Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety
Abstract
:1. Introduction
2. Conventional Methods of Antibiotic Detection and Food Contamination Limits
2.1. Microbiological Assays
2.2. Chromatographic Methods of Antibiotic Detection
2.3. Immunoassay
2.4. Other Analytical Methods for Antibiotic Detection
3. Nucleic Acid Aptamers
3.1. SELEX and the Basic Properties of Nucleic Acid Aptamers
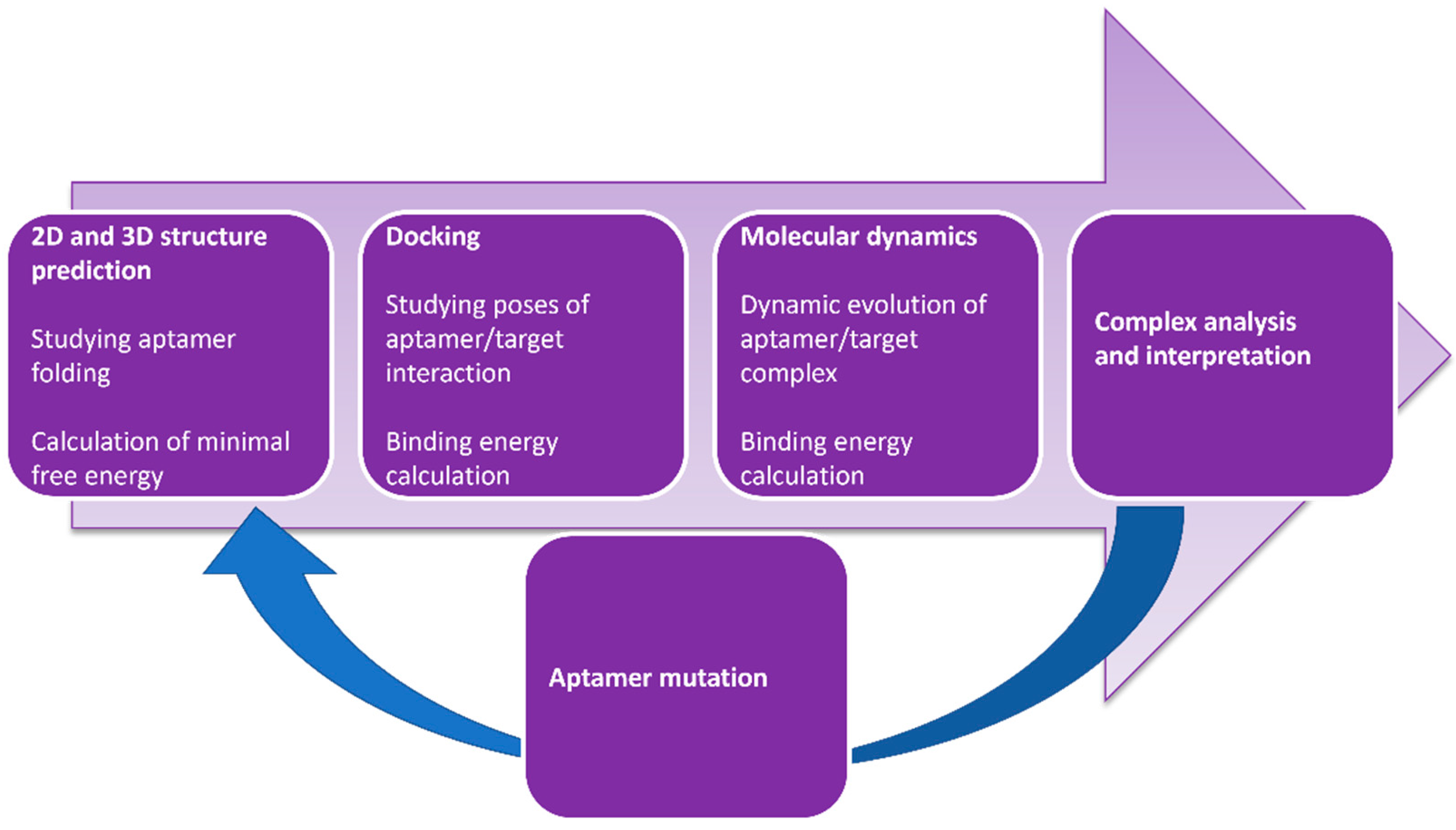
3.2. Computer Modeling of Aptamer–Antibiotic Complexes and Optimization of Aptamer Sequences
4. Principles of Biosensors and Electrochemical Methods of Antibiotic Detection
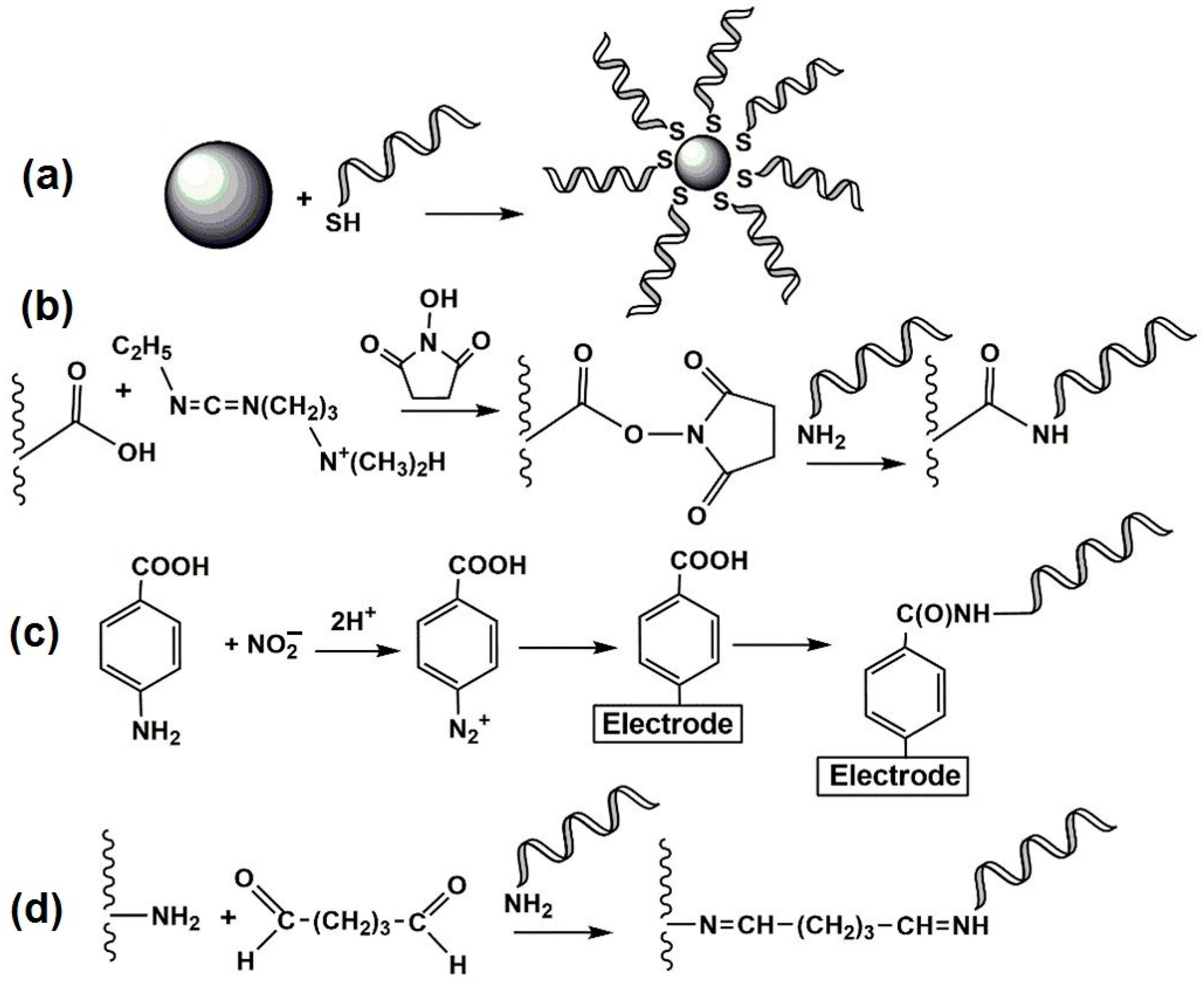
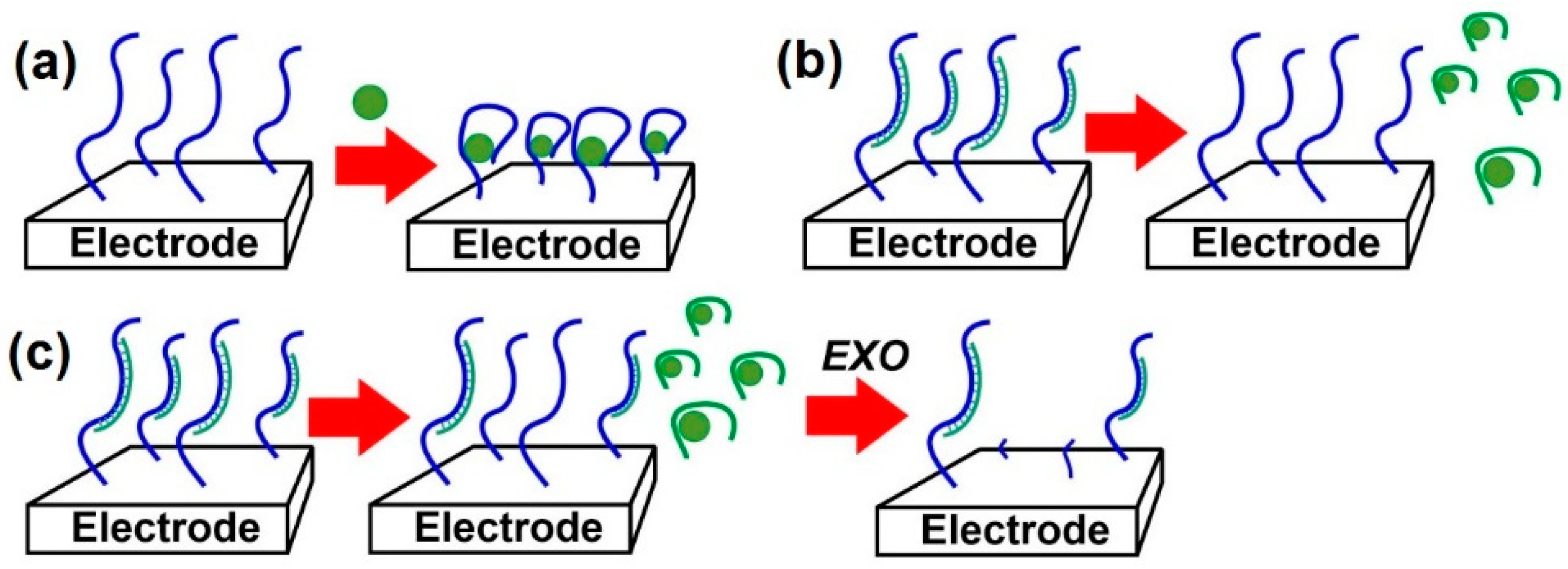
4.1. Aptamer Imobilization Protocols
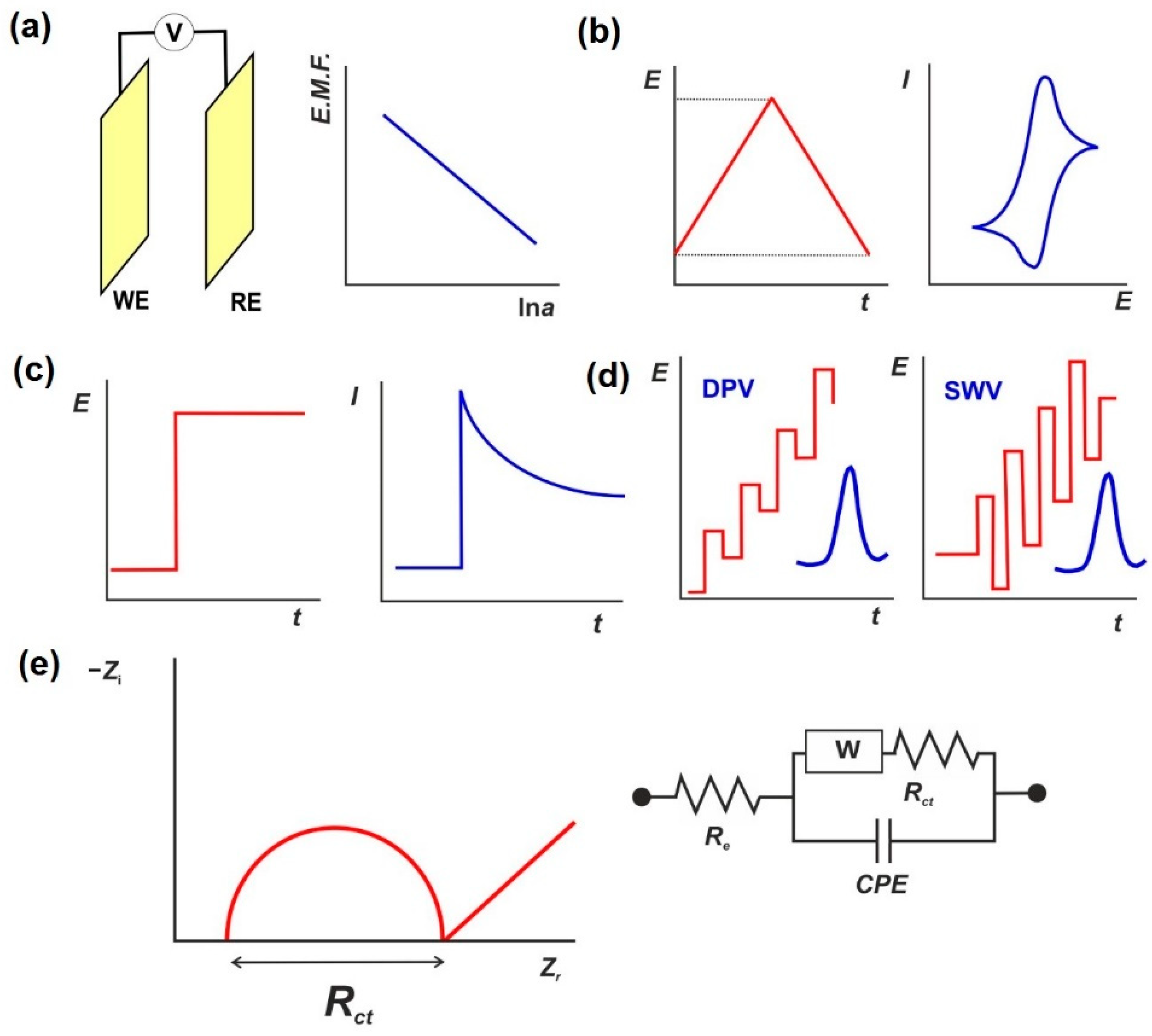
4.2. Electrochemical Signal Measurement Modes
4.3. Aptasensor Signal Measurements
5. Electrochemical Aptasensors for Antibiotic Detection
5.1. Surface-Layer Permeability Assessment
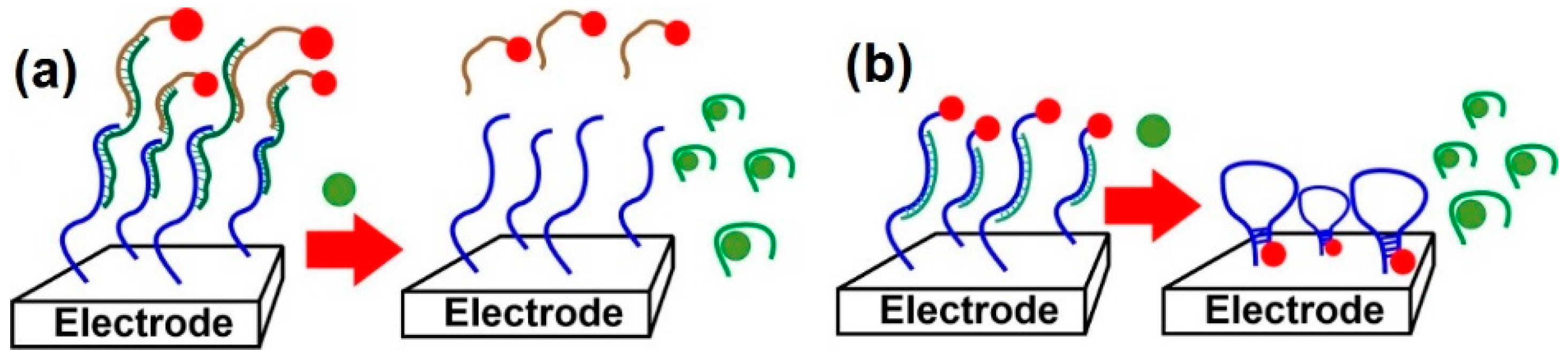
5.2. Lalbeled Aptamers/DNA Strands
5.3. Biochemical Amplification Protocols
5.4. Real-Sample Analysis
5.5. Recent Trends in Aptasensors Design
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banan, K.; Hatamabadi, D.; Afsharara, H.; Mostafiz, H.; Sadeghi, H.; Rashidi, S.; Beirami, A.D.; Shahbazi, M.-A.; Kecili, R.; Hussain, C.M.; et al. MIP-based extraction techniques for the determination of antibiotic residues in edible meat samples: Design, performance and recent developments. Trends Food Sci. Technol. 2022, 199, 164–179. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Antibiotic use in heavy pigs: Comparison between urine and muscle samples from food chain animals analysed by HPLC-MS/MS. Food Chem. 2017, 235, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hlabangana, L.; Memeza, S. Ion-pair isocratic simultaneous determination of broad spectrum antibiotics in environmental samples by HPLC with UV detection. Environ. Nanotechnol. Monit. Manag. 2018, 10, 104–111. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mishra, R.K.; Tang, X.; Marty, J.L. Detection of antibiotics in food: New achievements in the development of biosensors. Trends Anal. Chem. 2020, 127, 115883. [Google Scholar] [CrossRef]
- Banica, F.-G. Chemical Sensors and Biosensors. Fundamentals and Applications; Wiley: Chichester, UK, 2012; pp. 1–4. [Google Scholar]
- Evtugyn, G. Biosensors: Essentials; Springer: Heidelberg, Germany, 2014; pp. 1–20. [Google Scholar]
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Anal. Chim. Acta 2006, 568, 200–210. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-based biosensors for antibiotic detection: A review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.; Andersson, D.I. Selection of resistance at lethal and non-lethal antibiotic concentrations. Curr. Opin. Microbiol. 2012, 15, 555–560. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Larsson, D.G. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Long, H.; Miller, S.F.; Strauss, C.; Zhao, C.; Cheng, L.; Ye, Z.; Griffin, K.; Te, R.; Lee, H.; Chen, C.C.; et al. Antibiotic treatment enhances the genome-wide mutation rate of target cells. Proc. Natl. Acad. Sci. USA 2016, 113, E2498–E2505. [Google Scholar] [CrossRef] [Green Version]
- Almakki, A.; Esteves, K.; Vanhove, A.S.; Mosser, T.; Aujoulat, F.; Marchandin, H.; Toubiana, M.; Monfort, P.; Jumas-Bilak, E.; Licznar-Fajardo, P. A new methodology to assess antimicrobial resistance of bacteria in coastal waters; pilot study in a Mediterranean hydrosystem. Comptes Rendus Geosci. 2017, 349, 310–318. [Google Scholar] [CrossRef]
- European Commission. Commission regulation laying down a community procedure for the 751 establishment of maximum residue limits of veterinary medical products in foodstuffs of 752 animal origin. Off. J. 1997, 224, 1–8. [Google Scholar]
- Treiber, F.M.; Beranek-Knauer, H. Antimicrobial residues in food from animal origin—A review of the literature focusing on products collected in stores and markets worldwide. Antibiotics 2021, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Kukurova, I.; Hozova, B. The utilization of disk diffusion method and the Delvotest® for determining synergistic effects of cephalosporin combinations in milk. J. Food Nutr. Res. 2007, 46, 9–14. [Google Scholar]
- Piech, T.; Majer-Dziedzic, B.; Kostruba, A.; Grzelak, E.M.; Choma, I.M. Thin-layer chromatography—Direct bioautography as an alternative method for screening of antibiotic residues in milk: A comparative study. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 292–297. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Monette, C.E.; Bracero, S.; Saha, M.S. Methods for field measurement of antibiotic concentrations: Limitations and outlook. FEMS Microbiol. Ecol. 2018, 94, fiy105. [Google Scholar] [CrossRef]
- Kalunke, R.M.; Grasso, G.; D’Ovidio, R.; Dragone, R.; Frazzoli, C. Detection of ciprofloxacin residues in cow milk: A novel and rapid optical β-galactosidase-based screening assay. Microchem. J. 2018, 136, 128–132. [Google Scholar] [CrossRef]
- Le Breton, M.H.; Savoy-Perroud, M.C.; Diserens, J.M. Validation and comparison of the Copan Milk Test and Delvotest SP-NT for the detection of antimicrobials in milk. Anal. Chim. Acta 2007, 586, 280–283. [Google Scholar] [CrossRef]
- Hozová, B.; Kratmüllerová, M. Assay of antibiotic detection limits in cow’s milk model samples and comparison of sensitivity of various detection systems (disk diffusion method, Delvotest SP and Penzym S 100). Czech J. Food. Sci. 2001, 19, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Liu, Y.; Sha, J.; Zhang, Z.; Tu, Q.; Chen, P.; Wang, J. High-throughput microfluidic system for long-term bacterial colony monitoring and antibiotic testing in zero-flow environments. Biosens. Bioelectron. 2011, 26, 1993–1999. [Google Scholar] [CrossRef]
- Deiss, F.; Funes-Huacca, M.E.; Bal, J.; Tjhung, K.F.; Derda, R. Antimicrobial susceptibility assays in paper-based portable culture devices. Lab Chip 2014, 14, 167–171. [Google Scholar] [CrossRef]
- Bobbit, D.R.; Ng, K.W. Chromatographic analysis of antibiotic materials in food. J. Chromatogr. A 1992, 624, 153–170. [Google Scholar] [CrossRef]
- Giese, R.W. Electron-capture mass spectrometry: Recent advances. J. Chromatogr. A 2000, 892, 329–346. [Google Scholar] [CrossRef]
- Sasaki, K.; Takeda, M.; Uchiyama, M. Gas-liquid chromatographic determination of chloramphenicol in agricultural crops. J. Assoc. Anal. Chem. 1976, 59, 1118–1121. [Google Scholar] [CrossRef]
- Karageorgou, E.; Christoforidou, S.; Ioannidou, M.; Psomas, E.; Samouris, G. Detection of β-lactams and chloramphenicol residues in raw milk—Development and application of an HPLC-DAD method in comparison with microbial inhibition assays. Foods 2018, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Jha, R.R.; Singh, N.; Kumari, R.; Patel, D.K. Ultrasound-assisted emulsification microextraction based on a solidified floating organic droplet for the rapid determination of 19 antibiotics as environmental pollutants in hospital drainage and Gomti river water. J. Sep. Sci. 2017, 40, 2694–2702. [Google Scholar] [CrossRef]
- Nölting, B. Methods in Modern Biophysics, 2nd ed.; Springer: Heidelberg, Germany, 2006; pp. 37–48. [Google Scholar]
- Aga, D.S.; O’Connor, S.; Ensley, S.; Payero, J.O.; Snow, D.; Tarkalson, D. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2005, 53, 7165–7171. [Google Scholar] [CrossRef]
- Cháfer-Pericás, C.; Maquieira, A.; Puchades, R. Fast screening methods to detect antibiotic residues in food samples. Trends Anal. Chem. 2010, 29, 1038–1049. [Google Scholar] [CrossRef]
- Graham, D.W.; Olivares-Rieumont, S.; Knapp, C.W.; Lima, L.; Werner, D.; Bowen, E. Antibiotic resistance gene abundances associated with waste discharges to the Almendares River near Havana, Cuba. Environ. Sci. Technol. 2011, 45, 418–424. [Google Scholar] [CrossRef]
- Meyer, V.K.; Meloni, D.; Olivo, F.; Martlbauer, E.; Dietrich, R.; Niessner, R.; Seidel, M. Validation procedure for multiplex antibiotic immunoassays using flow-based chemiluminescence microarrays. Methods Mol. Biol. 2016, 1518, 195–212. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Aydin, S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.E. Practical understanding and use of surface enhanced Raman scattering/surface enhanced resonance Raman scattering in chemical and biological analysis. Chem. Soc. Rev. 2008, 37, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Li, D.W.; Zhai, W.L.; Li, Y.T.; Long, Y.T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2014, 181, 23–43. [Google Scholar] [CrossRef]
- Sierra-Rodero, M.; Fernández-Romero, J.M.; Gómez-Hens, A. Determination of aminoglycoside antibiotics using an on-chip microfluidic device with chemiluminescence detection. Microchim. Acta 2012, 179, 185–192. [Google Scholar] [CrossRef]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, M.B.; Stephenson, M.L.; Scott, J.F.; Hecht, L.I.; Zamecnik, P.C. A soluble ribonucleic acid intermediate in protein synthesis. J. Biol. Chem. 1958, 231, 241–257. [Google Scholar] [CrossRef]
- Carothers, J.M.; Szostak, J.W. In vitro selection of functional oligonucleotides and the origins of biochemical activity. In The Aptamer Handbook: Functional Oligonucleotides and Their Applications, 1st ed.; Klussmann, S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 1–28. [Google Scholar]
- Strehlitz, B.; Stoltenburg, R. SELEX and its recent optimizations. In Aptamers in Bioanalysis, 1st ed.; Mascini, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 31–59. [Google Scholar]
- Hianik, T. Advances in electrochemical and acoustic aptamer-based biosensors and immunosensors in diagnostics of leukemia. Biosensors 2021, 11, 177. [Google Scholar] [CrossRef]
- Hianik, T.; Grman, I.; Karpisova, I. The effect of DNA aptamer configuration on the sensitivity of detection thrombin at surface by acoustic method. Chem. Commun. 2009, 41, 6303–6305. [Google Scholar] [CrossRef]
- Lyu, C.; Khan, I.M.; Wang, Z. Capture-SELEX for aptamer selection: A short review. Talanta 2021, 229, 122274. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-M.; Go, M.-J.; Lee, J.; Na, D.; Yoo, S.-M. Recent advances in micro/nanomaterial-based aptamer selection strategies. Molecules 2021, 26, 5187. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent advances in SELEX technology and aptamer applications in biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Duan, N.; Wu, S.; Hao, L.; Xia, Y.; Ma, X.; Wang, Z. Graphene oxide-assisted non-immobilized SELEX of okdaic acid aptamer and the analytical application of aptasensor. Sci. Rep. 2016, 6, 21665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Chen, C.; Larcher, L.M.; Barrero, R.A.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50. [Google Scholar] [CrossRef]
- Hasegawa, H.; Savory, N.; Abe, K.; Ikebukuro, K. Methods for improving aptamer binding affinity. Molecules 2016, 21, 421. [Google Scholar] [CrossRef]
- Kalra, P.; Dhiman, A.; Cho, W.C.; Bruno, J.G.; Sharma, T.K. Simple methods and rational design for enhancing aptamer sensitivity and specificity. Front. Mol. Biosci. 2018, 5, 41. [Google Scholar] [CrossRef]
- Wu, Y.X.; Kwon, Y.J. Aptamers: The “Evolution” of SELEX. Methods 2016, 106, 21–28. [Google Scholar] [CrossRef]
- Kohlberger, M.; Gadermaier, G. SELEX: Critical factors and optimization strategies for successful aptamer selection. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef]
- Komarova, N.; Kuznetsov, A. Inside the black box: What makes SELEX better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zheng, X.; Jiao, B.; Wang, L. Post-SELEX optimization of aptamers. Anal. Bioanal. Chem. 2016, 408, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Lai, J.-C.; Horng, H.-E.; Liu, T.-C.; Hong, C.-Y. Generation of aptamers from a primer-free randomized ssDNA library using magnetic-assisted rapid aptamer selection. Sci. Rep. 2017, 7, 45478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dembowski, S.K.; Bowser, M.T. Microfluidic methods for aptamer selection and characterization. Analyst 2018, 143, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Ghulam, M.; Li, L.; Qu, F. Evolution of multi-functional capillary electrophoresis for high-efficiency selection of aptamers. Biotechnol. Adv. 2019, 37, 107432. [Google Scholar] [CrossRef]
- Liu, H.; Yu, J. Challenges of SELEX and demerits of aptamer-based methods. In Aptamers for Analytical Applications; Dong, Y., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 345–364. [Google Scholar]
