Decoding Physical and Cognitive Impacts of Particulate Matter Concentrations at Ultra-Fine Scales
Abstract
:1. Introduction
2. Materials and Methods
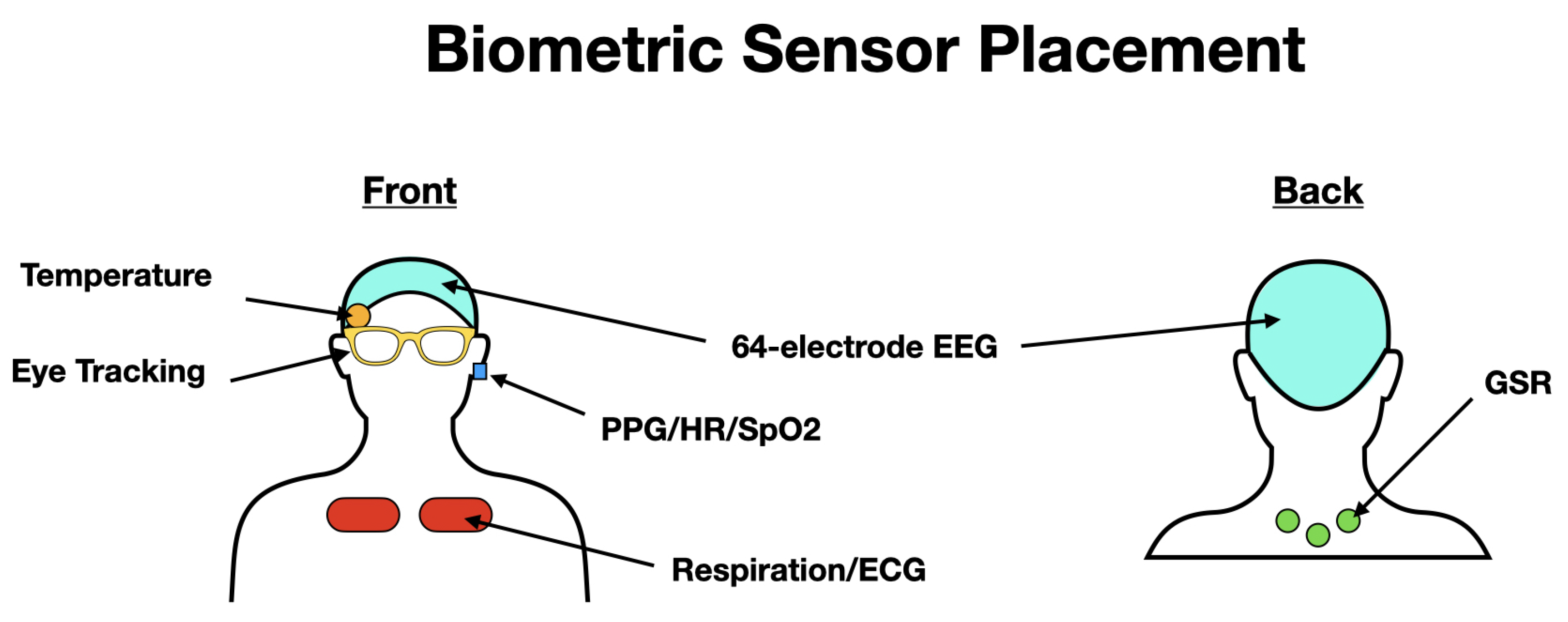
2.1. Holistic Sensing
