Useful High-Entropy Source on Spinel Oxides for Gas Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HEOs
2.2. Identification of the Product Phase of HEOs
2.3. Fabrication of Sensing Elements
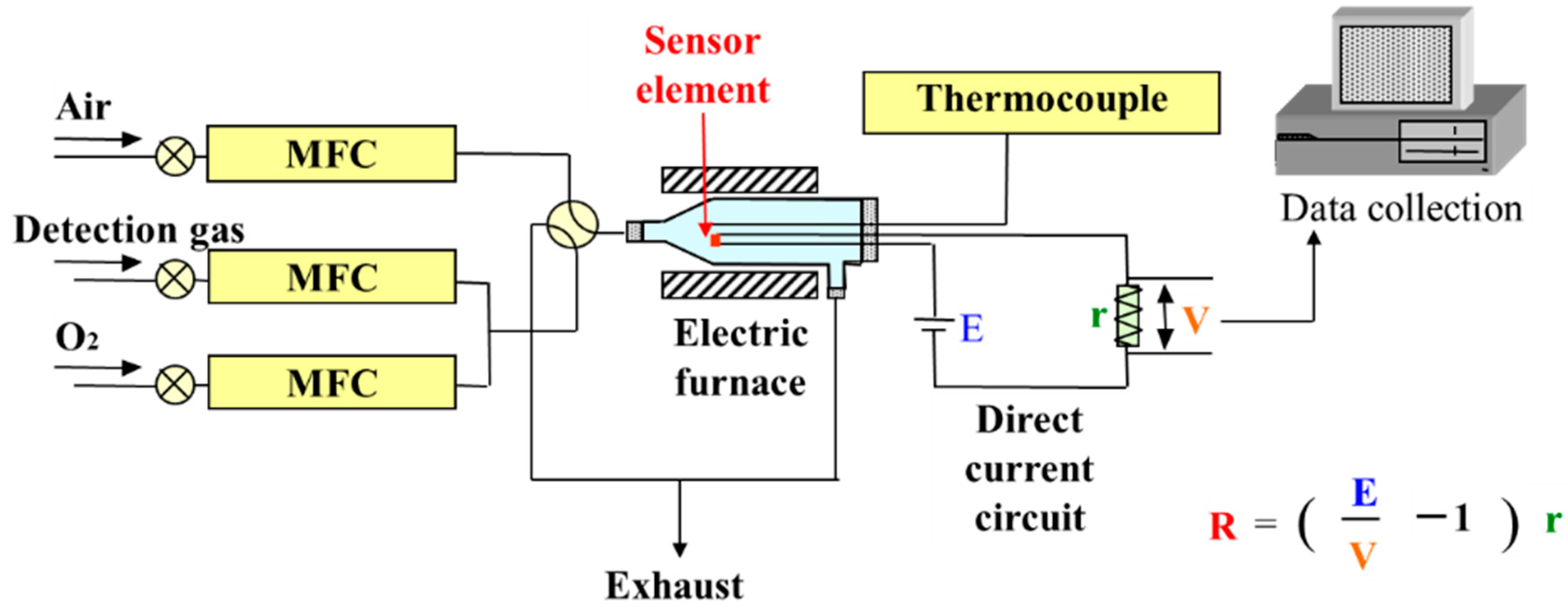
2.4. Gas Detection System
3. Results and Discussion
3.1. Identification of the Product Phase of HEOs
3.2. Electrical Properties of HEOs and Sensing Performance in Response to NO2, H2, NH3, and H2S
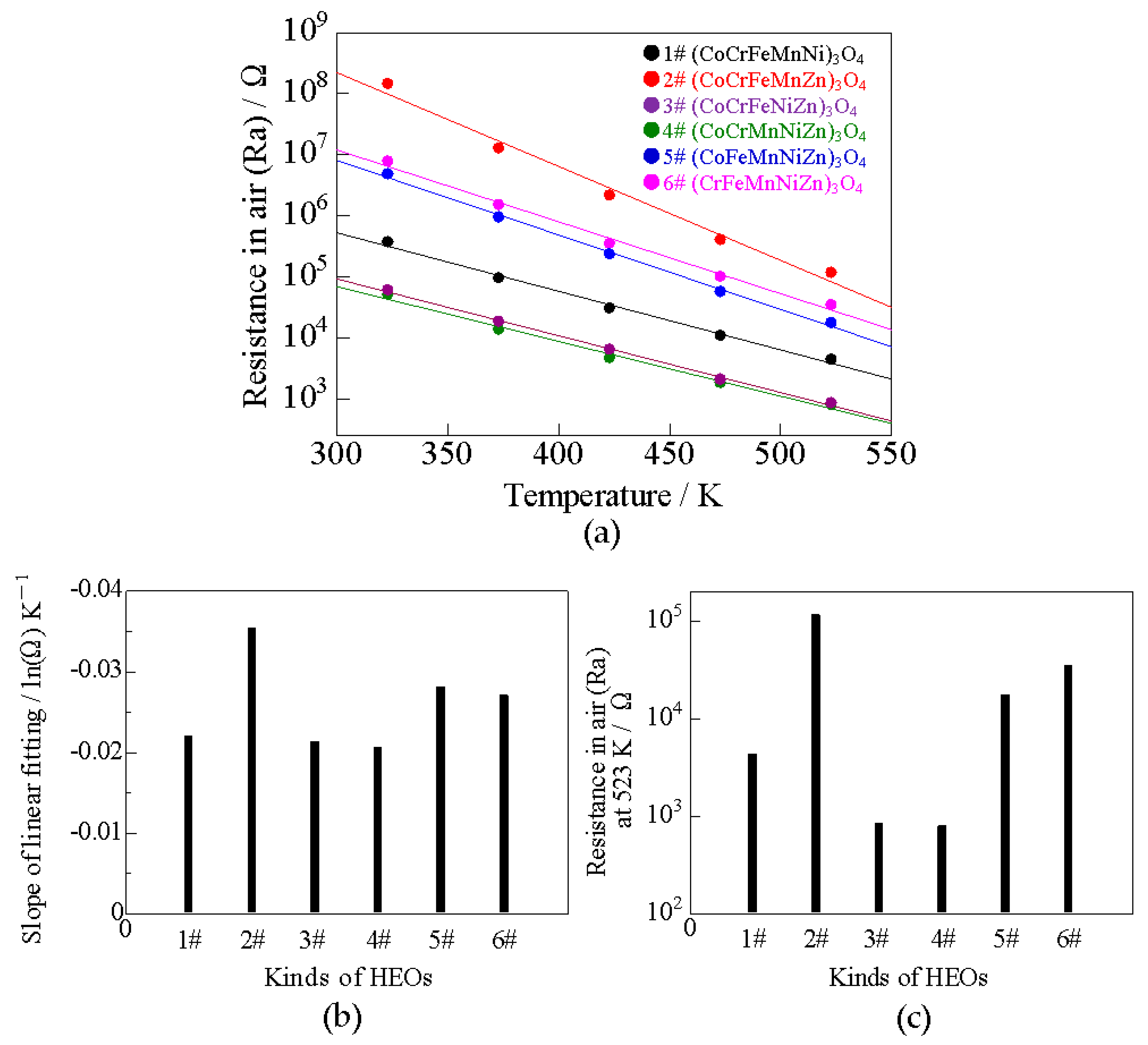
3.2.1. Electrical Properties of HEOs
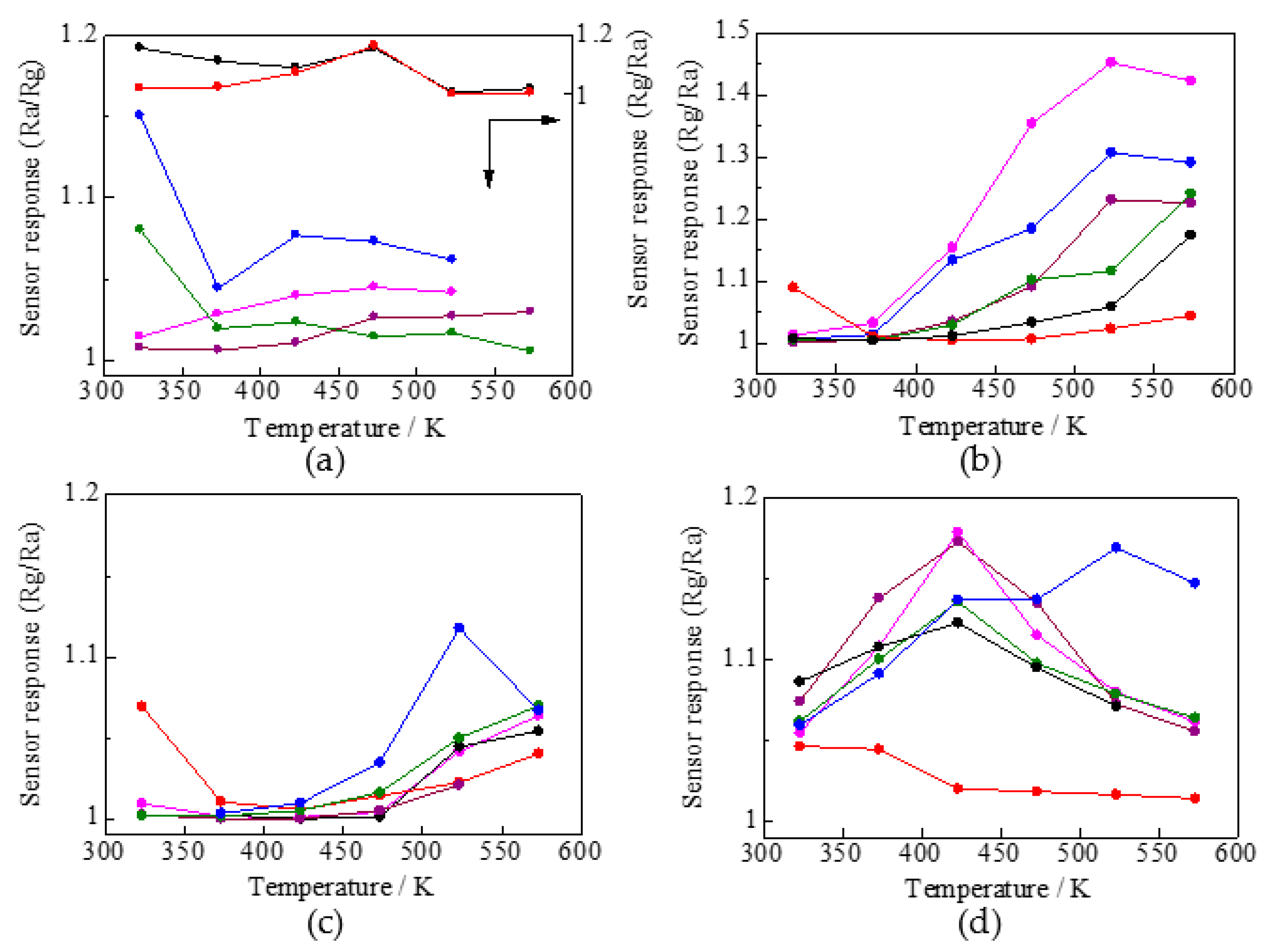
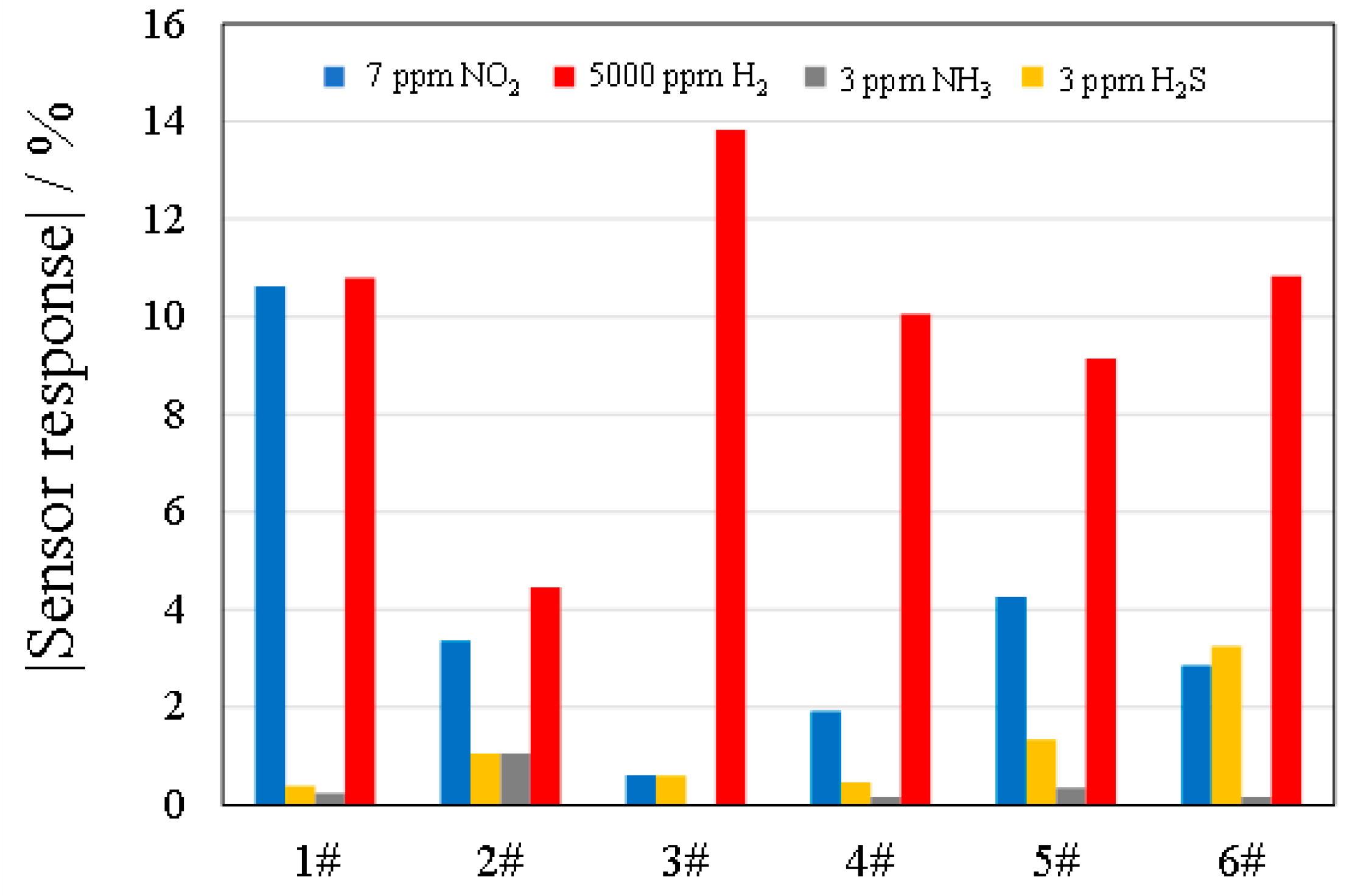
3.2.2. Sensing Performance in Response to NO2, H2, NH3, and H2S
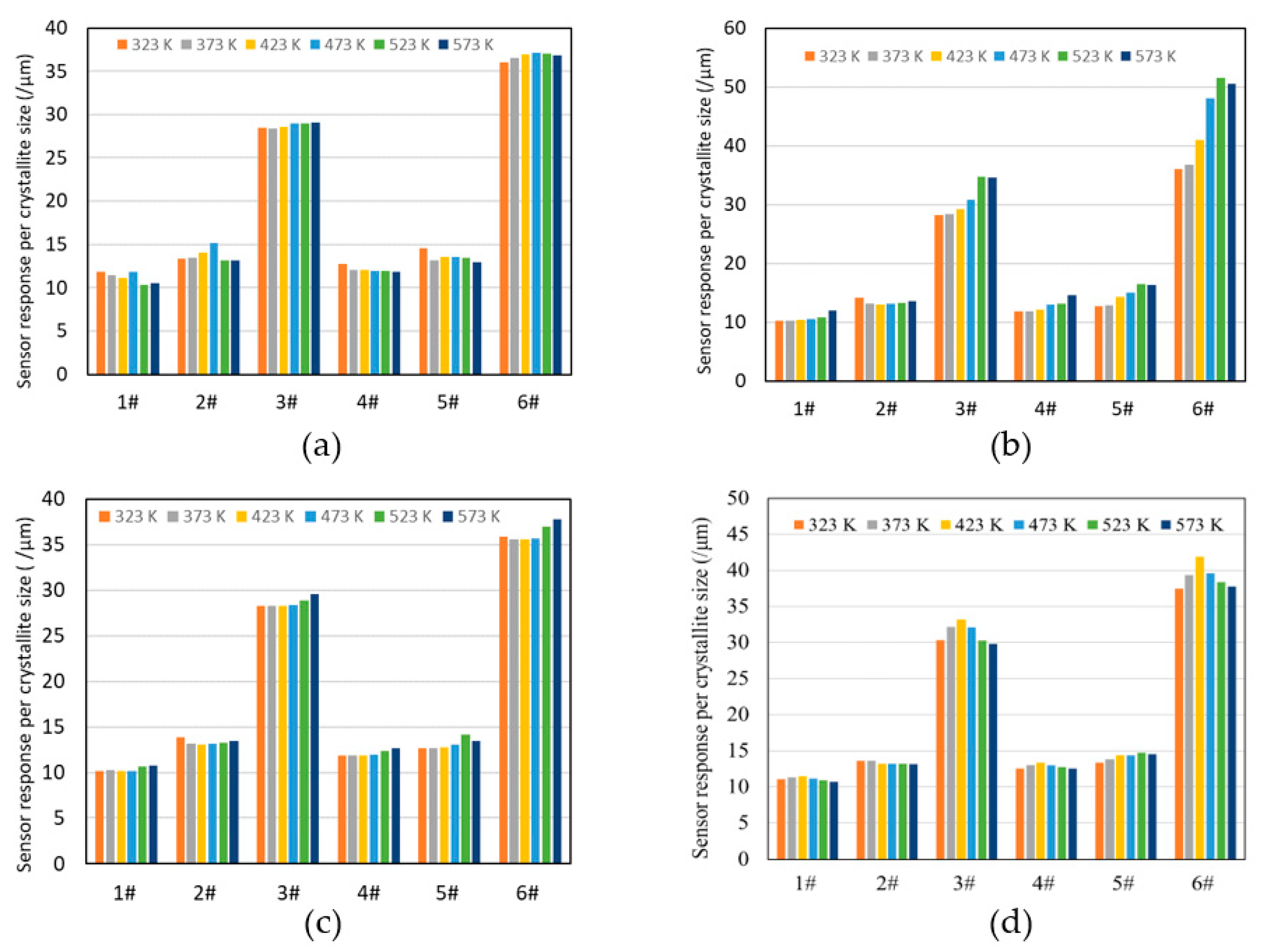
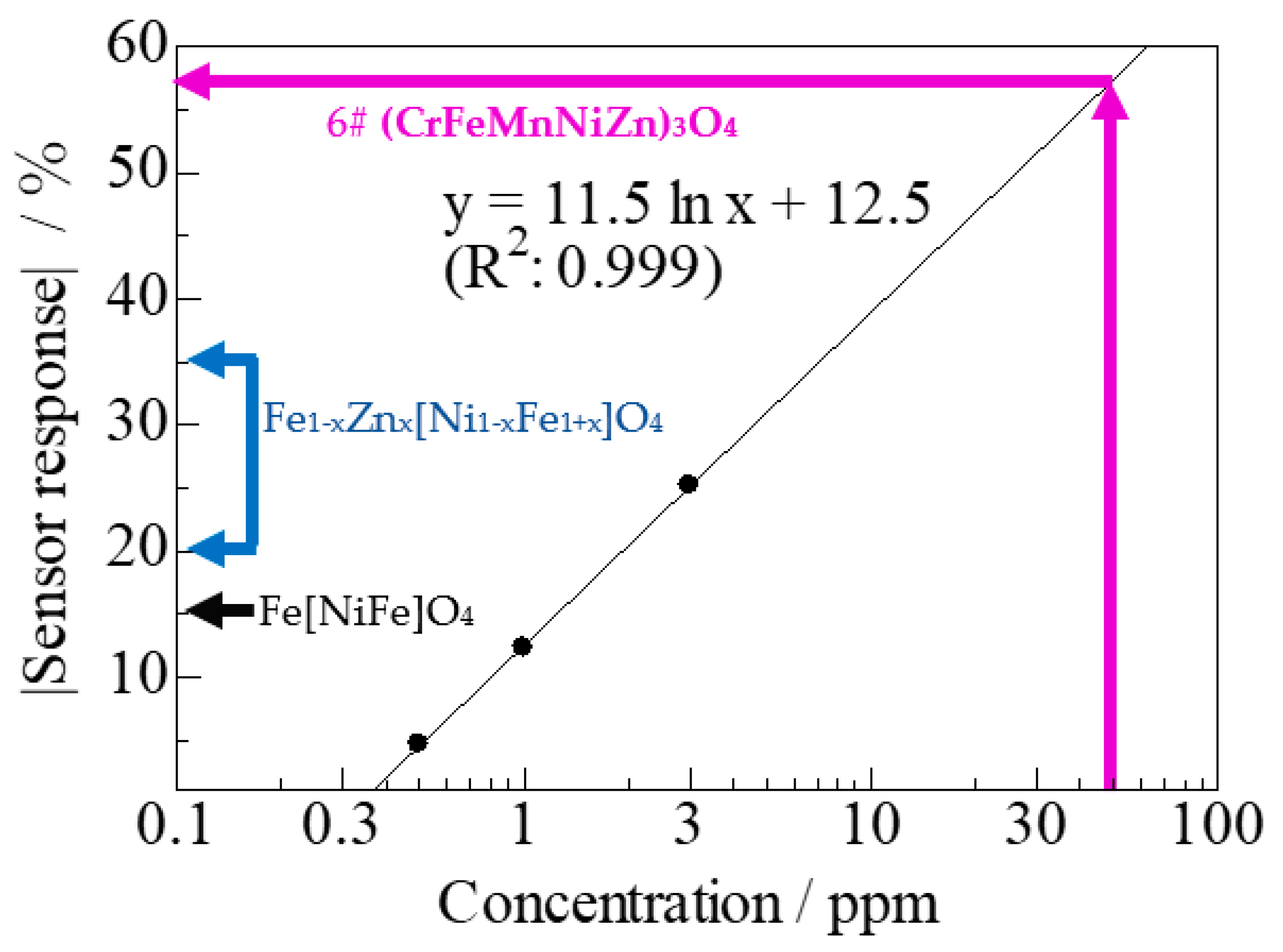
3.2.3. Dominance of HEO Cations in H2S Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Mao, A.; Xiang, H.-Z.; Zhang, Z.-G.; Kuramoto, K.; Zhang, H.; Jia, Y. A new class of spinel high-entropy oxides with controllable magnetic properties. J. Magn. Magn. Mater. 2020, 497, 165884. [Google Scholar] [CrossRef]