- Takahashi, M.; Wu, X.; Ho, M.; Chomchan, P.; Rossi, J.J.; Burnett, J.C.; Zhou, J. High throughput sequencing analysis of RNA libraries reveals the influences of initial library and PCR methods on SELEX efficiency. Sci. Rep. 2016, 6, 33697. [Google Scholar] [CrossRef] [Green Version]
- Gevertz, J.; Gan, H.H.; Schlick, T. In vitro RNA random pools are not structurally diverse: A computational analysis. RNA 2005, 11, 853–863. [Google Scholar] [CrossRef] [Green Version]
- Vorobyeva, M.; Davydova, A.; Vorobjev, P.; Pyshnyi, D.; Venyaminova, A. Key aspects of nucleic acid library design for in vitro selection. Int. J. Mol. Sci. 2018, 19, 470. [Google Scholar] [CrossRef] [Green Version]
- Ruff, K.M.; Snyder, T.M.; Liu, D.R. Enhanced functional potential of nucleic acid aptamer libraries patterned to increase secondary structure. J. Am. Chem. Soc. 2010, 132, 9453–9464. [Google Scholar] [CrossRef]
- Kimoto, M.; Yamashige, R.; Matsunaga, K.; Yokoyama, S.; Hirao, I. Generation of high-affinity DNA aptamers using an expanded genetic alphabet. Nat. Biotechnol. 2013, 31, 453–457. [Google Scholar] [CrossRef]
- Dellafiore, M.A.; Montserrat, J.M.; Iribarren, A.M. Modified nucleoside triphosphates for in vitro selection techniques. Front. Chem. 2016, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Röthlisberger, P.; Hollenstein, M. Aptamer chemistry. Adv. Drug Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef] [PubMed]
- De-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. SPR sensing of small molecules with modified RNA aptamers: Detection of neomycin B. Biosens. Bioelectron. 2009, 24, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, E.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Impedimetric aptasensor for tobramycin detection in human serum. Biosens. Bioelectron. 2011, 26, 2354–2360. [Google Scholar] [CrossRef]
- Han, S.R.; Yu, J.; Lee, S.-W. In Vitro Selection of RNA aptamers that selectively bind danofloxacin. Biochem. Biophys. Res. Commun. 2014, 448, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lis, J.T.; Shi, H. A Systematic study of the features critical for designing a high avidity multivalent aptamer. Nucleic Acid Ther. 2013, 23, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, A.; Anand, A.; Malhotra, A.; Khan, E.; Santra, V.; Kumar, A.; Sharma, T.K. Rational truncation of aptamer for cross-species application to detect krait envenomation. Sci. Rep. 2018, 8, 17795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Zhao, J.; Liu, N.; Yang, M.; Zhao, Q.; Li, C.; Liu, M. Structure-guided post-SELEX optimization of an ochratoxin A aptamer. Nucleic Acids Res. 2019, 47, 5963–5972. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Ahmad Raston, N.H.; Gu, M.B. An ultra-sensitive colorimetric detection of zetracyclines using the shortest aptamer with highly enhanced affinity. Chem. Commun. 2014, 50, 40–42. [Google Scholar] [CrossRef]
- Meng, F.; Ma, X.; Duan, N.; Wu, S.; Xia, Y.; Wang, Z.; Xu, B. Ultrasensitive SERS aptasensor for the detection of oxytetracycline based on a gold-enhanced nano-assembly. Talanta 2017, 165, 412–418. [Google Scholar] [CrossRef]
- Yue, F.; Li, F.; Kong, Q.; Guo, Y.; Sun, X. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications. Sci. Total Environ. 2021, 762, 143129. [Google Scholar] [CrossRef]
- Soheili, V.; Taghdisi, S.M.; Khayyat, M.H.; Abnous, K.; Bazzaz, B.S.F.; Ramezani, M. Colorimetric and ratiometric aggregation assay for streptomycin using gold manoparticles and a new and highly specific aptamer. Microchim. Acta 2016, 183, 1687–1697. [Google Scholar] [CrossRef]
- Sanford, A.A.; Rangel, A.E.; Feagin, T.A.; Lowery, R.G.; Argueta-Gonzalez, H.S.; Heemstra, J.M. RE-SELEX: Restriction enzyme-based evolution of structure-switching aptamer biosensors. Chem. Sci. 2021, 12, 11692–11702. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; McKeague, M.; Pitre, S.; Dumontier, M.; Green, J.; Golshani, A.; Derosa, M.C.; Dehne, F. Computational approaches toward the design of pools for the in vitro selection of complex aptamers. RNA 2010, 16, 2252–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beier, R.; Boschke, E.; Labudde, D. New strategies for evaluation and analysis of SELEX experiments. BioMed Res. Int. 2014, 2014, 849743. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, J. SPA-LN: A scoring function of ligand–nucleic acid interactions via optimizing both specificity and affinity. Nucleic Acids Res. 2017, 45, e110. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M. In silico approaches to RNA aptamer design. Biochimie 2018, 145, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Shieh, K.R.; Kratschmer, C.; Maier, K.E.; Greally, J.M.; Levy, M.; Golden, A. AptCompare: Optimized de novo motif discovery of RNA aptamers via HTS-SELEX. Bioinformatics 2020, 36, 2905–2906. [Google Scholar] [CrossRef]
- Ishida, R.; Adachi, T.; Yokota, A.; Yoshihara, H.; Aoki, K.; Nakamura, Y.; Hamada, M. RaptRanker: In silico RNA aptamer selection from HT-SELEX experiment based on local sequence and structure information. Nucleic Acids Res. 2020, 48, e82. [Google Scholar] [CrossRef]
- Caroli, J.; Forcato, M.; Bicciato, S. APTANI2: Update of aptamer selection through sequence-structure analysis. Bioinformatics 2020, 36, 2266–2268. [Google Scholar] [CrossRef]
- Krüger, A.; Zimbres, F.; Kronenberger, T.; Wrenger, C. Molecular modeling applied to nucleic acid-based molecule development. Biomolecules 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Bernat, V.; Disney, M.D. RNA structures as mediators of neurological diseases and as drug targets. Neuron 2015, 87, 28–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
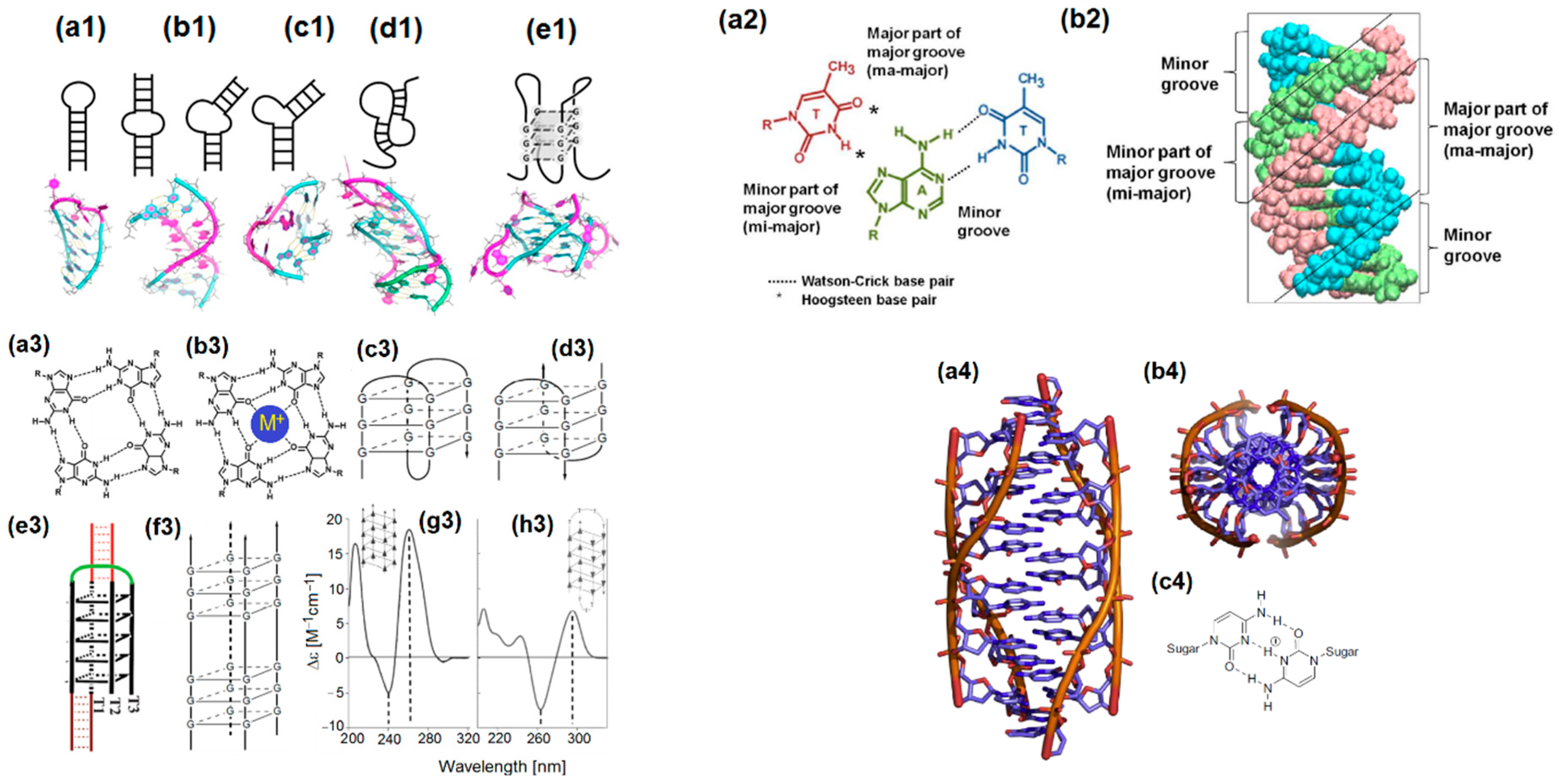
- Tateishi-Karimata, H.; Nakano, M.; Sugimoto, N. Comparable stability of Hoogsteen and Watson–Crick base pairs in ionic liquid choline dihydrogen phosphate. Sci. Rep. 2014, 4, 3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Zhou, Y.; Liu, J. G-quadruplex DNA for construction of biosensors. Trends Anal. Chem. 2020, 132, 116060. [Google Scholar] [CrossRef]
- Day, H.A.; Pavlou, P.; Waller, Z.A.E. i-Motif DNA: Structure, stability and targeting with ligands. Bioorg. Med. Chem. 2014, 22, 4407–4418. [Google Scholar] [CrossRef]
- Ho, P.S.; Megan, L.; Carter, M.L. DNA structure: Alphabet soup for the cellular soul. In DNA Replication-Current Advances; Seligmann, H., Ed.; IntechOpen: London, UK, 2011; pp. 1–28. [Google Scholar] [CrossRef]
- Buglak, A.A.; Samokhvalov, A.V.; Zherdev, A.V.; Dzantiev, B.B. Methods and applications of in silico aptamer design and modeling. Int. J. Mol. Sci. 2020, 21, 8420. [Google Scholar] [CrossRef]
- Lu, X.-J.; Olson, W.K. 3DNA: A versatile, integrated software system for the analysis, rebuilding and visualization of three-dimensional nucleic-acid structures. Nat. Protoc. 2008, 3, 1213–1227. [Google Scholar] [CrossRef]
- Popenda, M.; Szachniuk, M.; Antczak, M.; Purzycka, K.J.; Lukasiak, P.; Bartol, N.; Blazewicz, J.; Adamiak, R.W. Automated 3D structure composition for large RNAs. Nucleic Acids Res. 2012, 40, e112. [Google Scholar] [CrossRef]
- Jeddi, I.; Saiz, L. Three-dimensional modeling of single stranded DNA hairpins for aptamer-based biosensors. Sci. Rep. 2017, 7, 1178. [Google Scholar] [CrossRef] [Green Version]
- Blind, M.; Blank, M. Aptamer selection technology and recent advances. Mol. Ther. Nucleic Acids 2015, 4, e223. [Google Scholar] [CrossRef]
- Hoinka, J.; Berezhnoy, A.; Dao, P.; Sauna, Z.E.; Gilboa, E.; Przytycka, T.M. Large scale analysis of the mutational landscape in HT-SELEX improves aptamer discovery. Nucleic Acids Res. 2015, 43, 5699–5707. [Google Scholar] [CrossRef]
- Seelam, P.P.; Mitra, A.; Sharma, P. Pairing interactions between nucleobases and ligands in aptamer:ligand complexes of riboswitches: Crystal structure analysis, classification, optimal structures, and accurate interaction energies. RNA 2019, 25, 1274–1290. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.A.; Kung, R.W.; D’souza, S.; Wetmore, S.D. Anatomy of noncovalent interactions between the nucleobases or ribose and π-containing amino acids in RNA–protein complexes. Nucleic Acids Res. 2021, 49, 2213–2225. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the interface of nucleic acid aptamers and binding targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.R.; Weber, J.K.; Yin, W.; Huynh, T.; Duan, W.; Zhou, R. In silico design and validation of high-affinity RNA aptamers targeting epithelial cellular adhesion molecule dimers. Proc. Natl. Acad. Sci. USA 2020, 117, 8486–8493. [Google Scholar] [CrossRef] [PubMed]
- Navien, T.N.; Thevendran, R.; Hamdani, H.Y.; Tang, T.-H.; Citartan, M. In silico molecular docking in DNA aptamer development. Biochimie 2021, 180, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Schlick, T.; Pyle, A.M. Opportunities and challenges in RNA structural modeling and design. Biophys. J. 2017, 113, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Sabri, M.Z.; Hamid, A.A.A.; Hitam, S.M.S.; Rahim, M.Z.A. The assessment of three dimensional modelling design for single strand DNA aptamers for computational chemistry application. Biophys. Chem. 2020, 267, 106492. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, Z.; Liu, D.; Jiang, H.; Zhang, Z.-K.; Lu, A.; Zhang, B.-T.; Yu, Y.; Zhang, G. Structural biology for the molecular insight between aptamers and target proteins. Int. J. Mol. Sci. 2021, 22, 4093. [Google Scholar] [CrossRef]
- Kinghorn, A.; Fraser, L.; Liang, S.; Shiu, S.; Tanner, J. Aptamer bioinformatics. Int. J. Mol. Sci. 2017, 18, 2516. [Google Scholar] [CrossRef] [Green Version]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Nithin, C.; Mukherjee, S.; Bahadur, R.P. A Non-tedundant protein-RNA docking benchmark version 2.0. Proteins 2017, 85, 256–267. [Google Scholar] [CrossRef] [PubMed]
- PLUMED Consortium. Promoting transparency and reproducibility in enhanced molecular simulations. Nat. Methods 2019, 16, 670–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townshend, R.J.L.; Eismann, S.; Watkins, A.M.; Rangan, R.; Karelina, M.; Das, R.; Dror, R.O. Geometric deep kearning of RNA structure. Science 2021, 373, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Ferdousi, R. AptaNet as a deep learning approach for aptamer–protein interaction prediction. Sci. Rep. 2021, 11, 6074. [Google Scholar] [CrossRef]
- Sabri, M.Z.; Hamid, A.A.A.; Hitam, S.M.S.; Rahim, M.Z.A. In-silico selection of aptamer: A review on the revolutionary approach to understand the aptamer design and interaction through computational chemistry. Mater. Today Proc. 2019, 19, 1572–1581. [Google Scholar] [CrossRef]
- Bashir, A.; Yang, Q.; Wang, J.; Hoyer, S.; Chou, W.; McLean, C.; Davis, G.; Gong, Q.; Armstrong, Z.; Jang, J.; et al. Machine learning guided aptamer refinement and discovery. Nat. Commun. 2021, 12, 2366. [Google Scholar] [CrossRef]
- Yan, S.; Peck, J.M.; Ilgu, M.; Nilsen-Hamilton, M.; Lamm, M.H. Sampling performance of multiple independent molecular dynamics simulations of an RNA aptamer. ACS Omega 2020, 5, 20187–20201. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Tan, K.X.; Danquah, M.K.; Guo, H.; Turgeson, A. Advancing aptamers as molecular probes for cancer theranostic applications—the role of molecular dynamics simulation. Biotechnol. J. 2020, 15, 1900368. [Google Scholar] [CrossRef]
- Qi, S.; Duan, N.; Khan, I.M.; Dong, X.; Zhang, Y.; Wu, S.; Wang, Z. Strategies to manipulate the performance of aptamers in SELEX, Post-SELEX and microenvironment. Biotechnol. Adv. 2022, 55, 107902. [Google Scholar] [CrossRef]
- Ilgu, M.; Yan, S.; Khounlo, R.M.; Lamm, M.H.; Nilsen-Hamilton, M. Common secondary and tertiary structural features of aptamer–ligand interaction shared by RNA aptamers with different primary sequences. Molecules 2019, 24, 4535. [Google Scholar] [CrossRef] [Green Version]
- Khoshbin, Z.; Housaindokht, M.R. Computer-aided aptamer design for sulfadimethoxine antibiotic: Step by step mutation vased on MD simulation approach. J. Biomol. Struct. Dyn. 2020, 39, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Domin, G.; Findeiß, S.; Wachsmuth, M.; Will, S.; Stadler, P.F.; Mörl, M. Applicability of a computational design approach for synthetic riboswitches. Nucleic Acids Res. 2017, 45, 4108–4119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chushak, Y.; Stone, M.O. In silico selection of RNA aptamers. Nucleic Acids Res. 2009, 37, e87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khavani, M.; Izadyar, M.; Housaindokht, M.R. Theoretical design and experimental study on the gold nanoparticles based colorimetric aptasensors for detection of neomycin B. Sens. Actuat. B Chem. 2019, 300, 126947. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Enzyme-based biosensors: Tackling electron transfer issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef]