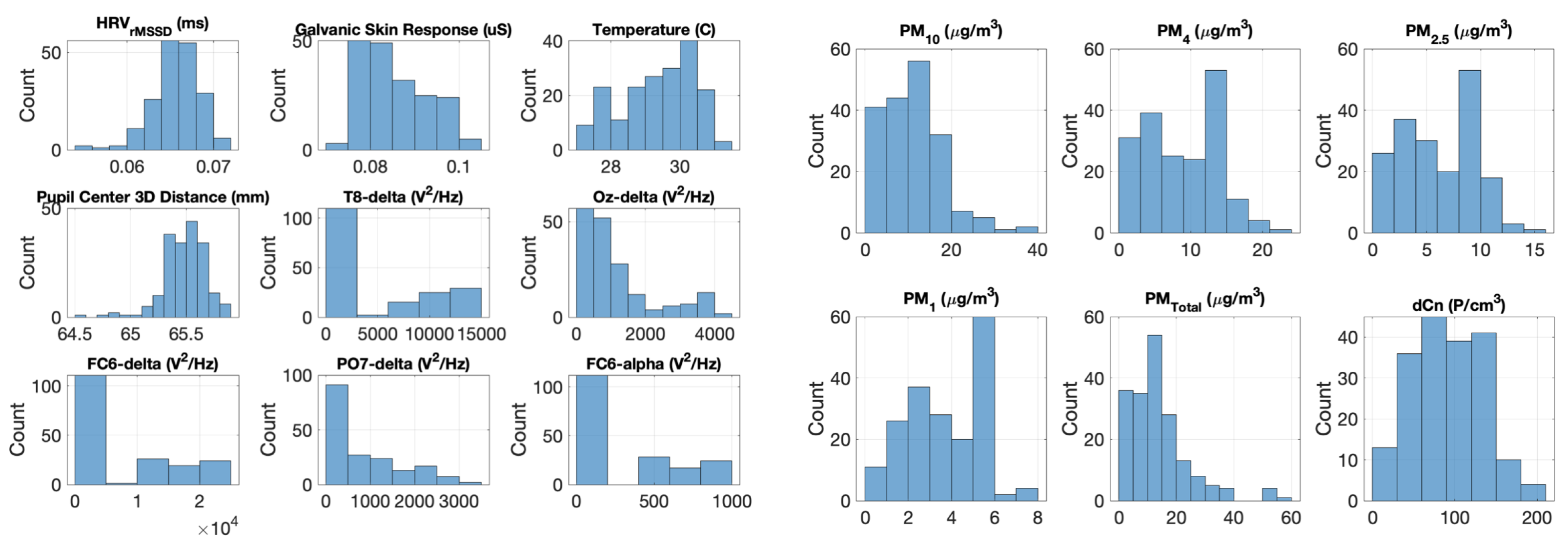
2.2. Data Collection
2.3. Model Development
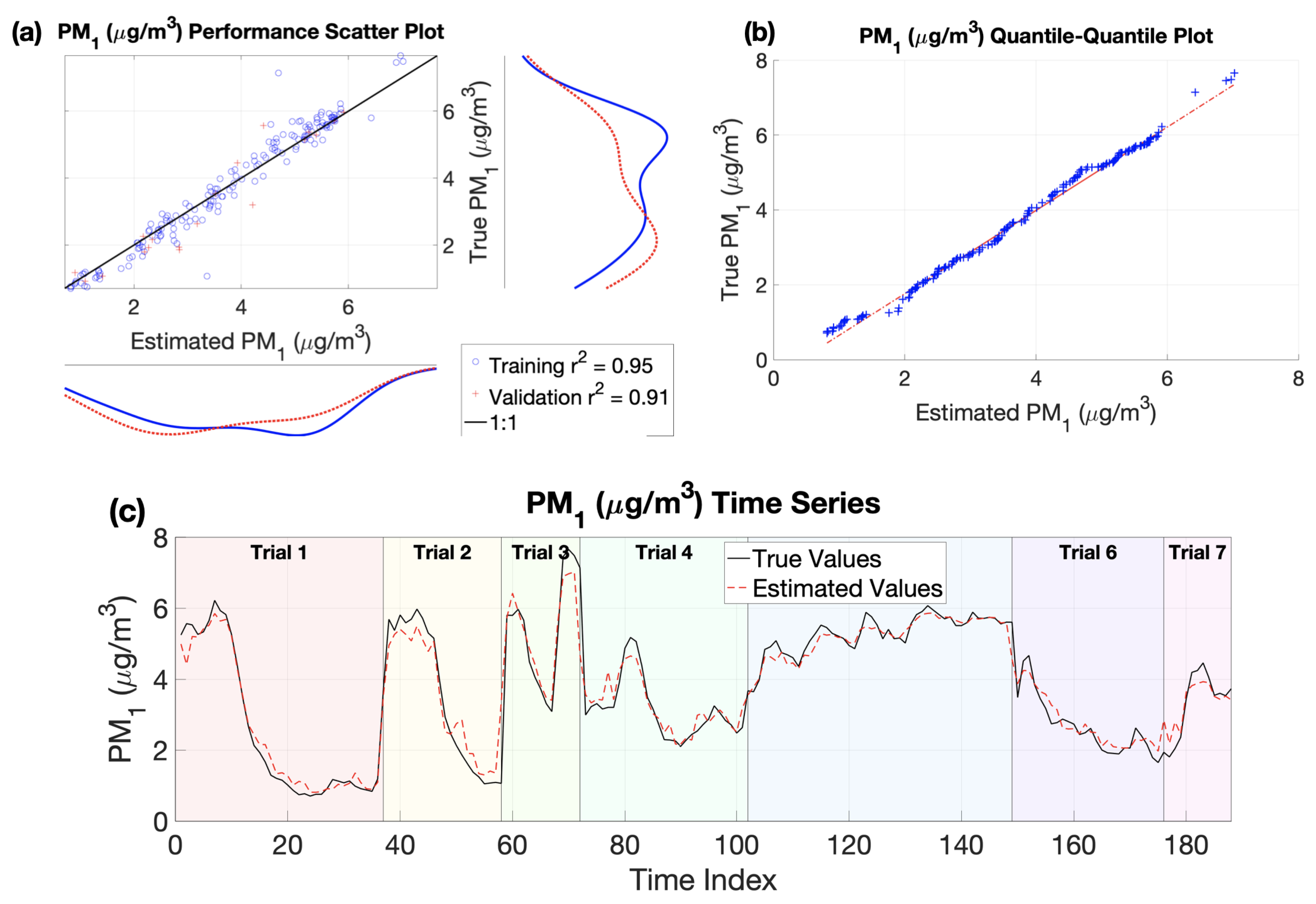
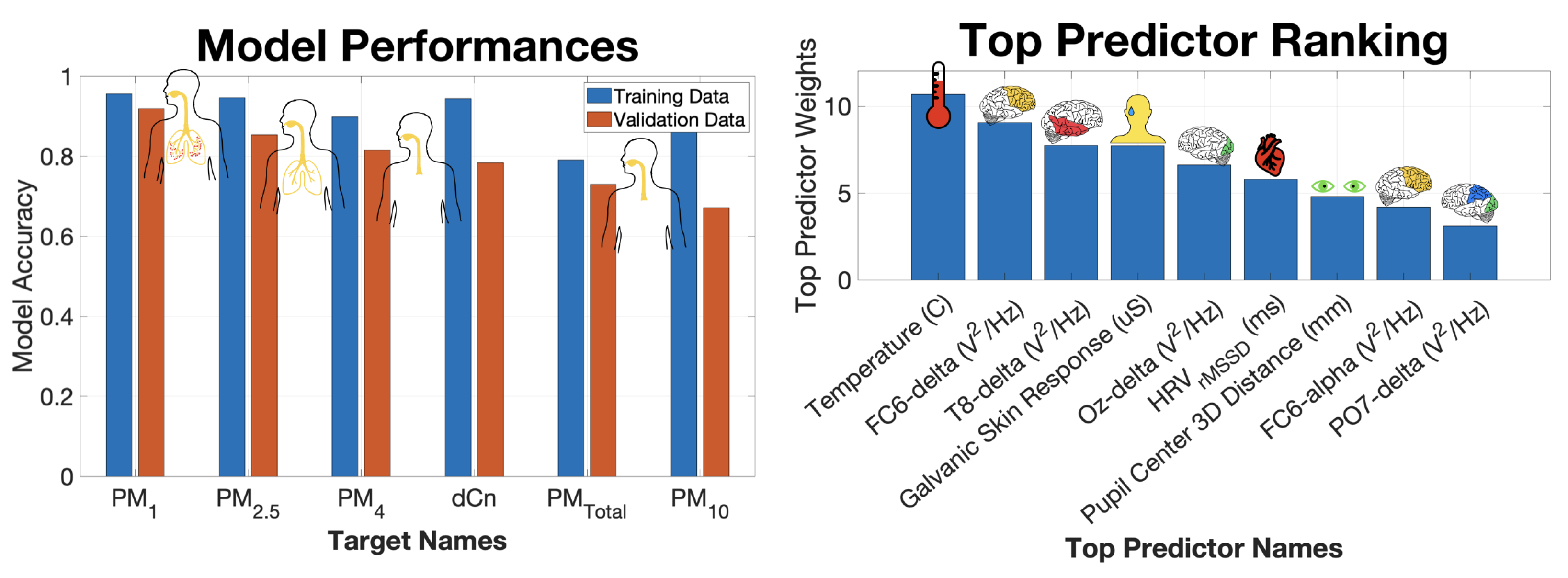
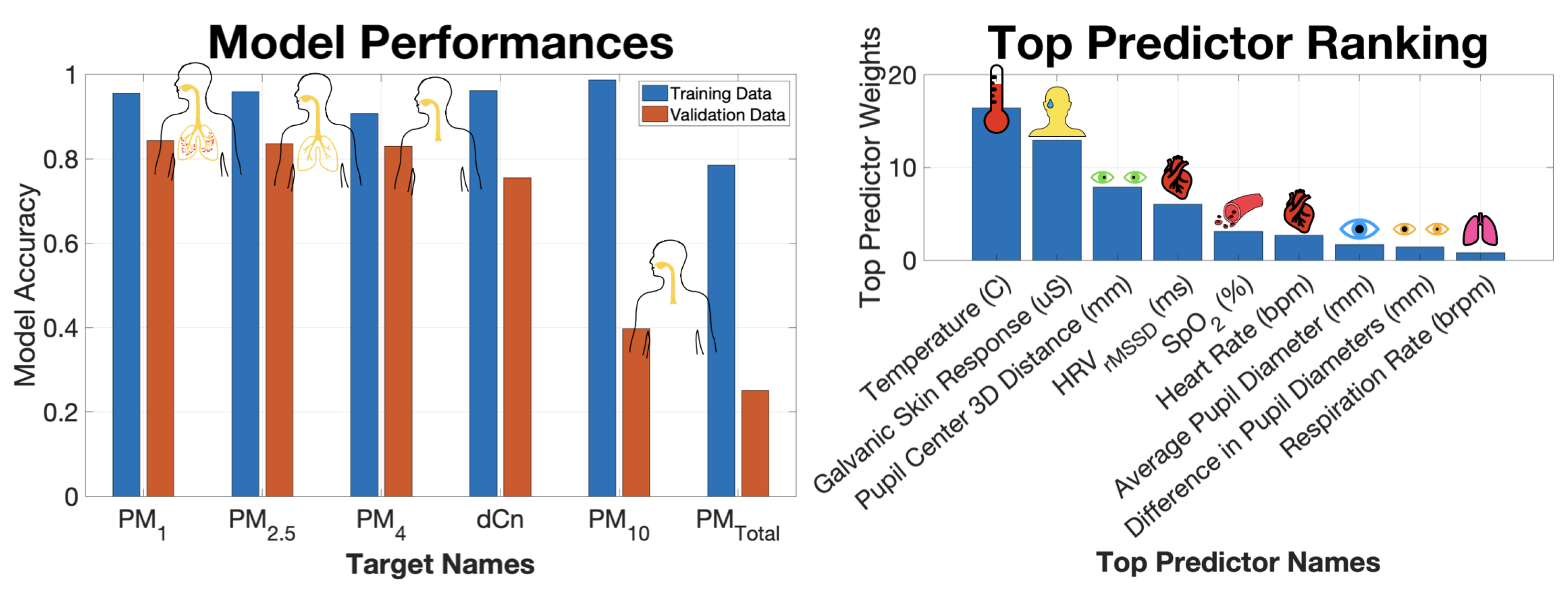
