Using an ATR-FTIR Technique to Detect Pathogens in Patients with Urinary Tract Infections: A Pilot Study
Abstract
:1. Introduction
2. Methods
2.1. Clinical Setting and Urine Culture
2.2. Measurements of Urine Samples with the ATR-FTIR Technique
2.3. Reagents
3. Results and Discussion
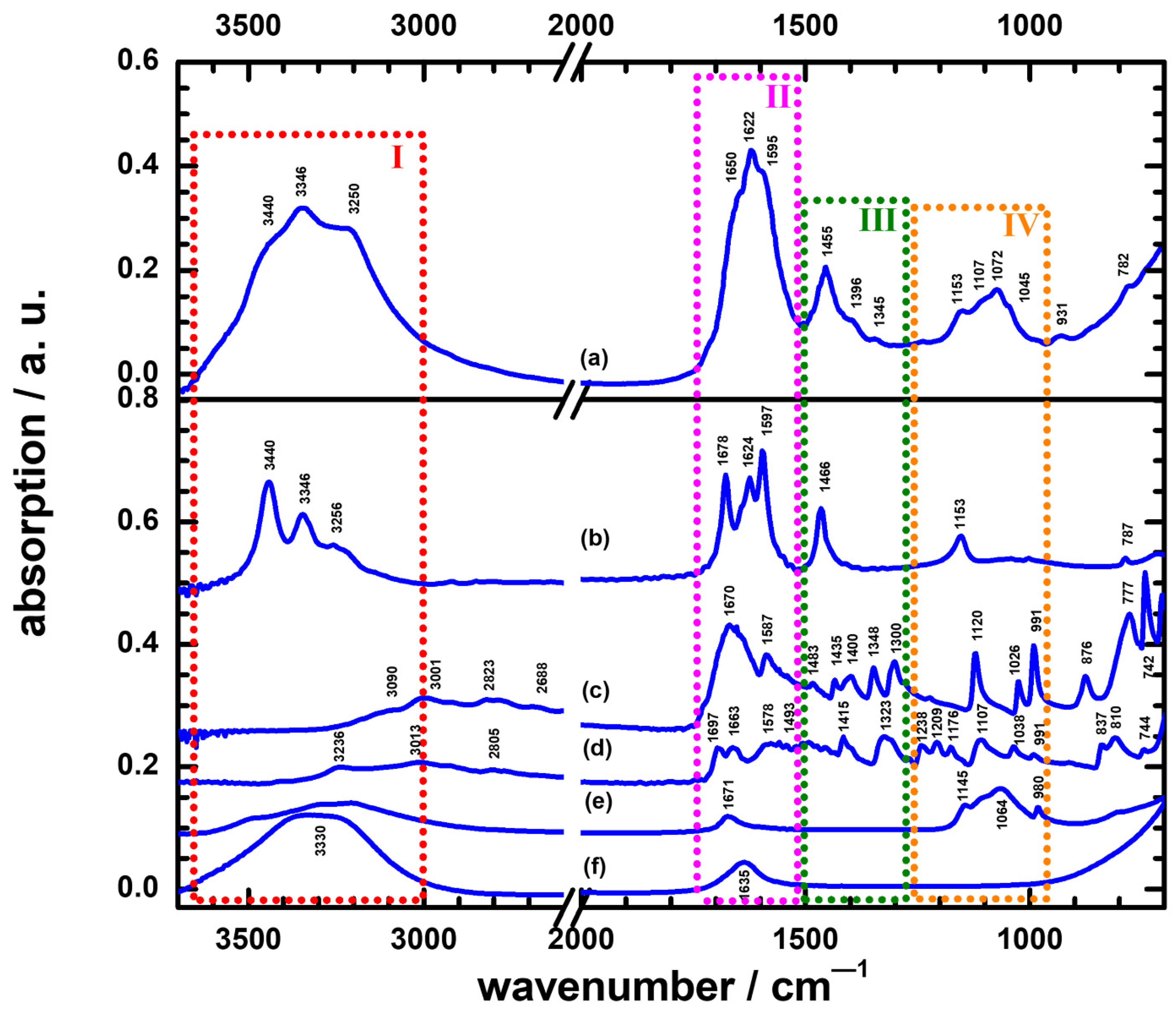
3.1. The Culture Result and ATR-FTIR Spectra of Urine
3.2. The Difference between the UTI and Non-UTI Groups in ATR-FTIR Spectra
3.3. The Characterization Method in ATR-FTIR Spectra to Discriminate Non-UTIs and UTIs with Yeast Species
3.4. The Characterization Method in ATR-FTIR Spectra to Discriminate Different Pathogens in UTI Groups
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. Open Forum Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.; Mach, K.E.; Shortliffe, L.M.D.; Banaei, N.; Wang, T.H.; Liao, J.C. New and developing diagnostic technologies for urinary tract infection. Nat. Rev. Urol. 2017, 14, 296–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simerville, J.A.; Maxted, W.C.; Pahira, J.J. Urinalysis: A comprehensive review. Am. Fam. Phys. 2005, 71, 1153–1162. [Google Scholar]
- Navarro, D.F.; Sullivan, F.; Azcoaga-Lorenzo, A.; Santiago, V.H. Point-of-care tests for urinary tract infections: Protocol for a systematic review and meta-analysis of diagnostic test accuracy. BMJ Open 2020, 10, e033424. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Gehani, M.; Kammili, N.; Bhardwaj, P.; Nag, V.; Devara, S.M.; Sharad, S. Clinical validation of innovative optical-sensor-based, low-cost, rapid diagnostic test to reduce antimicrobial resistance. J. Clin. Med. 2019, 8, 2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotsilkov, K.; Popova, C.; Boyanova, L.; Setchanova, L.; Mitov, I. Comparison of culture method and real-time PCR for detection of putative periodontopathogenic bacteria in deep periodontal pockets. Biotechnol. Equip. 2015, 29, 996–1022. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Jiang, Y.; Shi, B.; Wu, D.; Wu, W. Low-cost battery-powered and user-friendly real-time quantitative PCR system for the detection of multigene. Micromachines 2020, 11, 435. [Google Scholar] [CrossRef] [Green Version]
- Bangaoil, R.; Santillan, A.; Angeles, L.M.; Abanilla, L.; Lim, A., Jr.; Ramos, M.C.; Fellizar, A.; Guevarra, L., Jr.; Albano, P.M. ATR-FTIR spectroscopy as adjunct method to the microscopic examination of hematoxylin and eosin-stained tissues in diagnosing lung cancer. PLoS ONE 2020, 15, e0233626. [Google Scholar] [CrossRef]
- Sala, A.; Anderson, D.J.; Brennan, P.M.; Butler, H.J.; Cameron, J.M.; Jenkinson, M.D.; Rinaldi, C.; Theakstone, A.G.; Baker, M.J. Biofluid diagnostics by FTIR spectroscopy: A platform technology for cancer detection. Cancer Lett. 2020, 477, 122–130. [Google Scholar] [CrossRef]