- Hall, E.A.H.; Gooding, J.J.; Hall, C.E. Redox enzyme linked electrochemical sensors: Theory meets practice. Mikrochim. Acta 1995, 121, 119–145. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, M. Recent progress in electrochemical immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, S.; Guo, W.; Li, B.; Yang, Y.; Xie, B.; Li, K.; Zhang, L. Recent advances on functional nucleic-acid biosensors. Sensors 2021, 21, 7109. [Google Scholar] [CrossRef]
- Teles, F.; Fonseca, L. Trends in DNA biosensors. Talanta 2008, 77, 606–623. [Google Scholar] [CrossRef]
- Shaban, S.M.; Kim, D.-H. Recent advances in aptamer sensors. Sensors 2021, 21, 979. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhang, B.; Tang, J.; Liu, B.; Lai, W.; Tang, D. Sandwich-type immunosensors and immunoassays exploiting nanostructure labels: A review. Anal. Chim. Acta 2013, 758, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Xu, W.; Leng, X.; Wang, H.; Guo, Y.; Huang, J. A novel sandwich-type electrochemical aptasensor based on GR-3D Au and aptamer-AuNPs-HRP for sensitive detection of oxytetracycline. Biosens. Bioelectron. 2017, 88, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Anfossi, L.; Shen, L.; Cheng, L.; Wang, X. Non-competitive immunoassay for low-molecular-weight contaminant detection in food, feed and agricultural products: A mini-review. Trends Food Sci. Technol. 2018, 71, 181–187. [Google Scholar] [CrossRef]
- Prante, M.; Segal, E.; Scheper, T.; Bahnemann, J.; Walter, J. Aptasensors for point-of-care detection of small molecules. Biosensors 2020, 10, 108. [Google Scholar] [CrossRef]
- Mazaafrianto, D.N.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Recent microdevice-based aptamer sensors. Micromachines 2018, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yan, X.; Zhao, L.; Qi, X.; Wang, S.; Liang, X. An aptamer cocktail-based electrochemical aptasensor for direct capture and rapid detection of tetracycline in honey. Microchem. J. 2019, 150, 104179. [Google Scholar] [CrossRef]
- Su, Z.; Xu, H.; Xu, X.; Zhang, Y.; Ma, Y.; Li, C.; Xie, Q. Effective covalent immobilization of quinone and aptamer onto a gold electrode via thiol addition for sensitive and selective protein biosensing. Talanta 2017, 164, 244–248. [Google Scholar] [CrossRef]
- An, K.; Lu, X.; Wang, C.; Qian, J.; Chen, Q.; Hao, N.; Wang, K. Porous gold nanocages: High atom utilization for thiolated aptamer immobilization to well balance the simplicity, sensitivity, and cost of disposable aptasensors. Anal. Chem. 2019, 91, 8660–8666. [Google Scholar] [CrossRef]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Rabai, S.; Benounis, M.; Catanante, C.; Baraket, A.; Errachid, A.; Jaffrezic Renault, N.; Marty, J.-L.; Rhouati, A. Development of a label-free electrochemical aptasensor based on diazonium electrodeposition: Application to cadmium detection in water. Anal. Biochem. 2021, 612, 113956. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, F.; Noorbakhsh, A. Sensitive electrochemical prostate specific antigen aptasensor: Effect of carboxylic acid functionalized carbon nanotube and glutaraldehyde linker. Electroanalysis 2016, 28, 1134–1145. [Google Scholar] [CrossRef]
- Drummond, T.; Hill, M.; Barton, J. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Pohanka, M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Yildirim-Tirgil, N.; Lee, J.; Cho, H.; Lee, H.Y.; Somu, S.; Busnainac, A.; Gu, A.Z. A SWCNT based aptasensor system for antibiotic oxytetracycline detection in water samples. Anal. Methods 2019, 11, 2692–2699. [Google Scholar] [CrossRef]
- Melikishvili, S.; Piovarci, I.; Hianik, T. Advances in colorimetric assay based on AuNPs modified by proteins and nucleic acid aptamers. Chemosensors 2021, 9, 281. [Google Scholar] [CrossRef]
- Pehlivan, Z.S.; Torabfam, M.; Kurt, H.; Ow-Yang, C.; Hildebrandt, N.; Yüce, M. Aptamer and nanomaterial based FRET biosensors: A review on recent advances (2014–2019). Microchim. Acta 2019, 186, 563. [Google Scholar] [CrossRef]
- Düzgün, A.; Maroto, A.; Mairal, T.; O’Sullivan, K.; Rius, F.X. Solid-contact potentiometric aptasensor based on aptamer functionalized carbon nanotubes for the direct determination of proteins. Analyst 2010, 135, 1037–1041. [Google Scholar] [CrossRef]
- Lv, E.; Ding, J.; Qin, W. Potentiometric aptasensing of small molecules based on surface charge change. Sens. Actuators B 2018, 259, 463–466. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Zhang, F.; He, P. All-solid-state potentiometric aptasensing of streptomycin based on signal amplification by polycation. IEEE Sens. J. 2021, 21, 6300–6305. [Google Scholar] [CrossRef]
- Dahmen, E.A.M.F. Electroanalysis. Theory and Applications in Aqueous and Nonaqueous Media and in Automated Chemical Control. Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 1986; pp. 3–384. [Google Scholar]
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical impedance spectroscopy (EIS): Principles, construction, and biosensing applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Niazi, J.H.; Gu, M.B. Specific detection of oxytetracycline using DNA aptamer-immobilized interdigitated array electrode chip. Anal. Chim. Acta 2009, 634, 250–254. [Google Scholar] [CrossRef]
- Vichchulada, P.; Zhang, Q.; Lay, M.D. Recent progress in chemical detection with single-walled carbon nanotube networks. Analyst 2007, 132, 719–723. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M.; Oliveira-Brett, A.M. Nanostructured material–based electrochemical sensing of oxidative DNA damage biomarkers 8-oxoguanine and 8-oxodeoxyguanosine: A comprehensive review. Microchim. Acta 2021, 188, 58. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Y.; Zhang, X.; Zhou, J.; Xiong, E.; Li, X.; Chen, J. A novel electrochemical aptasensor for bisphenol A assay based on triple-signaling strategy. Biosens. Bioelectron. 2016, 79, 22–28. [Google Scholar] [CrossRef]
- Catanante, G.; Mishra, R.K.; Hayat, A.; Marty, J.-L. Sensitive analytical performance of folding based biosensor using methylene blue tagged aptamers. Talanta 2016, 153, 138–144. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Sun, X.; Guo, Y.; Zhao, W. A dual-signal amplification strategy for kanamycin based on ordered mesoporous carbon-chitosan/gold nanoparticles-streptavidin and ferrocene labelled DNA. Anal. Chim. Acta 2018, 1033, 185–192. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, R.; Chai, Y.; Zhuo, Y.; Hong, C.; Yang, X.; Su, H.; Qian, X. Highly sensitive, reusable electrochemical aptasensor for adenosine. Electrochim. Acta 2009, 54, 6207–6621. [Google Scholar] [CrossRef]
- Li, X.; Qi, H.; Shen, L.; Gao, Q.; Zhang, C. Electrochemical aptasensor for the determination of cocaine incorporating gold nanoparticles modification. Electroanalysis 2008, 20, 1475–1482. [Google Scholar] [CrossRef]
- Guo, Y.M.; Wang, X.Y.; Sun, X. A label-free electrochemical aptasensor based on electrodeposited gold nanoparticles and methylene blue for tetracycline detection. Int. J. Electrochem. Sci. 2015, 10, 3668–3679. [Google Scholar]
- Du, Y.; Li, B.; Wang, F.; Dong, S. Au nanoparticles grafted sandwich platform used amplified small molecule electrochemical aptasensor. Biosens. Bioelectron. 2009, 24, 1979–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Hu, G.; Wagberg, T.; Zhan, S.; Xu, H.; Zhou, P. Electrochemical aptasensor for tetracycline using a screen-printed carbon electrode modified with an alginate film containing reduced graphene oxide and magnetite (Fe3O4) nanoparticles. Microchim. Acta 2016, 183, 723–729. [Google Scholar] [CrossRef]
- Evtugyn, G.A.; Porfireva, A.V.; Hianik, T.; Cheburova, M.S.; Budnikov, H.C. Potentiometric DNA sensor based on electropolymerized phenothiazines for protein detection. Electroanalysis 2008, 20, 1300–1308. [Google Scholar] [CrossRef]
- Luan, Y.; Wang, N.; Li, C.; Guo, X.; Lu, A. Advances in the application of aptamer biosensors to the detection of aminoglycoside antibiotics. Antibiotics 2020, 9, 787. [Google Scholar] [CrossRef]
- Dong, X.; Yan, X.; Li, M.; Liu, H.; Li, J.; Wang, L.; Wang, K.; Lu, X.; Wang, S.; He, B. Ultrasensitive detection of chloramphenicol using electrochemical aptamer sensor: A mini review. Electrochem. Commun. 2020, 120, 106835. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Verdian, A.; Housaindokht, M.R.; Izadyar, M.; Rouhbakhsh, Z. Aptasensors as the future of antibiotics test kits—A case study of the aptamer application in the chloramphenicol detection. Biosens. Bioelectron. 2018, 122, 263–283. [Google Scholar] [CrossRef]
- Sadeghi, A.S.; Ansari, N.; Ramezanid, M.; Abnous, K.; Mohsenzadeh, M.; Taghdisi, S.M.; Alibolandi, M. Optical and electrochemical aptasensors for the detection of amphenicols. Biosens. Bioelectron. 2018, 118, 137–152. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Karimabadi, N.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Electrochemical and optical aptamer-based sensors for detection of tetracyclines. Trends Food Sci. Technol. 2018, 73, 45–57. [Google Scholar] [CrossRef]
- Hermouche, L.; Bendany, M.; Abbi, K.; El Hamdounia, Y.; Labjar, N.; El Mahi, M.; Lotfi, E.M.; Dalimi, M.; Dhiba, D.; El Hajjaji, S. Electrochemical sensors for tetracycline antibiotics detection based on carbon electrode materials modified by biological and chemical compounds: A review. Int. J. Environ. Anal. Chem. 2021. accepted. [Google Scholar] [CrossRef]
- Robati, R.Y.; Arab, A.; Ramezani, M.; Langroodi, F.A.; Abnous, K.; Taghdisi, S.M. Aptasensors for quantitative detection of kanamycin. Biosens. Bioelectron. 2016, 82, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Negahdary, M. Electrochemical aptasensors based on the gold nanostructures. Talanta 2020, 216, 120999. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, V. Contribution of nanomaterials to the development of electrochemical aptasensors for the detection of antimicrobial residues in food products. Chemosensors 2021, 9, 69. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Sun, C.; Li, Y. Recent advances in biosensors for antibiotic detection: Selectivity and signal amplification with nanomaterials. Food Chem. 2021, 361, 130109. [Google Scholar] [CrossRef]
- Han, C.; Li, R.; Li, H.; Liu, S.; Xu, C.; Wang, J.; Wang, Y.; Huang, J. Ultrasensitive voltammetric determination of kanamycin using a target-triggered cascade enzymatic recycling couple along with DNAzyme amplification. Microchim. Acta 2017, 184, 2941–2948. [Google Scholar] [CrossRef]
- Chen, M.; Gan, N.; Zhou, Y.; Li, T.; Xu, Q.; Cao, Y.; Chen, Y. A novel aptamer- metal ions- nanoscale MOF based electrochemical biocodes for multiple antibiotics detection and signal amplification. Sens. Actuators B 2017, 242, 1201–1209. [Google Scholar] [CrossRef]
- Sharma, A.; Istamboulie, G.; Hayat, A.; Catanante, G.; Bhanda, S.; Marty, J.L. Disposable and portable aptamer functionalized impedimetric sensor for detection of kanamycin residue in milk sample. Sens. Actuators B 2017, 245, 507–515. [Google Scholar] [CrossRef]
- Zeng, R.; Su, L.; Luo, Z.; Zhang, L.; Lu, M.; Tang, D. Ultrasensitive and label-free electrochemical aptasensor of kanamycin coupling with hybridization chain reaction and strand-displacement amplification. Anal. Chim. Acta 2018, 1038, 21–28. [Google Scholar] [CrossRef]
