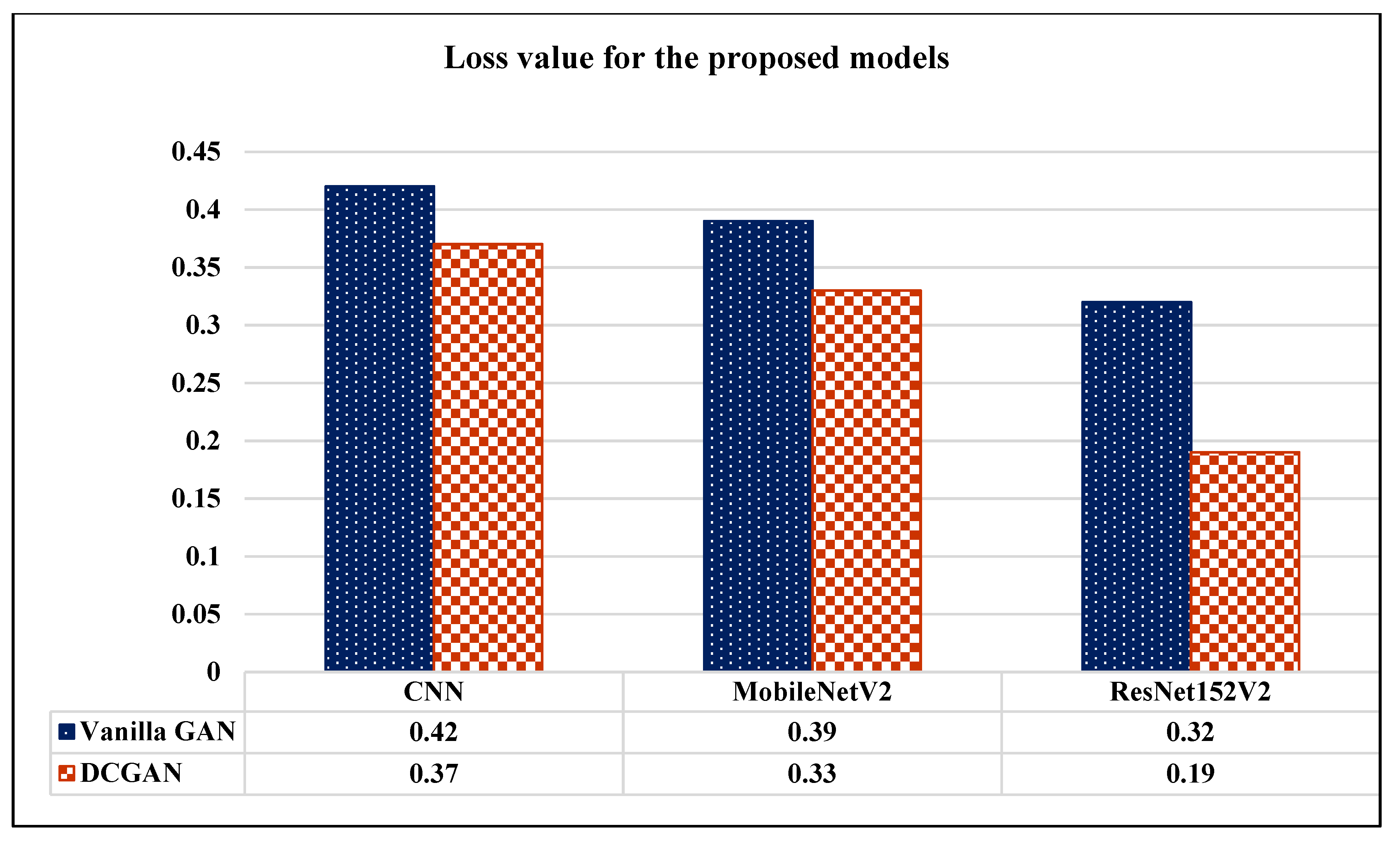
| Model | Vanilla GAN | DCGAN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Loss | Accuracy | Precision | Recall | AUC | Loss | Accuracy | Precision | Recall | AUC | |
| CNN | 0.42 | 94.84% | 93.24% | 95.49% | 95.29% | 0.37 | 96.63% | 97.01% | 96.83% | 98.14% |
| MobileNetV2 | 0.39 | 93.27% | 92.19% | 95.57% | 96.92% | 0.33 | 95.84% | 96.48% | 95.68% | 97.50% |
| ResNet152V2 | 0.32 | 97.94% | 96.91% | 97.03% | 96.65% | 0.19 | 99.09% | 99.12% | 99.08% | 99.51% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashedy, H.H.N.; Almansour, A.F.; Ibrahim, D.M.; Hammoudeh, M.A.A. BrainGAN: Brain MRI Image Generation and Classification Framework Using GAN Architectures and CNN Models. Sensors 2022, 22, 4297. https://doi.org/10.3390/s22114297
Alrashedy HHN, Almansour AF, Ibrahim DM, Hammoudeh MAA. BrainGAN: Brain MRI Image Generation and Classification Framework Using GAN Architectures and CNN Models. Sensors. 2022; 22(11):4297. https://doi.org/10.3390/s22114297
Chicago/Turabian StyleAlrashedy, Halima Hamid N., Atheer Fahad Almansour, Dina M. Ibrahim, and Mohammad Ali A. Hammoudeh. 2022. "BrainGAN: Brain MRI Image Generation and Classification Framework Using GAN Architectures and CNN Models" Sensors 22, no. 11: 4297. https://doi.org/10.3390/s22114297
APA StyleAlrashedy, H. H. N., Almansour, A. F., Ibrahim, D. M., & Hammoudeh, M. A. A. (2022). BrainGAN: Brain MRI Image Generation and Classification Framework Using GAN Architectures and CNN Models. Sensors, 22(11), 4297. https://doi.org/10.3390/s22114297