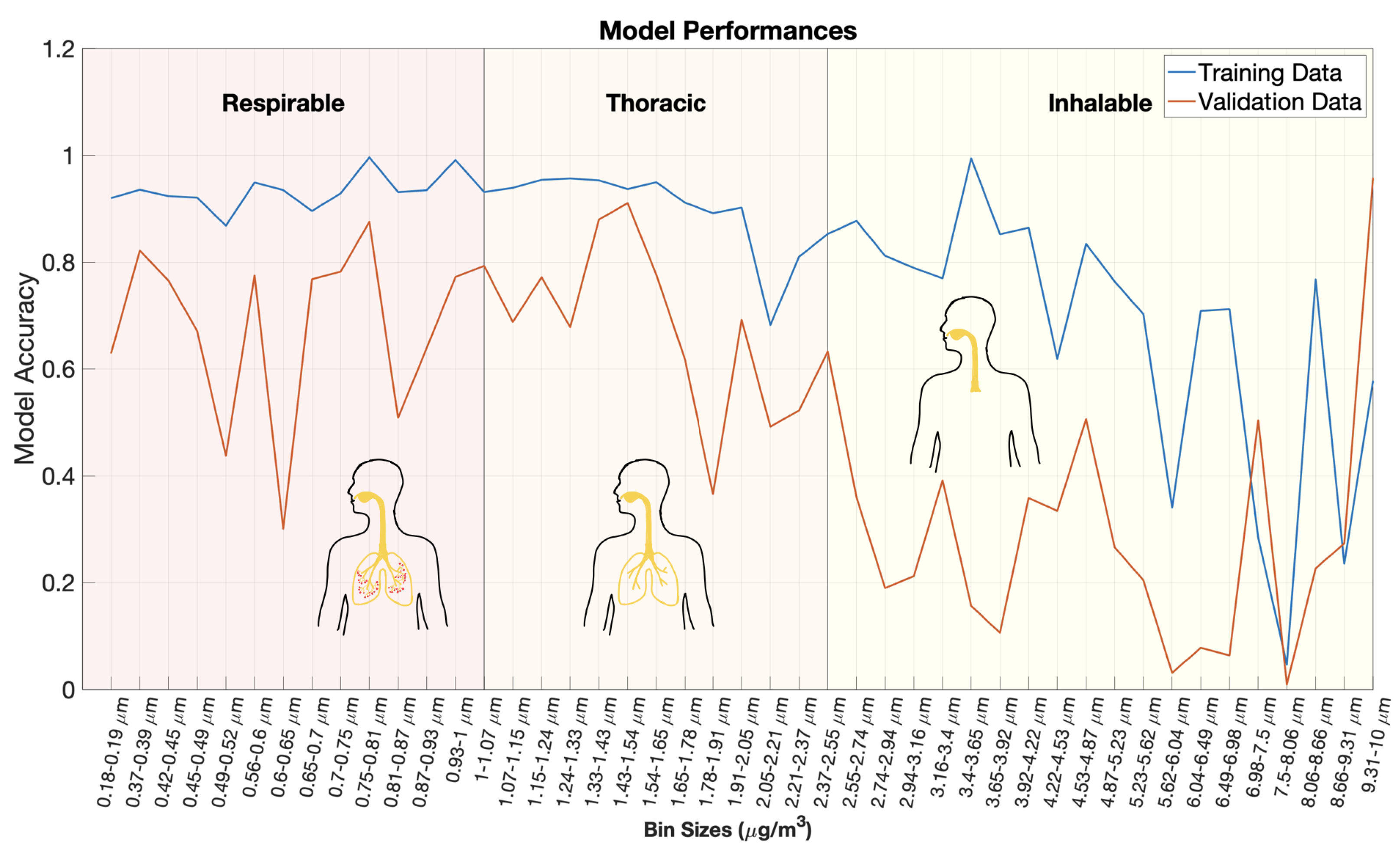
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| HR | Heart Rate |
| BP | Blood Pressure |
| EEG | Electroencephalography |
| GSR | Galvanic Skin Response |
| ECG | Electrocardiography |
| HRV | Heart Rate Variability |
| PPG | Photoplethysmogram |
| RR | Respiration Rate |
| PM | Particulate Matter |
| dCn | Total Particle Count Density |
| SpO | Blood Oxygen Saturation |
References
- WHO. Ambient (Outdoor) Air Pollution. Retrieved 24 January 2022. Available online: https://www.who.int/news-room/factsheets/ detail/ambient-(outdoor)-airquality-and-health (accessed on 29 May 2022).