- Hong, G.; Chen, X.; Cao, Y.; Dong, Y.; Wu, D.; Hu, F.; Gan, N. Enzyme- and label-free electrochemical aptasensor for kanamycin detection based on double stir bar-assisted toehold-mediated strand displacement reaction for dual-signal amplification. Biosens. Bioelectron. 2018, 112, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gan, N.; Li, T.; Zhou, Y.; Cao, Y.; Dong, Y. Electrochemical aptasensor for multi-antibiotics detection based on endonuclease and exonuclease assisted dual recycling amplification strategy. Talanta 2018, 179, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Sun, X.; Guo, Y. Multiplex electrochemical aptasensor for detecting multiple antibiotics residues based on carbon fiber and mesoporous carbon-gold nanoparticles. Sens. Actuators B 2018, 265, 217–226. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Wang, X.; Sun, X. Multiplexed aptasensor based on metal ions labels for simultaneous detection of multiple antibiotic residues in milk. Biosens. Bioelectron. 2018, 115, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lai, G.; Liu, S.; Yu, A. Ultrasensitive electrochemical aptasensing of kanamycin antibiotic by enzymatic signal amplification with a horseradish peroxidase-functionalized gold nanoprobe. Sens. Actuators B 2018, 273, 1762–1767. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.; Han, X.; Shi, W.; Liu, Y.; Yan, H.; Zhang, G. A signal-on electrochemical aptasensor for highly sensitive and specific detection of kanamycin based on target-induced signaling probe shifting mechanism. Sens. Actuators B 2018, 273, 480–487. [Google Scholar] [CrossRef]
- Han, X.; Yu, Z.; Li, F.; Shi, W.; Fu, C.; Yan, H.; Zhang, G. Two kanamycin electrochemical aptamer-based sensors using different signal transduction mechanisms: A comparison of electrochemical behavior and sensing performance. Bioelectrochemistry 2019, 129, 270–277. [Google Scholar] [CrossRef]
- He, B.; Yan, S. Voltammetric kanamycin aptasensor based on the use of thionine incorporated into Au@Pt core-shell nanoparticles. Microchim. Acta 2019, 186, 77. [Google Scholar] [CrossRef]
- Shen, Z.; He, L.; Cao, Y.; Hong, F.; Zhang, K.; Hu, F.; Lin, J.; Wu, D.; Gan, N. Multiplexed electrochemical aptasensor for antibiotics detection using metallic-encoded apoferritin probes and double stirring bars-assisted target recycling for signal amplification. Talanta 2019, 197, 491–499. [Google Scholar] [CrossRef]
- Huang, S.; Gan, N.; Zhang, X.; Wu, Y.; Shao, Y.; Jiang, Z.; Wang, Q. Portable fluoride-selective electrode as signal transducer for sensitive and selective detection of trace antibiotics in complex samples. Biosens. Bioelectron. 2019, 128, 113–121. [Google Scholar] [CrossRef]
- Yao, X.; Shen, J.; Liu, Q.; Fa, H.; Yang, M.; Hou, C. A novel electrochemical aptasensor for the sensitive detection of kanamycin based on UiO-66-NH2/MCA/MWCNT@rGONR nanocomposites. Anal. Methods 2020, 12, 4967–4976. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, H.; Zhang, H.; Chu, G.; Guo, Y.; Sun, X. Ultrasensitive electrochemiluminescence aptasensor for kanamycin detection based on silver nanoparticle-catalyzed chemiluminescent reaction between luminol and hydrogen peroxide. Sens. Actuators B 2020, 304, 127367. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, J.; Qu, X.; Li, S.; Zhao, Y.; Liu, S.; Wang, Y.; Huang, J.; Yu, J. Efficient strand displacement amplification via stepwise movement of a bipedal DNA walker on an electrode surface for ultrasensitive detection of antibiotics. Analyst 2020, 145, 2975–2981. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, W.; Wang, F.; Zhang, F.; Wang, Q.; He, P. Simultaneous detection of streptomycin and kanamycin based on an all solid-state potentiometric aptasensor array with a dual-internal calibration system. Sens. Actuators B 2020, 311, 127857. [Google Scholar] [CrossRef]
- Bi, H.; Wu, Y.; Wang, Y.; Liu, G.; Ning, G.; Xu, Z. A molecularly imprinted polymer combined with dual functional Au@Fe3O4 nanocomposites for sensitive detection of kanamycin. J. Electroanal. Chem. 2020, 870, 114216. [Google Scholar] [CrossRef]
- He, X.; Han, H.; Shi, W.; Dong, J.; Lu, X.; Yang, W.; Lu, X. A label-free electrochemical DNA biosensor for kanamycin detection based on diblock DNA with poly-cytosine as a high affinity anchor on graphene oxide. Anal. Methods 2020, 12, 3462–3469. [Google Scholar] [CrossRef]
- Kulikova, T.; Gorbatchuk, V.; Stoikov, I.; Rogov, A.; Evtugyn, G.; Hianik, T. Impedimetric determination of kanamycin in milk with aptasensor based on carbon black-oligolactide composite. Sensors 2020, 20, 4738. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, L.; Jiang, D.; Shan, X.; Wang, W.; Shiigi, H.; Chen, Z. Ultrasensitive ECL aptasensing of kanamycin based on synergistic promotion strategy using 3,4,9,10-perylenetetracar-boxylic-L-cysteine/Au@HKUST-1. Anal. Chim. Acta 2021, 1180, 338780. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Peng, J. Blue-light photoelectrochemical aptasensor for kanamycin based on synergistic strategy by Schottky junction and sensitization. Sens. Actuators B 2021, 340, 129898. [Google Scholar] [CrossRef]
- Li, F.; Wu, Y.; Chen, D.; Guo, Y.; Wang, X.; Sun, X. Sensitive dual-labeled electrochemical aptasensor for simultaneous detection of multi-antibiotics in milk. Int. J. Hydrog. Energy 2021, 46, 23301–23309. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Y.; Zhan, D.; Lai, G. Homogeneous biorecognition reaction-induced assembly of DNA nanostructures for ultrasensitive electrochemical detection of kanamycin antibiotic. Anal. Chim. Acta 2021, 1154, 338317. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Fang, X.; Li, Z.; Pu, H.; Chang, J.; Chen, J.; Mao, S. Ultratrace antibiotic sensing using aptamer/graphene-based field-effect transistors. Biosens. Bioelectron. 2019, 126, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Yuan, L.; Jin, K.; Han, X.; Tian, Y.; Zhou, N. Electrochemical detection of tobramycin based on enzymes-assisted dual signal amplification by using a novel truncated aptamer with high affinity. Biosens. Bioelectron. 2018, 122, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Duan, F.; Hu, B.; He, L.; Wang, M.; Zhou, N.; Jia, Q.; Zhang, Z. Bimetallic cerium/copper organic framework-derived cerium and copper oxides embedded by mesoporous carbon: Label-free aptasensor for ultrasensitive tobramycin detection. Anal. Chim. Acta 2019, 1047, 150–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Wei, X.; Gu, Q.; Chen, M.; Zhang, J.; Mo, S.; Wang, J.; Xue, L.; Ding, Y.; et al. Amplified electrochemical antibiotic aptasensing based on electrochemically deposited AuNPs coordinated with PEI-functionalized Fe-based metal-organic framework. Microchim. Acta 2021, 188, 286. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, G.; Guo, C.; Xu, M.; Wang, M.; Wang, C.; Zhang, Z.; Du, M. A new strategy for the development of efficient impedimetric tobramycin aptasensors with metallo-covalent organic frameworks (MCOFs). Food Chem. 2022, 366, 130575. [Google Scholar] [CrossRef]
- Wu, Y.; Bi, H.; Ning, G.; Xu, Z.; Liu, G.; Wang, Y.; Zhao, Y. Cyclodextrin subject-object recognition-based aptamer sensor for sensitive and selective detection of tetracycline. J. Solid State Electrochem. 2020, 24, 2365–2372. [Google Scholar] [CrossRef]
- Roushani, M.; Ghanbaria, K.; Hoseini, S.J. Designing an electrochemical aptasensor based on immobilization of the aptamer onto nanocomposite for detection of the streptomycin antibiotic. Microchem. J. 2018, 141, 96–103. [Google Scholar] [CrossRef]
- Ghanbari, K.; Roushani, M. A novel electrochemical aptasensor for highly sensitive and quantitative detection of the streptomycin antibiotic. Bioelectrochemistry 2018, 120, 43–48. [Google Scholar] [CrossRef]
- Roushani, M.; Ghanbari, K. A novel aptasensor based on gold nanorods/ZnS QDs-modified electrode for evaluation of streptomycin antibiotic. Anal. Methods 2018, 10, 5197–5204. [Google Scholar] [CrossRef]
- Roushani, M.; Ghanbari, K. An electrochemical aptasensor for streptomycin based on covalent attachment of the aptamer onto a mesoporous silica thin film-coated gold electrode. Microchim. Acta 2019, 186, 115. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.G.; Gu, H.W.; Yi, H.C.; He, Y.Q.; Chen, J.; Sun, W.Y. Sensitive detection of streptomycin in milk using a hybrid signal enhancement strategy of MOF-based bio-bar code and target recycling. Anal. Chim. Acta 2020, 1125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Hou, W.; Jiao, Y.; Guo, Y.; Sun, X.; Zhao, J.; Wang, X. Ultra-sensitive aptasensor based on IL and Fe3O4 nanoparticles for tetracycline detection. Int. J. Electrochem. Sci. 2017, 12, 7426–7434. [Google Scholar] [CrossRef]
- Zhan, X.; Hu, G.; Wagberg, T.; Zhang, C.D.; Zhou, P. A label-free electrochemical aptasensor for the rapid detection of tetracycline based on ordered mesoporous carbon-Fe3O4. Aust. J. Chem. 2018, 71, 170–176. [Google Scholar] [CrossRef]
- He, B.-S.; Yan, S. Electrochemical aptasensor based on aptamer complimentary strand conjugate and thionine for sensitive detection of tetracycline with multiwalled carbon nanotubes and gold nanoparticles amplification. Anal. Methods 2018, 10, 783–790. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, P.; Xu, J.; Li, L.; Yang, L.; Liu, X.; Liu, S.; Zhou, Y. Electrochemical aptasensor based on a novel flower-like TiO2 nanocomposite for the detection of tetracycline. Sens. Actuators B 2018, 258, 906–912. [Google Scholar] [CrossRef]
- Alawad, A.; Istamboulié, G.; Calas-Blanchard, C.; Noguer, T. A reagentless aptasensor based on intrinsic aptamer redox activity for the detection of tetracycline in water. Sens. Actuators B 2019, 288, 141–146. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Ning, G.; Wu, Y.; Wu, S.; Mao, S.; Liu, G.-Q. An electrochemical strategy for tetracycline detection coupled triple helix aptamer probe with catalyzed hairpin assembly signal amplification. Biosens. Bioelectron. 2019, 143, 111613. [Google Scholar] [CrossRef]
- Mohammad-Razdari, A.; Ghasemi-Varnamkhasti, M.; Rostami, S.; Izadi, Z.; Ensafi, A.A. Magnetic and gold nanocomposite as a novel aptasensor for early detection of tetracycline residues. J. Food Meas. Charact. 2021, 15, 3387–3396. [Google Scholar] [CrossRef]
- Song, J.; Huang, M.; Lin, X.; Li, S.F.Y.; Jiang, N.; Liu, Y.; Guo, H.; Li, Y. Novel Fe-based metal-organic framework (MOF) modified carbon nanofiber as a highly selective and sensitive electrochemical sensor for tetracycline detection. Chem. Eng. J. 2022, 427, 130913. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, K.; Jiang, H.; Zhang, H.; Liu, Q.; You, T. Photoelectrochemical aptasensor for sensitive detection of tetracycline in soil based on CdTe-BiOBr heterojunction: Improved photoactivity enabled by Z-scheme electron transfer pathway. J. Hazard. Mater. 2022, 424, 127498. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lin, X.; Jiang, N.; Huang, M. Carbon-doped WO3 electrochemical aptasensor based on Box-Behnken strategy for highly-sensitive detection of tetracycline. Food Chem. 2022, 367, 130564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Lu, S. Construction of Ce-MOF@COF hybrid nanostructure: Label-free aptasensor for the ultrasensitive detection of oxytetracycline residues in aqueous solution environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, L.; Dong, X.; Yan, X.; Li, M.; Yan, S.; Yan, D. Aptamer-based thin film gold electrode modified with gold nanoparticles and carboxylated multi-walled carbon nanotubes for detecting oxytetracycline in chicken samples. Food Chem. 2019, 300, 125179. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Yan, P.; Ouyang, Q.; Dong, J.; Qian, J.; Chen, J.; Xu, L.; Li, H. Co3O4 nanoparticles/graphitic carbon nitride heterojunction for photoelectrochemical aptasensor of oxytetracycline. Anal. Chim. Acta 2020, 1125, 299–307. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, Z.; Wang, T.; Zhang, M.; Ding, C.; Guo, Y.; Jiang, D.; Wang, K. Ternary Z-scheme heterojunction of Bi SPR-promoted BiVO4/g-C3N4 with effectively boosted photoelectrochemical activity for constructing oxytetracycline aptasensor. Biosens. Bioelectron. 2020, 166, 112453. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Zhu, Q.-Q.; Zhang, H.-W.; Yuan, R.; He, H. A porous organic framework composite embedded with Au nanoparticles: An ultrasensitive electrochemical aptasensor toward detection of oxytetracycline. J. Mater. Chem. C 2020, 8, 14075–14082. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, W.; Guo, Y.; Wang, X.; Zhang, F.; Yu, L.; Guo, C.; Fang, G. Sensitive and selective electrochemical aptasensor via diazonium-coupling reaction for label-free determination of oxytetracycline in milk samples. Sens. Actuators Rep. 2020, 2, 100009. [Google Scholar] [CrossRef]
- Blidar, A.; Hosu, O.; Feier, B.; Ştefan, G.; Bogdan, D.; Cristea, C. Gold-based nanostructured platforms for oxytetracycline detection from milk by a “signal-on” aptasensing approach. Food Chem. 2022, 371, 131127. [Google Scholar] [CrossRef]