- Ho, C.S.; Jean, N.; Hogan, C.A.; Blackmon, L.; Jeffrey, S.S.; Holodniy, M.; Banaei, N.; Saleh, A.A.E.; Ermon, S.; Dionne, J. Rapid identification of pathogenic bacteria using Raman spectroscopy and deep learning. Nat. Commun. 2019, 10, 4927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, S.; Hong, W.; Wang, P. A Review of Raman-Based Technologies for Bacterial Identification and Antimicrobial Susceptibility Testing. Photonics 2022, 9, 133. [Google Scholar] [CrossRef]
- Rousseau, A.N.; Faure, N.; Rol, F.; Sedaghat, Z.; Le Galudec, J.; Mallard, F.; Josso, Q. Fast Antibiotic Susceptibility Testing via Raman Microspectrometry on Single Bacteria: An MRSA Case Study. ACS Omega 2021, 6, 16273–16279. [Google Scholar] [CrossRef] [PubMed]
- Ciloglu, F.U.; Caliskan, A.; Saridag, A.M.; Kilic, I.H.; Tokmakci, M.; Kahraman, M.; Aydin, O. Drug-resistant Staphylococcus aureus bacteria detection by combining surface-enhanced Raman spectroscopy (SERS) and deep learning techniques. Sci. Rep. 2021, 11, 18444. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. MALDI-TOF MS for the Diagnosis of Infectious Diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Gai, Z.; Saleh, L.; Tang, J.; Gui, T.; Kullak-Ublick, G.A.; Wang, J. Thermoplasmonic-Assisted Cyclic Cleavage Amplification for Self-Validating Plasmonic Detection of SARS-CoV-2. ACS Nano 2021, 15, 7536–7546. [Google Scholar] [CrossRef]
- Gür, S.D.; Bakhshpour, M.; Denizli, A. Selective detection of Escherichia coli caused UTIs with surface imprinted plasmonic nanoscale sensor. Mater. Sci. Eng. C 2019, 104, 109869. [Google Scholar] [CrossRef]
- Farraia, M.V.; Rufo, J.C.; Paciência, I.; Mendes, F.; Delgado, L.; Moreira, A. The electronic nose technology in clinical diagnosis: A systematic review. Porto Biomed. J. 2019, 4, e42. [Google Scholar] [CrossRef]
- Flores-Gonzalez, J.; Cancino-Díaz, J.C.; Chavez-Galan, L. Flow Cytometry: From Experimental Design to Its Application in the Diagnosis and Monitoring of Respiratory Diseases. Int. J. Mol. Sci. 2020, 21, 8830. [Google Scholar] [CrossRef]
- Stobiecka, M.; Dworakowska, B.; Jakiela, S.; Lukasiak, A.; Chalupa, A.; Zembrzycki, K. Sensing of survivin mRNA in malignant astrocytes using graphene oxide nanocarrier-supported oligonucleotide molecular beacons. Sens. Actuators B Chem. 2016, 235, 136–145. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Hairpin-Hairpin Molecular Beacon Interactions for Detection of Survivin mRNA in Malignant SW480 Cells. ACS Appl. Mater. Interfaces 2018, 10, 17028–17039. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2020, 229, 115463. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Ratajczak, K.; Jakiela, S. Toward early cancer detection: Focus on biosensing systems and biosensors for an anti-apoptotic protein survivin and survivin mRNA. Biosens. Bioelectron. 2019, 137, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.D.; Morais, C.L.M.; Lima, K.M.G. ATR-FTIR spectroscopy for virus identification: A powerful alternative. Biomed. Spectrosc. Imaging 2020, 9, 103–118. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, A.; Awasthi, K.K.; Awasthi, K.; Awasthi, A. Biosensors for diagnosis of urinary tract infections: Advances and future challenges. Mater. Lett. X 2021, 10, 100077. [Google Scholar] [CrossRef]
- Qiu, G.; Ng, S.P.; Lawrence Wu, C.M. Differential phase-detecting localized surface plasmon resonance sensor with self-assembly gold nano-islands. Opt. Lett. 2015, 40, 1924–1927. [Google Scholar] [CrossRef]
- Heise, H.M.; Voigt, G.; Lampen, P.; Küpper, L.; Rudloff, S.; Werner, G. Multivariate calibration for the determination of analytes in urine using mid-infrared attenuated total reflection spectroscopy. Appl. Spectrosc. 2001, 55, 434–443. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, treatment, and prevention of catheter-associated urinary tract infection. Top. Spinal Cord Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Yang, S.-P.; Chen, Y.-Y.; Hsu, H.-S.; Wang, F.-D.; Chen, L.-Y.; Fung, C.-P. A risk factor analysis of healthcare-associated fungal infections in an intensive care unit: A retrospective cohort study. BMC Infect. Dis. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenbeke, M.; Bruyne, S.D.; Boelens, J.; Oyaert, M.; Glorieux, G.; Biesen, W.V.; Linjala, J.; Delanghe, J.R.; Speeckaert, M.M. Exploring the possibilities of infrared spectroscopy for urine sediment examination and detection of pathogenic bacteria in urinary tract infections. Clin. Chem. Lab. Med. 2020, 58, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-L.; Liu, W.-F.; Tseng, H.-Y.; Yeh, Y.-T.; Tseng, W.-T.; Chou, Y.-Y.; Huang, X.-R.; Hsu, H.-C.; Ho, L.-I.; Pan, S.-W. Quantitative determination of leukocyte esterase with a paper-based device. RSC Adv. 2020, 10, 27042–27049. [Google Scholar] [CrossRef] [PubMed]
- Cheesbrough, M. District Laboratory Practice in Tropical Countries, Part 2; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Takamura, A.; Watanabe, K.; Akutsu, T.; Ozawa, T. Soft and robust identification of body fluid using Fourier transform infrared spectroscopy and chemometric strategies for forensic analysis. Sci. Rep. 2018, 8, 8459. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E. Catheter-related urinary tract infection. Drugs Aging 2005, 22, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Ou, T.Y.; Teng, S.O.; Chen, F.