- Gopal-Reddy, C.V.; Manorama, S.V.; Rao, V.J. Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite. Sens. Actuators B 1999, 55, 90–95. [Google Scholar] [CrossRef]
- Chen, N.-S.; Yang, X.-J.; Liu, E.-S.; Huang, J.-L. Reducing gas-sensing properties of ferrite compounds MFe2O4 (M = Cu, Zn, Cd and Mg). Sens. Actuators B 2000, 66, 178–180. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Liu, Z.-M.; Yang, Y.; Yang, H.-F.; Shen, G.-L.; Yu, R.-Q. Simple synthesis of MgFe2O4 nanoparticles as gas sensing materials. Sens. Actuators B 2005, 107, 600–604. [Google Scholar] [CrossRef]
- Hankare, P.P.; Jadhav, S.D.; Sankpal, U.B.; Patil, R.P.; Sasikala, R.; Mulla, I.S. Gas sensing properties of magnesium ferrite prepared by co-precipitation method. J. Alloy. Compd. 2009, 488, 270–272. [Google Scholar] [CrossRef]
- Cristina de Oliveira, R.; Pontes Ribeiro, R.A.; Cruvinel, G.H.; Ciola Amoresi, R.A.; Carvalho, M.H.; Aparecido de Oliveira, A.J.; Carvalho de Oliveira, M.; Ricardo de Lazaro, S.; Fernando da Silva, L.; Catto, A.C.; et al. Role of surfaces in the magnetic and ozone gas-sensing properties of ZnFe2O4 nanoparticles: Theoretical and experimental insights. ACS Appl. Mater. Interfaces 2021, 13, 4605–4617. [Google Scholar] [CrossRef] [PubMed]
- Hashishin, T.; Onoda, H.; Sanada, T.; Fujioka, D.; Kojima, K.; Naka, T. Magnesium Ferrite Sensor for H2S Detection. Sens. Mater. 2016, 28, 1229–1236. [Google Scholar]
- Sakaguchi, C.; Nara, Y.; Hashishin, T.; Abe, H.; Matsuda, M.; Tsurekawa, S.; Kubota, H. Direct observation of potential phase at joining interface between p-MgO and n-MgFe2O4. Sci. Rep. 2020, 10, 17055. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, S.; Chen, Y.; Naka, T.; Hashishin, T.; Maruyama, J.; Abe, H. Bottom-up Synthesis of 2D Layered High-Entropy Transition Metal Hydroxides. Nanoscale Adv. 2022, 4, 2468. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of Röntgen rays. Nachr. Ges. Wiss. Göttingen 1918, 2, 96–100. [Google Scholar]
- Shriver, D.F.; Atkins, P.W. Shriver and Atkins’ Inorganic Chemistry; W. H. Freeman and Company: New York, NY, USA, 2006. [Google Scholar]
- Hu, E.; Bak, S.-M.; Liu, Y.; Liu, J.; Yu, X.; Zhou, Y.-N.; Zhou, J.; Khalifah, P.; Ariyoshi, K.; Nam, K.-W.; et al. Utilizing Environmental Friendly Iron as a Substitution Element in Spinel Structured Cathode Materials for Safer High Energy Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501662. [Google Scholar] [CrossRef]
- Hu, E.; Wang, X.; Yu, X.; Yang, X.-Q. Probing the Complexities of Structural Changes in Layered Oxide Cathode Materials for Li-Ion Batteries during Fast Charge–Discharge Cycling and Heating. Acc. Chem. Res. 2018, 51, 290–298. [Google Scholar] [CrossRef]
- Toroker, M.C.; Carter, E.A. Hole Transport in Nonstoichiometric and Doped Wüstite. J. Phys. Chem. C 2012, 116, 17403–17413. [Google Scholar] [CrossRef]
- Van Daal, H.J.; Bosman, A.J. Hall Effect in CoO, NiO, and α-Fe2O3. Phys. Rev. 1967, 158, 736–747. [Google Scholar] [CrossRef]
- Fan, J.C.; Sreekanth, K.M.; Xie, Z.; Chang, S.L.; Rao, K.V. p-Type ZnO materials: Theory, growth, properties and devices. Prog. Mater. Sci. 2013, 58, 874–985. [Google Scholar] [CrossRef]
- De Wit, H.J.; Crevecoeur, C. n-type Hall effect in MnO. Phys. Lett. A 1967, 25, 393–394. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wu, X.-H.; Zhou, Z.-L. Novel high sensitivity and selectivity semiconductor gas sensor based on the p+n combined structure. Solid-State Electron. 2000, 44, 1603–1607. [Google Scholar] [CrossRef]
- Peng, R.; Shrestha, K.; Mishra, G.; Baltrusaitis, J.; Wu, C.-M.; Koodali, R.T. Efficient photocatalytic hydrogen evolution system by assembling earth abundant NixOy nanoclusters in cubic MCM-48 mesoporous materials. RSC Adv. 2016, 6, 59169–59180. [Google Scholar] [CrossRef]