- Zhu, Q.-Q.; Zhang, W.-W.; Zhang, H.-W.; Yuan, R.; He, H. Elaborately manufacturing an electrochemical aptasensor based on gold nanoparticle/COF composites for amplified detection performance. J. Mater. Chem. C 2020, 8, 16984–16991. [Google Scholar] [CrossRef]
- Mahmoudpour, M.; Kholafazad-Kordasht, H.; Dolatabadi, J.E.N.; Hasanzadeh, M.; Rad, A.H.; Torbati, M. Sensitive aptasensing of ciprofloxacin residues in raw milk samples using reduced graphene oxide and nanogold-functionalized poly(amidoamine) dendrimer: An innovative apta-platform towards electroanalysis of antibiotics. Anal. Chim. Acta 2021, 1174, 338736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Q.; Zhang, M.; You, F.; Hao, N.; Ding, C.; Wang, K. Simultaneous detection of enrofloxacin and ciprofloxacin in milk using a bias potentials controlling-based photoelectrochemical aptasensor. J. Hazard. Mater. 2021, 416, 125988. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Geng, L.; Wang, Q.; Wang, Y. Photoelectrochemical aptasensing of ofloxacin based on the use of a TiO2 nanotube array co-sensitized with a nanocomposite prepared from polydopamine and Ag2S nanoparticles. Microchim. Acta 2019, 186, 430. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhang, X.; Xue, X.; Xu, Y.; Ding, H.; Yan, T.; Yan, L.; Wei, Q. [Ru(bpy)3]2+@Ce-UiO-66/Mn:Bi2S3 heterojunction and its exceptional photoelectrochemical aptasensing properties for ofloxacin detection. ACS Appl. Bio Mater. 2021, 4, 7186–7194. [Google Scholar] [CrossRef]
- Wang, M.; Hu, M.; Liu, J.; Guo, C.; Peng, D.; Jia, Q.; He, L.; Zhang, Z.; Du, M. Covalent organic framework-based electrochemical aptasensors for the ultrasensitive detection of antibiotics. Biosens. Bioelectron. 2019, 132, 8–16. [Google Scholar] [CrossRef]
- Hu, M.; Liang, G.; Chen, K.; Zhu, L.; Xu, M.; Wang, M.; Li, J.; He, L.; Zhang, Z.; Du, M. Conjugated bimetallic cobalt/iron polyphthalocyanine as an electrochemical aptasensing platform for impedimetric determination of enrofloxacin in diverse environments. Microchim. Acta 2021, 188, 432. [Google Scholar] [CrossRef]
- Li, S.; He, B.; Liang, Y.; Wang, J.; Jiao, Q.; Liu, Y.; Guo, R.; Wei, M.; Jin, H. Sensitive electrochemical aptasensor for determination of sulfaquinoxaline based on AuPd NPs@UiO-66-NH2/CoSe2 and RecJf exonuclease-assisted signal amplification. Anal. Chim. Acta 2021, 1182, 338948. [Google Scholar] [CrossRef]
- He, B.; Li, M. A novel electrochemical aptasensor based on gold electrode decorated Ag@Au core-shell nanoparticles for sulfamethazine determination. Anal. Bioanal. Chem. 2018, 410, 7671–7678. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, C.; Zhu, Y.; Wang, Q.; Geng, L. Signal-on electrochemical aptasensor for sensitive detection of sulfamethazine based on carbon quantum dots/tungsten disulfide nanocomposites. Electrochim. Acta 2021, 393, 139054. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, X.; Guo, Q.; Wang, Y.; Lu, Z.; Ou, H.; Luo, Z.; Lou, X. Second generation of signaling-probe displacement electrochemical aptasensor for detection of picomolar ampicillin and sulfadimethoxine. Sens. Actuators B 2017, 253, 1129–1136. [Google Scholar] [CrossRef]
- Dang, X.; Zhao, H.; Wang, X.; Sailijiang, T.; Chen, S.; Quan, X. Photoelectrochemical aptasensor for sulfadimethoxine using g-C3N4 quantum dots modified with reduced graphene oxide. Microchim. Acta 2018, 185, 345. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Razdari, A.; Ghasemi-Varnamkhasti, M.; Izadi, Z.; Rostami, S.; Ensafi, A.A.; Siadat, M.; Losson, E. Detection of sulfadimethoxine in meat samples using a novel electrochemical biosensor as a rapid analysis method. J. Food Compos. Anal. 2019, 82, 103252. [Google Scholar] [CrossRef]
- Mohammad-Razdari, A.; Ghasemi-Varnamkhasti, M.; Izadi, Z.; Ensafi, A.A.; Rostami, S.; Siadat, M. An impedimetric aptasensor for ultrasensitive detection of Penicillin G based on the use of reduced graphene oxide and gold nanoparticles. Microchim. Acta 2019, 186, 372. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, S.-Q.; Han, Z.-Y.; Tian, X.-H.; Zhang, W.-W.; Li, C.-P.; Du, M. Construction of electrochemical aptasensors with Ag(I) metal−organic frameworks toward high-efficient detection of ultra-trace penicillin. Appl. Surf. Sci. 2020, 531, 147342. [Google Scholar] [CrossRef]
- Yu, Z.; Cui, P.; Xiang, Y.; Li, B.; Han, X.; Shi, W.; Yan, H.; Zhang, G. Developing a fast electrochemical aptasensor method for the quantitative detection of penicillin G residue in milk with high sensitivity and good anti-fouling ability. Microchem. J. 2020, 157, 105077. [Google Scholar] [CrossRef]
- Vafaye, S.E.; Rahman, A.; Safaeian, S.; Adabi, M. An electrochemical aptasensor based on electrospun carbon nanofber mat and gold nanoparticles for the sensitive detection of penicillin in milk. J. Food Meas. Charact. 2021, 15, 876–882. [Google Scholar] [CrossRef]
- Wang, J.; Ma, K.; Yin, H.; Zhou, Y.; Ai, S. Aptamer based voltammetric determination of ampicillin using a single-stranded DNA binding protein and DNA functionalized gold nanoparticles. Microchim. Acta 2018, 185, 68. [Google Scholar] [CrossRef]
- Wang, T.; Yin, H.; Zhang, Y.; Wang, L.; Du, Y.; Zhuge, Y.; Ai, S. Electrochemical aptasensor for ampicillin detection based on the protective effect of aptamer-antibiotic conjugate towards DpnII and Exo III digestion. Talanta 2019, 197, 42–48. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Qu, X.; Li, S.; Zhao, Y.; Liu, S.; Huang, J. Exonuclease III-powered DNA walking machine for label-free and ultrasensitive electrochemical sensing of antibiotic. Sens. Actuators B 2019, 297, 126771. [Google Scholar] [CrossRef]
- Li, F.; Zhu, J.; Li, R.; Liu, Y.; Li, Z.; Kang, H. Magnetic bead-based electrochemical aptasensor doped with multi-wall carbon nanotubes for the detection of ampicillin in milk. Int. J. Electrochem. Sci. 2020, 15, 7520–7530. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, X.; Ren, X.; Lu, Y.; Li, J.; Sun, M.; Yan, L.; Wei, Q.; Ju, H. Fabrication of N-GQDs and AgBiS2 dual-sensitized ZIFs-derived hollow ZnxCo3-xO4 dodecahedron for sensitive photoelectrochemical aptasensing of ampicillin. Sens. Actuators B 2020, 320, 128387. [Google Scholar] [CrossRef]
- Yan, T.; Feng, Y.; Ren, X.; Li, J.; Lu, Y.; Sun, M.; Yan, L.; Wei, Q.; Ju, F. Fabrication of CDs hybrid MIL-68(In) derived In2O3 hollow tubular heterojunction and their exceptional self-powered PEC aptasensing properties for ampicillin detecting. J. Mater. 2021, 7, 721–727. [Google Scholar] [CrossRef]
- Yuan, R.; Yan, Z.; Shaga, A.; He, H. Design and fabrication of an electrochemical sensing platform based on a porous organic polymer for ultrasensitive ampicillin detection. Sens. Actuators B 2021, 327, 128949. [Google Scholar] [CrossRef]
- Hamami, M.; Bouaziz, M.; Raouafi, N.; Bendounan, A.; Korri-Youssouf, H. MoS2/PPy nanocomposite as a transducer for electrochemical aptasensor of ampicillin in river water. Biosensors 2021, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, M.; Jiang, N.; Zheng, S.; Mu, T.; Meng, L.; Liu, Y.; Liu, J.; Chen, G. Ultrasensitive detection of amoxicillin by TiO2-g-C3N4@AuNPs impedimetric aptasensor: Fabrication, optimization, and mechanism. J. Hazard. Mater. 2020, 391, 122024. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, R.; Eissa, S.; Alotaibi, A.; Siddiqua, A.; Alsager, O.A.; Zourob, M. In vitro selection of DNA aptamers and their integration in a competitive voltammetric biosensor for azlocillin determination in waste water. Anal. Chim. Acta 2020, 1101, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yang, J.; Chen, B.; Zeng, S.; Zheng, D.; Chen, Y.; Gao, W. Design of ultrathin nanosheet subunits ZnIn2S4 hollow nanocages with enhanced photoelectric conversion for ultrasensitive photoelectrochemical sensing. Biosens. Bioelectron. 2021, 175, 112873. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, F.; Qin, X.; Wang, Q. Visible light photoelectrochemical aptasensor for chloramphenicol by using a TiO2 nanorod array sensitized with Eu(III) -doped CdS quantum dots. Microchim. Acta 2018, 185, 161. [Google Scholar] [CrossRef]
- Sui, C.; Zhou, Y.; Wang, M.; Yin, H.; Wang, P.; Ai, S. Aptamer-based photoelectrochemical biosensor for antibiotic detection using ferrocene modified DNA as both aptamer and electron donor. Sens. Actuators B 2018, 266, 514–521. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, J.; Wang, L.; Duan, X.; Wang, Y.; Xiang, Y.; Li, G. Sensitive detection of chloramphenicol based on Ag-DNAzyme-mediated signal amplification modulated by DNA/metal ion interaction. Biosens. Bioelectron. 2019, 127, 45–49. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Q.; Geng, L.; Shu, X.; Wang, Y. A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 2019, 197, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Rahmati, Z.; Hoseini, S.J.; Fath, R.H. Impedimetric ultrasensitive detection of chloramphenicol based on aptamer MIP using a glassy carbon electrode modified by 3-ampy-RGO and silver nanoparticle. Colloids Surf. B 2019, 183, 110451. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xia, T.; Bai, W.; Ji, J.; Wang, H.; Huang, Y.; Deng, S.; Ma, K.; Su, Y.; Wan, Y. Handheld aptasensor for sandwiched detection of chloramphenicol. Chem. Res. Chin. Univ. 2020, 36, 291–295. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmati, Z.; Farokhi, S.; Hoseini, S.J.; Fath, R.H. The development of an electrochemical nanoaptasensor to sensing chloramphenicol using a nanocomposite consisting of graphene oxide functionalized with (3-Aminopropyl) triethoxysilane and silver nanoparticles. Mater. Sci. Eng. C 2020, 108, 110388. [Google Scholar] [CrossRef]
- Lu, M.; Cao, C.; Wang, F.; Liu, C. A polyethyleneimine reduced graphene oxide/gold nanocubes based electrochemical aptasensor for chloramphenicol detection using single-stranded DNA-binding protein. Mater. Des. 2021, 199, 109409. [Google Scholar] [CrossRef]
- He, B.; Wang, S. An electrochemical aptasensor based on PEI-C3N4/AuNWs for determination of chloramphenicol via exonuclease-assisted signal amplification. Microchim. Acta 2021, 188, 22. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Zhu, Q.-Q.; Yuan, R.; He, H. Crystal engineering of MOF@COF core-shell composites for ultra-sensitively electrochemical detection. Sens. Actuators B 2021, 329, 129144. [Google Scholar] [CrossRef]
- Wang, S.; He, B.; Liang, Y.; Jin, H.; Wei, M.; Ren, W.; Suo, Z.; Wang, J. Exonuclease III-driven dual-amplified electrochemical aptasensor based on PDDA-Gr/PtPd@Ni-Co hollow nanoboxes for chloramphenicol detection. J. ACS Appl. Mater. Interfaces 2021, 13, 26362–26372. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Wu, M.; Jia, C.; Feng, S.; Zhao, J.; Liang, L. Sensitivity of photoelctrochemical aptasensor using spiral nanorods for detecting antibiotic levels in experimental and real samples. Talanta 2022, 237, 122930. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, D.; Shan, X.; Wang, W.; Xu, F.; Shiigi, H.; Chen, Z. Ternary electrochemiluminescence biosensor based on black phosphorus quantum dots doped perylene derivative and metal organic frameworks as a coreaction accelerator for the detection of chloramphenicol. Microchem. J. 2022, 172, 106927. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 2011, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Liu, B.; Zhang, D.; Zhang, C.; Guo, Y.; Chu, X.; Wang, W.; Wang, H.; Yan, X.; et al. Signal enhancing strategies in aptasensors for the detection of small molecular contaminants by nanomaterials and nucleic acid amplification. Talanta 2022, 236, 122866. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Svendsen, W.E.; Molin, S. Electrochemical detection of pyocyanin as a biomarker for Pseudomonas aeruginosa: A focused review. Sensors 2020, 20, 5218. [Google Scholar] [CrossRef] [PubMed]
| Substance | MRL [µg/kg] | Substance | MRL [µg/kg] |
|---|---|---|---|
| β-lactam antibiotics | Sulfonamide antibiotics | ||
| Penicillin G | 4 | Sulfamethazine | 100 |
| Amoxicillin | 4 | Sulfadiazine | 100 |
| Ampicillin | 4 | Sulfadimethocxine | 100 |
| Oxacillin | 30 | Sulfaquinoxaline | 100 |
| Cloxacilliin | 30 | Sulfapyridine | 100 |
| Dicloxacillin | 30 | Sulfamethoxypyridazine | 100 |
| Nafcillin | 30 | Sulfamerazine | 100 |
| Cephquinome | 20 | Sulfachloropyridazine | 100 |
| Ceftiofur | 100 | Tetracycline antibiotics | |
| Cefazolin | 50 | Tetracycline | 100 |
| Cefacetrile | 125 | Oxytetracycline | 100 |
| Cefaperazone | 50 | Chlorotetracycline | 100 |