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef] [PubMed]
- Daellenbach, K.R.; Uzu, G.; Jiang, J.; Cassagnes, L.E.; Leni, Z.; Vlachou, A.; Stefenelli, G.; Canonaco, F.; Weber, S.; Segers, A.; et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 2020, 587, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Brook, R.D.; Burnett, R.T.; Dockery, D.W. How is cardiovascular disease mortality risk affected by duration and intensity of fine particulate matter exposure? An integration of the epidemiologic evidence. Air Qual. Atmos. Health 2010, 4, 5–14. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brook, R.D.; Bard, R.L.; Kaplan, M.J.; Yalavarthi, S.; Morishita, M.; Dvonch, J.T.; Wang, L.; Yang, H.Y.; Spino, C.; Mukherjee, B.; et al. The effect of acute exposure to coarse particulate matter air pollution in a rural location on circulating endothelial progenitor cells: Results from a randomized controlled study. Inhal. Toxicol. 2013, 25, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schikowski, T.; Altuğ, H. The role of air pollution in cognitive impairment and decline. Neurochem. Int. 2020, 136, 104708. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavy, V.; Ebenstein, A.; Roth, S. The Impact of Short Term Exposure to Ambient Air Pollution on Cognitive Performance and Human Capital Formation. 2014. Available online: https://www.nber.org/system/files/working_papers/w20648/w20648.pdf (accessed on 29 May 2022).
- Chatzidiakou, L.; Mumovic, D.; Dockrell, J. The Effects of Thermal Conditions and Indoor Air Quality on Health, Comfort and Cognitive Performance of Students; University college London Institute for Environmental Design and Engineering: London, UK, 2015. [Google Scholar]
- Zhang, X.; Chen, X.; Zhang, X. The impact of exposure to air pollution on cognitive performance. Proc. Natl. Acad. Sci. USA 2018, 115, 9193–9197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehab, M.A.; Pope, F.D. Effects of short-term exposure to particulate matter air pollution on cognitive performance. Sci. Rep. 2019, 9, 8237. [Google Scholar] [CrossRef] [PubMed]
- Künn, S.; Palacios, J.; Pestel, N.; Indoor Air Quality and Cognitive Performance. IZA Discussion Paper No. 12632. Available online: http://dx.doi.org/10.2139/ssrn.3460848 (accessed on 29 May 2022).