- Gulsoy, G.; Was, G.S. Surface oxidation of Alloy 617 in low oxygen partial pressure He–CO–CO2 environments at 750–850 °C. Corros. Sci. 2015, 90, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.K.; Saha, B.; Satpati, B.; Banerjee, S. Structural and electrochemical analysis of a novel co-electrodeposited Mn2O3–Au nanocomposite thin film. Dalton Trans. 2015, 44, 9158–9169. [Google Scholar] [CrossRef]
- Kapse, V.D.; Ghosh, S.A.; Raghuwanshi, F.C.; Kapse, S.D. Nanocrystalline spinel Ni0.6Zn0.4Fe2O4: A novel material for H2S sensing. Mater. Chem. Phys. 2009, 113, 638–644. [Google Scholar] [CrossRef]


| Crystal Plane | Crystallite Size/nm | |||||
|---|---|---|---|---|---|---|
| 1# | 2# | 3# | 4# | 5# | 6# | |
| 111 | 175.8 | 155.8 | 28.8 | 227.4 | 140.3 | 48.6 |
| 220 | 20.5 | 18.2 | 29.5 | 27.2 | 17.3 | 25.3 |
| 311 | 22.8 | 15.1 | 20.2 | 20.7 | 16.0 | 23.1 |
| 222 | 250.6 | 153.8 | 81.2 | 148.8 | 274.3 | 20.8 |
| 400 | 31.5 | 66.2 | 15.1 | 29.9 | 48.6 | 23.0 |
| 422 | 207.2 | 135.2 | 66.6 | 163.7 | 69.2 | 46.6 |
| 511 | 49.4 | 44.1 | 23.0 | 26.3 | 31.5 | 21.5 |
| 440 | 27.8 | 27.8 | 18.9 | 34.6 | 36.2 | 16.2 |
| Average | 98.2 | 77.0 | 35.4 | 84.8 | 79.2 | 28.2 |
| Spinel | A site | Mn2+ | Fe2+ | Co2+ | Ni2+ | Zn2+ |
|---|---|---|---|---|---|---|
| B site | dm | d5 | d6 | d7 | d8 | d10 |
| Cr3+ | d3 | 0 | 0 | 0 | 0 | 0 |
| Fe3+ | d5 | 0.1 | 0.5 | 0.5 | 0.5 | 0 |
| Mn3+ | d4 | N/A | N/A | N/A | N/A | 0 |
| Co3+ | d6 | N/A | N/A | N/A | 0 | 0 |
| (a) | ||||||
| Spinel | A Site | Mn2+ | Fe2+ | Co2+ | Ni2+ | Zn2+ |
| B site | dm | d5 | d6 | d7 | d8 | d10 |
| Cr3+ | d3 | L | L | L | L | M |
| Fe3+ | d5 | L | L | L | L | H |
| Mn3+ | d4 | L | L | L | L | H |
| Co3+ | d6 | L | L | L | L | H |
| Ni3+ | d7 | L | L | L | L | M |
| (b) | ||||||
| Migration level | 1# | 2# | 3# | 4# | 5# | 6# |
| without Zn | without Ni | without Mn | without Fe | without Cr | without Co | |
| H | 0 | 3 | 2 | 2 | 3 | 2 |
| M | 0 | 1 | 2 | 2 | 1 | 2 |
| L | 20 | 12 | 12 | 12 | 16 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashishin, T.; Taniguchi, H.; Li, F.; Abe, H. Useful High-Entropy Source on Spinel Oxides for Gas Detection. Sensors 2022, 22, 4233. https://doi.org/10.3390/s22114233
Hashishin T, Taniguchi H, Li F, Abe H. Useful High-Entropy Source on Spinel Oxides for Gas Detection. Sensors. 2022; 22(11):4233. https://doi.org/10.3390/s22114233
Chicago/Turabian StyleHashishin, Takeshi, Haruka Taniguchi, Fei Li, and Hiroya Abe. 2022. "Useful High-Entropy Source on Spinel Oxides for Gas Detection" Sensors 22, no. 11: 4233. https://doi.org/10.3390/s22114233
APA StyleHashishin, T., Taniguchi, H., Li, F., & Abe, H. (2022). Useful High-Entropy Source on Spinel Oxides for Gas Detection. Sensors, 22(11), 4233. https://doi.org/10.3390/s22114233