| Cephapirin | 60 | Doxycycline | 0 |
| Aminoglycoside antibiotics | |||
| Kanamycin | 0.4 | ||
| Aptamer | Surface Layer | Detection Mode | Real Sample | Dynamic Range, LOD | Ref. |
|---|---|---|---|---|---|
| Kanamycin | |||||
| 5′-TGG TGG GGG TTG AGG CTA AGC CG-3′ | Au electrode covered with auxiliary DNA hairpin | Target-triggered cascade enzymatic recycling couple with DNAzyme amplification initiated by aptamer–analyte interaction. Hemin is used as a redox probe and H2O2 reduction current as a signal measured by DPV | Milk | 1 pM–10 nM, 0.5 pM | [175] |
| 5′-NH2-(CH2)6-TGG GGG TTG AGG CTA AGC CGA C-3′ | Bare GCE | Zr MOF (UiO-66-NH2) saturated with Pb2+ ions as label of auxiliary DNA hybridized with the aptamer on the surface of magnetic beads. Target interaction released it, and Pb2+ was determined via DPV in stripping voltammetry mode | Milk | 0.002 nM–100 nM, 0.16 pM | [176] |
| 5′-TGG GGG TTG AGG CTA AGC CGA-3′-NH2 | Screen-printed graphite electrode modified with aptamer via diazonium salt grafting and carbodiimide binding | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk | 1.2–75 ng/mL, 0.11 ng/mL | [177] |
| 5′-TGG GGG TTG AGG CTA AGC CGA CTC AGA GAT CCA TAT GGA ACC CCC A-3′ | Au electrode with covalently attached hairpin DNA containing aptamer sequence (underlined) | Interaction with analyte triggers strand displacement amplification with hybridization chain reaction, followed by intercalation of the MB molecules, which signal is determined by DPV | Milk | 0.05–200 pM, 36 fM | [178] |
| 5′-TCA GCG GGG AGG AAG AGA TGG GGG TTG AGG CTA AGC CGA GGA GTA-3′ | Au bars modified with hybridized DNA probe and auxiliary DNA sequence initiating recycling hybridization synthesis. Aptamer sequence is underlined | Interaction with analyte-activated, toehold-mediated strand displacement reaction increased the MB quantities accumulated in double-stranded products. DPV signal increased with analyte concentration | Milk | 0.05 pM–50.0 nM, 16.0 fM | [179] |
| 5′-HS-(CH2)6-TGG GGG TTG AGG CTA AGC CGA CCG TAA-3′ | GCE modified with hybridized DNA probe and auxiliary DNA sequence initiating recycling hybridization synthesis with transfer of the MOF-labeled sequences and release of MB. Aptamer sequence is underlined | Interaction with analyte-activated strand displacement reaction resulted in release of MB recorded with SWV | Milk, fish | 0.1 pM–50 nM, 35 fM | [180] |
| 5′-NH2-AGA TGG GGG TTG AGG CTA AGC CGA-3′ | Screen-printed carbon electrodes modified with ordered mesoporous carbon fibers mixed with Au nanoparticles, followed by auxiliary DNA adsorption and their hybridization with CdS particles bearing aptamer | Interaction with analyte resulted in release of CdS containing aggregates, followed by DPV measurement of Cd2+ signal with stripping voltammetry | Milk | 0.1–1000 nM, 87.3 pM | [181] |
| 5′-NH2-AGA TGG GGG TTG AGG CTA AGC CGA-3′ | Screen-printed electrode modified MWCNTs and Au nanoparticles bearing aptamer labeled with CdS nanochains | Interaction with analyte resulted in release of CdS containing aggregates, followed by DPV measurement of Cd2+ signal with stripping voltammetry | Milk | 0.1–100 nM, 74.50 pM | [182] |
| 5′-SH-(CH2)6-TCG GCT TAG CCT CAA CCC CCA-3′ | Au electrode covered with capturing DNA hybridized with biotinylated aptamer and saturated with MB. | Interaction with analyte removes aptamer from the electrode interface; Au nanoparticles modified with streptavidin and HRP are added. DPV measurement of the signal related to the enzymatic MB oxidation after addition of H2O2. | Milk | 2.0 pg/mL–100 ng/mL, 0.88 pg/mL | [183] |
| 5′-ACT TCT CGC AAG ATG GGG GTT GAG GCT AAG CCG AAT ACT CCA GT-Fc-3′ | Au electrode covered with mesoporous carbon-biotinylated Au nanoparticles and streptavidin conjugate of auxiliary DNA | DPV measurement of the Fc signal increased with the analyte concentration due to hybridization of the analyte–aptamer complex with auxiliary DNA on the electrode surface | Milk | 0.1 nM–4 μM, 35.69 pM | [159] |
| 5′-TGG GGG TTG AGG CTA AGC CGA GTC AC-3′ | Au electrode covered with assistant DNA probe partially hybridized with aptamer | Interaction with analyte removes aptamer from the electrode and signaling DNA probe bearing MB is attached. DPV measurement of the MB signal increased with analyte concentration | Human serum, river water, milk | 10 pM–1.0 μM, 3.3 pM | [184] |
| 5′-TGG GGG TTG AGG CTA AGC CGA-3′ | Au electrode modified with partially complementary aptamer and auxiliary DNA bearing MB label | SWV of the MB signal increased after analyte binding and conformational changes in the labeled DNA sequence (DNA folding). Alternatively, labeled DNA sequence displaces aptamer (labeled sequence shift) | Milk, tap water | 10.0 nM–10.0 μM, 3.0 nM (DNA folding) 200.0 pM–1.0 μM, 60.0 pM (labeled sequence shift) | [185] |
| 5′-AGA TGG GGG TTG AGG CTA AGC CGA-3′ | Au electrode modified with binding DNA bonded to the aptamer labeled with thionine saturated Au@Pt core–shell nanoparticles | When aptamer is bonded to analyte, signaling DNA labeled with Au@Pt core–shell nanoparticles and thionine replaced that in the complex; DPV signal of thionine increased with analyte concentration | Chicken | 1 pM–1 μM, 0.16 pM | [186] |
| 5′-AGA TCC TAG GAG GCA CAT GTA AGA GTA GAT GGG GGT TGA GGC TAA GCC GAT AGC TA-3′ | Au bars for accumulation of the apoferritin particles loaded with Pb2+ ions bonded to the specific aptamer | Release of Pb2+ ions in acidic media and their detection by SWV stripping voltammetry | Milk, fish | 0.05 pM–50 nM, 18 fM | [187] |
| 5′-TGG GGG TTG AGG CTA AGC CGA CGC GCG CG-(CH2)6-3′ | Au bars for accumulation of the aptamer–MOF bearing F− ions | Release of F− ions measured potentiometrically with ISE | Milk, fish, urine, blood serum | 1.0–200 nM, 0.35 nM | [188] |
| 3′-NH2-(CH2)6-TCT GGG GGT TGA GGC TAA GCC GAC AG-5′ | GCE covered with Zr containing MOF (UiO-66-NH2), melamine–cyanuric acid COF and MWCNT@rGO COF; DNA–aptamer hybrid is attached to the modifier | SWV of the MB signal reducing after removal of the aptamer–analyte complex from the sensor surface | Fish meat, milk | 25–900 nM, 13 nM | [189] |
| 5′-NH2-(CH2)6-AGA TGG GGG TTG AGG CTA AGC CGA-3′ | Pt electrode covered with Ag nanoparticles and aptamer | ECL signal of luminal H2O2 system decreased with increased analyte concentration due to luminescence quenching by aptamer–analyte complexation | Milk | 0.5–100 ng/mL, 0.06 ng/mL | [190] |
| 5′-TAG CCT TTT TTT GGG GGT TGA GGC TAA GCC GAC-3′ | Au electrode modified with thiolated oligonucleotides involved in binding of auxiliary DNA labeled with MB, which yield is activated by binding target analyte with aptamer sequence of the DNA hairpin (underlined part of the sequence) | DPV signal of the MB accumulated in the surface layer of ds-DNA formed in the bipedal DNA machine activated by aptamer–analyte interaction | Drinking water | 10 fM–100 pM, 7.1 fM | [191] |
| 5′-SH-AGA TGG GGG TTG AGG CTA AGC CGA-3′ | Four-channel, screen-printed carbon electrode modified with rGO and dendritic Au nanostructures with attached thiolated aptamer | Potentiometric signal against electrode covered with polyA oligonucleotide | Milk | 10 pM–1 μM, 5.24 pM | [192] |
| 5′- (COOH)-TGG GGG TTG AGG CTA AGC CGA AAA AAA A-3′ | GCE covered with rGO–Au nanoparticles in chitosan film and the MIP obtained from electropolymerization of 3-aminophenylboronic acid in the presence of the analyte | DPV signal of ferrocene attached to the Au@Fe3O4 particles covered with thiolated cyclodextrin and aptamer. Signal detection after accumulation of the particles on the analyte adsorbed on the MIP layer | Milk, tap water, artesian water, groundwater | 10–500 nM, 1.87 nM | [193] |
| 5′-TGG GGG TTG AGG CTA AGC CGA CCC CCC CCC CCC CCC-3′ | GCE covered with MWCNTs and GO containing adsorbed aptamer. | DPV of the [Fe(CN)6]3−/4− redox probe. | Milk | 0.05 pM–100 nM, 0.0476 pM | [194] |
| 3′-NH2-TGG GGG TTG AGG CTA AGC CGA-C-5′ | GCE covered with carbon black and Calix [4] arene-bearing lactic fragments, aminated aptamer covalently attached via carbodiimide binding | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, yogurt | 0.7–50 nM, 0.3 nM | [195] |
| 5′-AGA TGG GGG TTG AGG CTA AGC CGA-3′ | GCE modified with perylene derivative and aptamer adsorbed on Au@Cu2O heterostructures | ECL signal of S2O82− reduction increased with the analyte concentration due to removal of aptamer from the sensor surface | Milk | 0.1 pM–10 nM, 0.042 pM | [196] |
| 5′-SH-(CH2)6-TGG GGG TTG AGG CTA AGC CGA-3′ | ITO glass modified with CuO/Pd nanocomposite and thiolated aptamer hybridized with auxiliary DNA conjugated with CdS QD | Photoelectrochemical signal recorded after aptamer–analyte interaction and auxiliary DNA removal | Milk | 0.1–500 nM, 20 pM | [197] |
| 5′-Bio-ACC GCG GGG UUG CGG ACC GGG AGC UCC AGC-NH2-3′ | Au electrode modified with Au nanoparticles and CdS-aptamer conjugated via streptavidin–biotin binding | DPV, increase in the ferricyanide peak due to removal of aptamer–analyte complex from the surface | Milk | 1–400 nM; 0.12 nM | [198] |
| 5′-TGG GGG TTG AGG CTA AGC CGA GGA GTA-3′ | Au electrode covered with auxiliary DNA complementary to DNA fragment of a hairpin DNA coupled to the aptamer | DPV signal of HRP reaction with MB and H2O2. Enzyme is coupled to the auxiliary DNA on the electrode after amplification of the aptamer–hairpin DNA structures in the presence of exonuclease EXO-1 | Milk, honey | 0.05 pg/mL–10 ng/mL, 9.1 fg/mL | [199] |
| Tobramycin | |||||
| 5′-HS-GGCA CGAG GUUU AGCU ACAC UCGU GCC-3′ | Graphene-based field-effect transistor with aptamer in the channel area. | Drain current. | Water | 0.3 nM | [200] |
| 5′-AAA AAA GAC TAG GCA CTA GTC AAA AAA CCC CGA TCC TAG TCT TTC CC-3′ | Au electrode modified with signaling DNA. | Analyte initiates multiple recycling via strand displacement DNA polymerization; final product interacts with signaling DNA, and the quantity of hybridized structure is determined with the current of [Ru(NH3)6]3+ redox probe in DPV mode | Milk, water | 10–200 nM, 5.13 nM | [201] |
| 5′-ACU UGG UUU AGG UAA UGA GU-3′ | Au electrode modified with calcinated CeO2/CuOx MOF and aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe. | Human serum, milk | 0.01 pg/ mL–10 ng/ mL, 2.0 fg/ mL | [202] |
| 5′-Bio-GGC ACG AGG UUU AGC UAC ACU CGU GCC NH2-3′ | Au electrode modified with Au nanoparticles and PbS–aptamer conjugated via streptavidin–biotin binding | DPV, increase in the ferricyanide peak due to removal of aptamer–analyte complex from the surface | Milk | 1–10,000 nM, 0.49 nM | [198] |
| 5′-GGG ACT TGG TTT AGG TAA TGA GTC CC-3′ | GCE modified with poly(ethylene imine), Fe MOF, and Au nanoparticles with thiolated binding DNA complementary to the aptamer | Aptamer competitively reacts either with analyte or binding DNA. After that, free binding DNA is hybridized with signaling DNA bearing MB. DPV signal of MB increases with the analyte concentration | Milk | 100 pM–500 nM, 56 pM | [203] |
| 5′-ACU UGG UUU AGG UAA UGA GU-3′ | Au electrode modified with CoNi metal–covalent organic frameworks based on phthalocyanine tetra-amine and phenanthroline derivatives | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, chicken eggs | 0.1 fg/mL–1 pg/mL, 0.07 fg/mL | [204] |
| Streptomycin | |||||
| 5′-GTT TGT GTA TTA CAG TTA TGT TAC CCT CAT TTT TCT GAA-C-3′ | Au electrode covered with thiolated β-cyclodextrin and aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, tap and lake water, bacteria culture medium | 0.01–100 nM, 0.008 nM | [205] |
| 5′-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G-SH-3′ | GCE covered with graphene QDs modified with amino and thiol groups, Au nanoparticles, and Ag nanoparticles with attached aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Human serum | 0.01–812.21 pg/mL, 0.0033 pg/mL | [206] |
| 5′-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G-SH-3′. | GCE covered with thiolated graphene QDs, and Au nanoparticles with attached aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Human serum | 0.1–700 pg/mL, 0.033 pg/mL | [207] |
| 5′-NH2-GGG GTC TGG TGT TCT GCT TTG TTC TGT CGG GTC GT-3′ | Screen-printed carbon electrodes modified with ordered mesoporous carbon fibers mixed with Au nanoparticles, followed by auxiliary DNA adsorption and their hybridization with PbS particles bearing aptamer | Interaction with analyte resulted in release of PbS containing aggregates followed by DPV. measurement of Pb2+ signal with stripping voltammetry | Milk | 0.1–1000 nM, 45.0 pM | [181] |