- The Royal Society. Machine learning: The power and promise of computers that learn by example. In Report by the Royal Society; The Royal Society: London, UK, 2017; Volume 66. [Google Scholar] [CrossRef] [Green Version]
- Buteau, S.; Goldberg, M.S. A structured review of panel studies used to investigate associations between ambient air pollution and heart rate variability. Environ. Res. 2016, 148, 207–247. [Google Scholar] [CrossRef] [PubMed]
- Amoabeng Nti, A.A.; Robins, T.G.; Mensah, J.A.; Dwomoh, D.; Kwarteng, L.; Takyi, S.A.; Acquah, A.; Basu, N.; Batterman, S.; Fobil, J.N. Personal exposure to particulate matter and heart rate variability among informal electronic waste workers at Agbogbloshie: A longitudinal study. BMC Public Health 2021, 21, 2161. [Google Scholar] [CrossRef] [PubMed]
- Santos, U.d.P.; Braga, A.L.F.; Giorgi, D.M.A.; Pereira, L.A.A.; Grupi, C.J.; Lin, C.A.; Bussacos, M.A.; Zanetta, D.M.T.; Saldiva, P.H.d.N.; Filho, M.T. Effects of air pollution on blood pressure and heart rate variability: A panel study of vehicular traffic controllers in the city of São Paulo, Brazil. Eur. Heart J. 2005, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; Wadsworth, Inc.: Belmont, CA, USA, 1984. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Ho, T.K. The random subspace method for constructing decision forests. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 832–844. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.H. Greedy Function Approximation: A Gradient Boosting Machine; The Annals of Statistics; Institute of Mathematical Statistics: London, UK, 2001; Volume 29, pp. 1189–1232. Available online: http://www.jstor.org/stable/2699986 (accessed on 29 May 2022).
- Freund, Y.; Schapire, R.E. A Decision-Theoretic Generalization of On-Line Learning and an Application to Boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef] [Green Version]
- Probst, P.; Wright, M.N.; Boulesteix, A.L. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef] [Green Version]
- Poh, T.Y.; Ali, N.A.T.B.M.; Aogáin, M.M.; Kathawala, M.H.; Setyawati, M.I.; Ng, K.W.; Chotirmall, S.H. Inhaled nanomaterials and the respiratory microbiome: Clinical, immunological and toxicological perspectives. Part. Fibre Toxicol. 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; Kiderman, A.; Szczupak, M.; Ashmore, R.C.; Hoffer, M.E. Patterns of Pupillary Activity during Binocular Disparity Resolution. Front. Neurol. 2018, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Li, J.; Ngai, G.; Leong, H.V.; Bulling, A. Moment-to-Moment Detection of Internal Thought from Eye Vergence Behaviour. arXiv 2019, arXiv:1901.06572. [Google Scholar]
- World Health Organization. Occupational and Environmental Health Team. Hazard prevention and Control in the Work Environment: Airborne Dust. World Health Organization. 1999. Available online: https://apps.who.int/iris/handle/10665/66147 (accessed on 29 May 2022).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talebi, S.; Lary, D.J.; Wijeratne, L.O.H.; Fernando, B.; Lary, T.; Lary, M.; Sadler, J.; Sridhar, A.; Waczak, J.; Aker, A.; et al. Decoding Physical and Cognitive Impacts of Particulate Matter Concentrations at Ultra-Fine Scales. Sensors 2022, 22, 4240. https://doi.org/10.3390/s22114240
Talebi S, Lary DJ, Wijeratne LOH, Fernando B, Lary T, Lary M, Sadler J, Sridhar A, Waczak J, Aker A, et al. Decoding Physical and Cognitive Impacts of Particulate Matter Concentrations at Ultra-Fine Scales. Sensors. 2022; 22(11):4240. https://doi.org/10.3390/s22114240
Chicago/Turabian StyleTalebi, Shawhin, David J. Lary, Lakitha O. H. Wijeratne, Bharana Fernando, Tatiana Lary, Matthew Lary, John Sadler, Arjun Sridhar, John Waczak, Adam Aker, and et al. 2022. "Decoding Physical and Cognitive Impacts of Particulate Matter Concentrations at Ultra-Fine Scales" Sensors 22, no. 11: 4240. https://doi.org/10.3390/s22114240
APA StyleTalebi, S., Lary, D. J., Wijeratne, L. O. H., Fernando, B., Lary, T., Lary, M., Sadler, J., Sridhar, A., Waczak, J., Aker, A., & Zhang, Y. (2022). Decoding Physical and Cognitive Impacts of Particulate Matter Concentrations at Ultra-Fine Scales. Sensors, 22(11), 4240. https://doi.org/10.3390/s22114240