| 5′-NH2-GGG GTC TGG TGT TCT GCT TTG TTC TGT CGG GTC GT-3′ | Screen-printed electrode modified MWCNTs and Au nanoparticles bearing aptamer labeled with PbS nanochains | Interaction with analyte resulted in release of PbS containing aggregates, followed by DPV measurement of Pb2+ signal with stripping voltammetry | Milk | 0.1–100 nM, 36.45 pM | [182] |
| 5′-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G-SH-3′ | GCE covered with thiourea capped CdS QDs and covalently attached aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, blood serum | 1 fg/mL–1.111 pg/mL, 1.111 pg/mL–11.1111 ng/mL, 0.35 fg/mL | [208] |
| 5′-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC G-SH-3′. | Au electrode modified with mesoporous silica film and aptamer | DPV and EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, blood serum | 1 fg/mL–6.2 ng/mL, 0.33 fg/mL | [209] |
| 5′-GGG GTC TGG TGT TCT GCT TTG TTC TGT CGG GTC GT-3′ | Screen-printed carbon electrode modified with Au nanoparticles with attached auxiliary DNA hybridized with aptamer | Interaction with analyte initiated removal of aptamer from the electrode surface by endonuclease-assisted cleavage. After that, MOF-based biobarcode was attached, and its quantity was determined via DPV signal of [Ru(NH3)6]3+ redox probe | Milk | 0.005–150 ng/mL, 2.6 pg/mL | [210] |
| 5′-SH-TAG GGA ATT CGT CGA CGG ATC CGG GGT CTG GTG TTC TGC TTT GTT CTG TCG GGT CGT CTG CAG GTC GAC GCA TGC GCC-G-3′ | Four-channel screen-printed carbon electrode modified with rGO and dendritic Au nanostructures with attached thiolated aptamer | Potentiometric signal against electrode covered with polyA oligonucleotide | Milk | 10 pM–10 μM, 9.66 pM | [191] |
| Tetracycline | |||||
| 5′-NH2-(CH2)6-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G-3′ | Screen-printed, carbon electrode modified with N-octylpyridinium hexafluorophosphate and Fe3O4 dispersed in chitosan. Aptamer attached to the modifier | Peak currents of the [Fe(CN)6]3−/4− redox probe were measured using cyclic voltammetry | Milk | 1.0 nM–10 mM, 1.0 nM | [211] |
| 5′-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G-3′ | Screen-printed, carbon electrode covered with ordered mesoporous carbon–Fe3O4 composite and physically adsorbed aptamer | DPV signal of thionine as redox probe added to the aptasensor | - | 5 nM–10 μM, 0.8 nM | [212] |
| 5′-HS-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G-3′. | Au electrode modified with the monolayer of thiolated aptamer | MWCNTs with loaded Au nanoparticles were modified with the auxiliary DNA complementary to aptamer and saturated with thionine. In the interaction with analyte, it left the electrode, and DPV signal of thionine decreased with increased analyte concentration | Chicken | 0.1 nM–1 μM, 0.06 nM | [213] |
| 5′-SH-(CH2)6-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G-3′ | GCE modified with the MoS2–TiO2 nanocomposite and aptamer | First, aptasensor was treated with analyte solution. Then, signaling biotinylated DNA interacted with aptamer molecules that were not involved in the reaction with analyte. After that, avidin–HRP conjugate was added, and the enzyme activity quantified by DPV signal of the hydroquinone–benzoquinone redox pair interacted with HRP in the presence of H2O2 | Milk | 0.15 nM–6.0 μM, 0.05 nM | [214] |
| 5′-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G-3′ | Screen-printed, carbon electrode grafted with diazonium salt. Aptamer attached to carboxylic groups by carbodiimide binding | LSV with the [Fe(CN)6]3−/4− redox probe | Lake water | 0.05–20 μg/L 0.035 μg/L | [215] |
| 5′-TCT CTC GGT GGT GTC CTC TCT-3′ | Au electrode covered with thiolated β-cyclodextrin | Triple helix aptamer probes interact with analyte by releasing trigger probe initiating catalytic hybridization of two hairpins bearing Fc units. Afterward, exonuclease III demolished hybridization products and released Fc accumulated in the cyclodextrin moiety. DPV signal of Fc increased with the analyte concentration | Milk | 0.2 nM–100 nM, 0.13 nM | [216] |
| 5′-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT G-3′ and 5′-GTT TGT GTA TTA CAG TTA TGT TAC CCT CAT TTT TCT GAA-C-3′ | Screen-printed Au electrode modified with equimolar mixture of two thiolated aptamers | SWV measurements with the [Fe(CN)6]3−/4− redox probe | Honey | 0.01–1000 ng/mL, 0.0073 ng/mL | [135] |
| 5′-SH-CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG-3′ | Pre-oxidized pencil graphite electrode covered with magnetic nanoparticles with loaded Au layer and attached thiolated aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk of cow, sheep, goat, and water buffalo | 1.0 pM–1.0 μM, 0.03 pM | [217] |
| 5′-SH-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT-G-3′ | Screen-printed carbon electrode covered with carbonized Fe-based MOF, Au nanoparticles with attached thiolated aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Tap water, lake water | 0.1 nM–100 μM, 0.01 nM | [218] |
| 5′-NH2-(CH2)6-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG TCC CAC TGC GCG TGG ATC CGA GCT CCA CGT-G-3′ | ITO electrode covered with CdTe–BiOBr heterojunction, chitosan, and physically adsorbed aptamer | Photocurrent measured at −0.2 V | Soil | 10–1500 pM, 9.25 pM | [219] |
| 5′ -SH-CGT ACG GAA TTC GCT AGC CCC CCG GCA GGC CAC GGC TTG GGT TGG-TCC-CAC-TGC- GCG-TGG ATC CGA GCT CCA CGT-G-3′ | GCE covered with composite of WO3 and MWCNTs followed by electrodeposition of Au nanoparticles and attachment of thiolated aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Tap, pond, and river water, milk, honey, black tea | 0.1–100 nM, 0.048 nM | [220] |
| Oxytetracycline | |||||
| 5′-CGT ACG GAA TTC GCT AGC CGA GGC ACA GTC GCT GGT GCC TAC CTG GTT GCC GTT GTG TGG ATC CGA GCT CCA CGT-G-3′ | Au electrode covered with Ce-based MOF obtained from melamine and cyanuric acid and physically adsorbed aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, urine, river water | 0.1–0.5 ng/ mL, 17.4 fg/ mL | [221] |
| 5′-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCG AGC TCC ACG-TG-3′ | Resistive sensor with semiconductive SWCNT modified with aptamer | Measurement of the resistance shift after hybridization of excessive aptamer remained free after the contact with analyte solution | Real wastewater samples | 10–75 μg/L, 1.125 μg/L | [143] |
| 5′-SH-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCG AGC TCC ACG TG-3′ | Thin-film Au electrode modified with monolayer of thiolated aptamer. | Carboxylated MWCNTs were loaded with Au nanoparticles and thionine and modified with auxiliary DNA hybridized with the aptamer attached to the electrode. Interaction with analyte released MWCNT–DNA composite from the electrode and decreases the thionine current measured by DPV | Chicken | 1 × 10−13–1 × 10−5 g/mL, 3.1 × 10−14 g/mL | [222] |
| 5′-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCC GAG CTC CAC GTG-3′ | ITO glass electrode modified with Co3O4/g-C3N4 heterojunction and aptamer physically adsorbed on the layer | Photocurrent measurement | Water samples | 0.01–500 nM, 3.5 pM | [223] |
| 5′-NH2-CGT ACG GAA TTC GCT AGC GGG CGG GGG TGC TGG GGG AAT GGA GTG CTG CGT GCT GCG GGG ATC CGA GCT CCA CGT-G-3′. | ITO glass electrode modified with Bi/BiVO4/g-C3N4 heterojunction and aptamer attached by glutaraldehyde binding | Photocurrent measurement | Milk | 0.01–1000 nM, 3.3 pM | [224] |
| 5′-NH2-(CH2)6-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCG AGC TCC ACG-TG-3′ | Au electrode modified with porous organic framework synthesized from 1,3,5-tris-bromomethyl-2,4,6-trimethylbenzene and tetraphenylethylene with electrodeposited Au nanoparticles and aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, human serum, urine, river water | 1.0 × 10−5– 1.0 ng/mL, 3.19 fg/mL | [225] |
| 5′-NH2-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCG AGC TCC ACG TG-3′ | GCE grafted with diazonium salt, followed by aptamer attachment by carbodiimide binding | DPV measurements with the [Fe(CN)6]3−/4− redox probe | Milk | 1.0 × 10−9– 1.0 × 10−4 g/mL, 2.29×10−10 g/mL | [226] |
| 5′-Fc-GGA ATT CGC TAG CAC GTT GAC GCT GGT GCC CGG TTG TGG TGC GAG TGT TGT GTG GAT CCG AGC TCC ACG TG-(CH2)6-SH-3′ | Screen-printed carbon electrodes covered with a thin Au layer obtained electrochemically in different modes. Thiolated aptamer with terminal Fc group attached to Au by Au–S bonding | DPV measurement of Fc signal after analyte–aptamer binding and aptamer structure folding | Milk | 50 nM–1.2 μM, 8.7 nM | [227] |
| Ciprofloxacin | |||||
| 5′-ATA CCA GCT TAT TCA ATT GCA GGG TAT CTG AGG CTT GAT CTA CTA AAT GTC GTG GGG CAT TGC TAT TGG CGT TGA TAC GTA CAA TCG TAA TCA GTT AG-3′ | Au electrode covered with the COF based on 1,3,5-tris(4-aminophenyl)benzene and 2,5- dimethoxyterephaldehyde with electrodeposited Au nanoparticles and attached aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | Milk, human serum, river water, urine | 3.02 × 10–5–1.51 nM, 7.06 fM | [228] |
| 5′-NH2-ATA CCA GCT TAT TCA ATT GCA GGG TAT CTG AGG CTT GAT CTA CTA AAT GTC GTG GGG CAT TGC TAT TGG CGT TGA TAC GTA CAA TCG TAA TCA GTT AG-3′ | GCE covered with rGO, aminated dendrimer PAMAM and Au nanoparticles, aptamer reduced graphene oxide | DPV measurements with [Fe(CN)6]3−/4− redox probe | Milk | 1 nM–1 mM, 1 nM | [229] |
| 5′-NH2-ATA-CCA-GCT-TAT- TCA-ATT-GCA-GGG-TAT-CTG-AGG-CTT-GAT-CTA-CTA-AAT-GTC-GTG-GGG-CAT-TGC-TAT-TGG-CGT-TGA-TAC-GTA-CAA-TCG-TAA-TCA-GTT-AG-3′ | ITO glass electrode modified with rGO doped with Bi3+/black anatase TiO2 and adsorbed aptamer | Anodic photocurrent | Milk | 0.01–1000 ng/mL, 3.3 pg/mL | [230] |
| Ofloxacin | |||||
| 5′-SH-ATA CCA GCT TAT TCA ATT AGT TGT GTA TTG AGG TTT GAT CTA GGC ATA GTC AAC AGA GCA CGA TCG ATC TGG CTT GTT CTA CAA TCG TAA TCA GTT AG-3′. | Ti foils anodized and covered with Ag2S and self-polymerized polydopamine film. Aptamer immobilized via physical adsorption | Photocurrent measurement | Milk | 5.0 pM–100 nM, 0.75 pM | [231] |
| 5′-NH2-(CH2)6-ATA CCA GCT TAT TCA ATT AGT TGT GTA TTG AGG TTT GAT CTA GGC ATA GTC AAC AGA GCA CGA TCG ATC TGG CTT GTT CTA CAA TCG TAA TCA GTT AG-3′. | ITO glass electrode modified with [Ru(bpy)3]2+@Ce-UiO-66/Mn:Bi2S3 heterojunction followed by immobilization of aptamer in chitosan matrix by glutaraldehyde linking | [Ru(bpy)3]2+ photocatalytic current | Tap water, lake water | 0.01−100 nM, 6 pM | [232] |
| Enrofloxacin | |||||
| 5′-CCC ATC AGG GGG CTA GGC TAA CAC GGT TCG GCT CTC TGA GCC CGG GTT ATT TCA GGG GGA-3′ | Au electrode modified with the COF based on 1,3,6,8-tetrakis(4-formylphenyl)pyrene and melamine, aptamer physically adsorbed on the surface | EIS measurements with [Fe(CN)6]3−/4− redox probe | Human serum | 0.01 pg/mL–2 ng/mL, 6.07 fg/mL | [233] |
| 5′-CCC ATC AGG GGG CTA GGC TAA CAC GGT TCG GCT CTC TGA GCC CGG GTT ATT TCA GGG GGA-3′ | Au electrode modified with Co/Fe polyphtalocyanine and physically adsorbed aptamer | EIS measurements with [Fe(CN)6]3−/4− redox probe | River water, milk, pork | 0.1 fg/mL–100 pg/mL, 0.06 fg/mL | [234] |
| 5′-NH2-CCC ATC AGG GGG CTA GGC TAA CAC GGT TCG GCT CTC TGA GCC CGG GTT ATT TCA GGG GGA-3′ | ITO glass electrode modified with rGO doped with Bi3+/black anatase TiO2 and adsorbed aptamer | Anodic photocurrent | Milk | 0.01–10,000 ng/mL, 3.3 pg/mL | [230] |
| Sulfaquinoxaline | |||||
| 5′-GTA ACC CTG CCA CAT CCA ACC CCC ATG TTG GCT CTT AC-3′ | Au electrode modified with poly(ethylene imine)–CoSe2 and Zr-based MOF UiO-66–NH2 with chemically synthesized Au–Pd nanoparticles. On them, thiolated auxiliary DNA is covalently attached and hybridized with the aptamer | Interaction with analyte results in release of the aptamer from the electrode interface. Treatment with the RecJf exonuclease reduced the length of auxiliary DNA. After that, DPV signal of [Fe(CN)6]3−/4− redox probe was measured | Pork | 1 pg/mL–100 ng/mL, 0.547 pg/mL | [235] |
| Sulfamethazine | |||||
| 5′-SH-TTA GCT TAT GCG TTG GCC GGG ATA AGG ATC CAG CCG TTG TAG ATT TGC GTT CTA ACT CTC-Fc-3′ | Au electrode modified with Ag@Au core–shell nanoparticles and thiolated aptamer | DPV signal of Fc label | Pork | 0.1–50 ng/mL, 0.033 ng/mL | [236] |
| 5′-NH2-TTA GCT TAT GCG TTG GCC GGG ATA AGG ATC CAG CCG TTG TAG ATT TGC GTT CTA ACT CTC-3′ | GCE covered with WS2–carbon QDs, followed by aptamer adsorption | After interaction with the analyte, poly(ethylene imine) was added and the current of the [Ru(NH3)6]3+ redox probe was measured in DPV mode | Pork | 10 pM–1.0 µM, 4.0 pM | [237] |
| Sulfadimethoxine | |||||
| 5′-GAG GGC AAC GAG TGT TTA TAG-3′ | Au electrode covered with thiolated capturing DNA partially hybridized with aptamer | Interaction with analyte removed aptamer–analyte complex from the electrode interface. SWV signal of the [Fe(CN)6]3−/4− redox probe | Lake water | 1 nM–1 mM, 1 nM | [238] |
| 5′-GAG GGC AAC GAG TGT TTA TAG A-3′ | ITO glass Electrode modified with GO, g-C3N4 QDs, and aptamer | Photocurrent measurements | Tap, lake, and wastewater | 0.5–80 nM, 0.1 nM | [239] |
| 5′-SH-GAG GGC AAC GAG TGT TTA TAG A-3′ | Pencil graphite electrode modified with rGO, electrodeposited Au nanoparticles, and thiolated aptamer | EIS signal of the [Fe(CN)6]3−/4− redox probe | Fish, chicken, beef | 1.0 fM–10 μM, 0.37 fM | [240] |
| Penicillin | |||||
| 5′-SH-TTA GTT GGG GTT CAG TTG G-3′ | Pencil graphite electrode modified with rGO and electrodeposited Au nanoparticles with thiolated aptamer | EIS signal of the [Fe(CN)6]3−/4− redox probe | Milk of cow, sheep, goat and water buffalo | 1.0 fM–10 μM, 0.8 fM | [241] |
| 5′-NH2-CTG AAT TGG ATC TCT CTT CTT GAG CGA TCT CCA CA-3′ | Au electrode covered with Ag-based COF with immobilized aptamer | EIS signal of the [Fe(CN)6]3−/4− redox probe | Milk | 0.001–0.5 ng/mL, 0.849 pg/mL | [242] |
| 5′-GGG TCT GAG GAG TGC GCG GTG CCA GTG AGT-3′ | Au electrode bearing thiolated aptamer with terminal MB group | SWV signal of the MB as redox label | Buffer Milk | 0.3 nM 1.7 nM | [243] |
| 5′-SH-(CH2)6-CTG AAT TGG ATC TCT CTT CTT GAG CGA TCT CCA CA-3′ | Electrospun carbon nanofiber mat covered with Au nanoparticles and attached thiolated aptamer | LSV signal of the [Fe(CN)6]3−/4− redox probe | Milk | 1–400 ng/mL, 0.6 ng/mL | [244] |
| Ampicillin | |||||
| 5′-SH-TGG GGG TTG AGG CTA AGC CGA C-3 | GCE covered Au nanoparticles and aptamer bonded via Au–S interaction | Auxiliary DNA complementary to aptamer is first attached to the Au nanoparticles and then involved in the reaction with ssDNA binding protein that blocked the electrode surface. In the reaction with analyte, blocking particles are released and the DPV signal of the [Fe(CN)6]3− ions increased | Milk | 1 pM–5 nM, 0.38 pM | [245] |
| 5′-TTA GTT GGG GTT CAG TTG G-3′ | Au electrode modified with the COF based on 1,3,6,8-tetrakis(4-formylphenyl)pyrene and melamine, aptamer physically adsorbed on the surface | EIS signal of the [Fe(CN)6]3−/4− redox probe | Human serum | 0.01 pg/mL–2 ng/mL, 6.07 fg/mL | [233] |
| 5′-NH2-(CH2)6-TAG CTA TCG GCT TAG CCT CAA CCC CCA TCT ACT CTT ACA TGT GCC TCC TAG GAT CT-3′ | Au bars for accumulation of the apoferritin particles loaded with Cd2+ ions bonded to the specific aptamer | Release of Cd2+ ions in acidic media and their detection by SWV stripping voltammetry | Milk, fish | 0.05 pM–50 nM, 15 fM | [187] |
| 5′-TTG ATC GCG GGC GGT TGT ATA GCG G-3′ | GCE covered with the monolayer of auxiliary DNA complementary to the aptamer | Aptamer is first mixed with the analyte and then added to the electrode. In the absence of the analyte, hybrid part of the layer is digested by exonuclease. In the opposite case, DNA monolayer is protected. The removal of DNA from the surface is determined by DPV signal of the [Fe(CN)6]3−/4− redox probe | Milk, water | 0.1–100 nM, 0.032 nM | [246] |
| HS-TTT TTT TTG TGG GTA GGG GGG GGT TGG GGC GGC C-3′ | Au electrode covered with DNA walker-locking probe and DNA track via Au–S binding | DNA walker and locking probe form the duplex protected from exonuclease. Enzymatic amplification is triggered by the analyte specifically bonded to aptamer (underlined part of the sequence). As a result of the cycle, hemin binding part of the sequence interacts with them and biocatalytic reaction of H2O2 reduction is detected in DPV mode | Milk | 1 pM to 10 nM, 0.76 pM | [247] |
| 5′–Bio–GCG GGC GGT TGT ATA GCG G–3′ | Magnetic GCE | Magnetic beads modified with ampicillin are involved in the reaction with biotinylated aptamer. After reaction, HRP–streptavidin conjugate is attached and HRP detected by linear scan voltammetry using hydroquinone/benzoquinone pair | Milk | 0.1 pM–10 nM, 0.1 pM | [248] |
| 5′-NH2-(CH2)6-TTT TGC GGG CGG TTG TAT AGC GG-3′ | ITO glass electrode modified with N-doped graphene QDs and AgBiS2 dual-sensitized Zn/Co bimetallic oxide dodecahedron. Aptamer covalently attached by carbodiimide binding | Photocurrent measurements | Tap and lake waters | 0.5 pM–10 nM, 0.25 pM | [249] |
| 5′-NH2-(CH2)6-TTT TGC GGG CGG TTG TAT AGC GG-3′ | ITO glass electrode covered with In2O3-In2S suspension followed by immobilization of aptamer in chitosan matrix by glutaraldehyde binding | Photocurrent measurements | Lake water | 0.001–300 ng/mL, 0.06 pg/mL | [250] |
| 5′-TTA GTT GGG GTT CAG TTG G-3′ | Au electrode covered with porous organic polymer prepared by the coupled polymerization of tetraphenylpyrene and dibromo-p-xylene | EIS signal of the [Fe(CN)6]3−/4− redox probe | Milk, river water, human serum | 1 × 10–5–5 ng/mL, 1.33 × 10–6 ng/mL | [251] |
| 5′-NH2-(CH2)6-GCG GGC GGT TGT ATA GCG G-3′ | Screen-printed carbon electrode modified with MoS2 nanospheres covered with electro-synthesized polypyrrole. Then, ethylene diamine was electrochemically attached to the polypyrrole and then 1,4-naphthoquinone and aptamer were covalently attached by carbodiimide binding | SWV signal of the naphtaquinone redox label | River water | LOD 0.28 pM | [252] |
| Amoxicillin | |||||
| 5′- SH-TTA GTT GGG GTT CAG TTG G-3′ | GCE modified with TiO2-g-C3N4@AuNPs and thiolated aptamer | EIS signal of the [Fe(CN)6]3−/4− redox probe | Wastewater | 0.5–3 nM, 0.2 nM | [253] |
| Azlocillin | |||||
| 5′-CAG GAA GAC AAC TCC GAC TAG AAT TGA TAA TCA AGA ATT CGT CTG GGG GGA ATG TGC G-3′ | Au electrode covered with cysteine monolayer and covalently attached aptamer | DPV signal of the [Fe(CN)6]3−/4− redox probe | Tap and wastewaters | 1 pg/mL–100 mg/mL, 1.2 pg/mL | [254] |
| Lincomycin | |||||
| 5′-NH2-(CH2)6-CGC GTG ATG TGG TCG ATG CGA TAC GGT GAG TCG CGC CAC GGC TAC ACA CGT CTC AGC GA-3′ | ITO glass electrode modified with TiO2 and hollow ZnIn2S4 nanocages | Photocurrent measurements | Milk | 0.1 pM–0.1 nM, 0.084 pM | [255] |
| Chloramphenicol | |||||
| 5′-ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | FTO electrode modified with TiO2 nanorods, CdS:Eu3+ QDs, and aptamer | Photocurrent measurements | Milk | 1.0 pM–3.0 nM, 0.36 pM | [256] |
| 5′-AGC AGC ACA GAG GTC AGA TGC ACT CGG ACC CCA TTC TCC TTC CAT CCC TCA TCC GTC CAC CCT ATG CGT GCT ACC GTG AA-Fc-3′ | ITO glass electrode covered with g-C3N4 nanosheets and aptamer | Photocurrent measurements | Water | 1 pM–100 nM, 0.22 pM | [257] |
| 5′-ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | Au electrode with hairpin DNA immobilized via terminal SH group | Analyte interacts with aptamer, which structure is stabilized by Ag+ ions. Their release activates DNAzyme that catalyzed removal of the DNA hairpin from the surface. This deceased charge transfer resistance measured by SWV with [Fe(CN)6]3−/4− redox probe | Milk | 1 pg/ mL–40 ng/ mL, 0.4 pg/mL | [258] |
| 5′-ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | Anodized Ti foil covered with Au QDs aptamer | Photocurrent measurements | Honey | 0.5–100 nM, 57.9 pM | [259] |
| 5′-NH2-ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | GCE covered with 3-amino ethylpyridine and Ag nanoparticles with thiolated aptamer. After saturation with analyte, resorcinol was polymerized on the surface to obtain MIPs. | Interaction of the MIP polymer with analyte was assessed with EIS signal of the [Fe(CN)6]3−/4− redox probe | Milk | 1.0 pM–1.0 nM, 0.3 pM | [260] |
| 5′-SH-ACT TCA GTG AGT TGT CCC ACG GTC GGC-3′ | Screen-printed, carbon electrode modified with Au and hairpin aptamer | The reaction with analyte results in conformational changes followed by the binding of signaling biotinylated aptamer and finally streptavidin–HRP conjugate. LSV/amperometric signal of tetramethylbenzidine as HRP substrate in the presence of H2O2 | - | 10 nM–10 μM, 1 nM | [261] |
| 5′-NH2-ACT TCA GAG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | GCE covered with GO modified with 3-aminopropyltriethoxysilan followed by deposition of chemically synthesized Ag nanoparticles and thiolated aptamer bonded via AG–S interaction | DPV signal of the [Fe(CN)6]3−/4− redox probe | Milk, honey | 1–100 ng/mL, 0.70 ng/mL | [262] |
| 5′-SH (CH2)6-ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | Au electrode covered with poly(ethylene imine)–rGO composite and electrodeposited Au nanoparticles with attached thiolated aptamer | EIS signal of the [Fe(CN)6]3−/4− redox probe enhanced by binding of specific protein to free aptamer molecules | Chicken | 5 pM–1 μM, 2.08 pM | [263] |
| 5′-TGT GTA CTT CAG TGA GTT GTC CCA CGG TCG GCG AGT CGG TGG TAG-3′ | Au electrode covered with composite of Au nanowires and g-C3N4 in PEI matrix followed by immobilization of thiolated auxiliary DNA and hybridization with an aptamer | Interaction with analyte removed aptamer from the duplex with auxiliary DNA. After that, Recjf exonuclease cleaves DNA strands, and [Fe(CN)6]3−/4− DPV signal is measured | Milk | 100 fM–1 μM, 2.96 fM | [264] |
| 5′--NH2-(CH2)6-ACC TGG GGG AGT ATT GCG GAG GAA GGT-3′ | Au electrode covered with UiO-66–NH2@COF particles based on 2-aminoterephthalic acid, 1,3,5-tris (4-aminophenyl)benzene, and 2,5-dimethoxy terephaldehyde followed by aptamer physical adsorption | EIS signal of the [Fe(CN)6]3−/4− redox probe | Milk, river water, human serum, urine | 31 fM–15.5 nM, 20 fM | [265] |
| 5′-AGC AGC ACA GAG GTC AGA TGA CTT CAG TGA GTT GTC CCA CGG TCG GCG AGT CGG TGG TAG CCT ATG CGT GCT ACC GTG AA-3′ | Au electrode modified with PDDA–graphene sheets and PtPd@Ni–Co MOF particles and covalently attached to capturing DNA | Aptamer–auxiliary DNA helix interacts with analyte and released auxiliary DNA into exonuclease III amplification cycle with hairpin DNA to form trigger DNA involved in the reaction with capturing DNA on the electrode interface. After that, signaling DNA bearing UiO-66 MOFs with MB molecules is attached and the MB signal measured in DPV mode | Honey | 10 fM−10 nM, 0.985 fM | [266] |
| 5′--SH-(CH2)6-AGC AGC ACA GAG GTC AGA TGC ACT CGG ACC CCA TTC TCC TTC CAT CCC TCA TCC GTC CAC CCT ATG CGT GCT ACC GTG AA-3′ | FTO electrode modified with MoS2 nanoarray | Photocurrent measurements | Milk | 0.1 pM–1 μM, 0.1 pM | [267] |
| 5′-ACT TCA GTG AGT TGT CCC ACG GTC GGC GAG TCG GTG GTA G-3′ | GCE modified with black phosphorus–Co-Ni/MOF–perylene derivative composite and physically adsorbed aptamer | ECL signal of persulfate cathodic reduction | Tap water | 0.1 pM–1.0 μM, 0.29 fM | [268] |
| Method | Specificity | Special Requirements | Detection Time | Cost |
|---|---|---|---|---|
| Microbiological | Low | Specialized bacteriological laboratory | Minimum 3 h | Modest |
| HPLC | High | Organic solvents | <1 h | High |
| GC | High | Sample should be transformed into gaseous phase | <1 h | High |
| MS | High | Sample should be transformed into ionized state | <1 h | High |
| MS–HPLC | High | Organic solvent, transformation of the sample into ionized state. | <1 h | High |
| MS–GC | High | Sample should be transformed into ionized state | <1 h | High |
| ELISA | High | Sandwich assay using expensive antibodies | <1 h | High |
| Electrochemical aptasensors | High | Antifouling surfaces for minimizing interferences with food matrix | <1 h | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evtugyn, G.; Porfireva, A.; Tsekenis, G.; Oravczova, V.; Hianik, T. Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors 2022, 22, 3684. https://doi.org/10.3390/s22103684
Evtugyn G, Porfireva A, Tsekenis G, Oravczova V, Hianik T. Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors. 2022; 22(10):3684. https://doi.org/10.3390/s22103684
Chicago/Turabian StyleEvtugyn, Gennady, Anna Porfireva, George Tsekenis, Veronika Oravczova, and Tibor Hianik. 2022. "Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety" Sensors 22, no. 10: 3684. https://doi.org/10.3390/s22103684
APA StyleEvtugyn, G., Porfireva, A., Tsekenis, G., Oravczova, V., & Hianik, T. (2022). Electrochemical Aptasensors for Antibiotics Detection: Recent Achievements and Applications for Monitoring Food Safety. Sensors, 22(10), 3684. https://doi.org/10.3390/s22103684