-L.; Hsieh, T.C.; Lee, W.S. Hospital-acquired urinary tract infections in patients with diabetes and urinary catheterization. J. Exp. Clin. Med. 2014, 6, 90–93. [Google Scholar] [CrossRef]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Blahovec, J.; Hejlová, A.; Čopίková, J. Yeast ß(1-3),(1-6)-D-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Salman, A.; Tsror, L.; Pomerantz, A.; Moreh, R.; Mordechai, R.; Huleihel, M. FTIR spectroscopy for detection and identification of fungal phytopathogenes. Spectroscopy 2010, 24, 261–267. [Google Scholar] [CrossRef]
- Nitosetein, T.; Wongwattanakul, M.; Chonanant, C.; Leelayuwat, C.; Charoensri, N.; Jearanaikoon, P.; Lulitanond, A.; Wood, B.R.; Tippayawat, P.; Heraud, P. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy combined with chemometric modelling for the classification of clinically relevant Enterococci. J. Appl. Microbiol. 2021, 130, 982–993. [Google Scholar] [CrossRef]
- Sharaha, U.; Rodriguez-Diaz, E.; Sagi, O.; Riesenberg, K.; Salman, A.; Bigio, I.J.; Huleihel, M. Fast and reliable determination of Escherichia coli susceptibility to antibiotics: Infrared microscopy in tandem with machine learning algorithms. J. Biophotonics 2019, 12, e201800478. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, M.S.; Lee, Y.-L.; Jie, E.Y.; Kim, S.-G.; Kim, S.W. Rapid tool for identification of bacterial strains using Fourier transform infrared spectroscopy on genomic DNA. J. Appl. Microbiol. 2018, 126, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Soler-Arango, J.; Figoli, C.; Muraca, G.; Bosch, A.; Brelles-Mariño, G. The Pseudomonas aeruginosa biofilm matrix and cells are drastically impacted by gas discharge plasma treatment: A comprehensive model explaining plasmamediated biofilm eradication. PLoS ONE 2019, 14, e0216817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWhirter, M.J.; McQuillan, A.J.; Bremer, P.J. Influence of ionic strength and pH on the first 60 min of Pseudomonas aeruginosa attachment to ZnSe and to TiO2 monitored by ATR-IR spectroscopy. Colloids Surf. B 2002, 26, 365–372. [Google Scholar] [CrossRef]
- Martak, D.; Valot, B.; Sauget, M.; Cholley, P.; Thouverez, M.; Bertrand, X.; Hocquet, D. Fourier-Transform InfraRed Spectroscopy Can Quickly Type Gram-Negative Bacilli Responsible for Hospital Outbreaks. Front. Microbiol. 2019, 10, 1440. [Google Scholar] [CrossRef] [Green Version]


| Diagnosis | Group | Culture Result | Type |
|---|---|---|---|
| Non-UTI (n = 11) | 1 | No growth (n = 11) | |
| UTI (n = 7) | 2 | E. coli (n = 1) | GN bacterium |
| 3 | Yeast (n = 4) | Fungi | |
| 4 | P. aeruginosa (n = 1) | GN bacterium | |
| 5 | E. faecium (n =1) | GP bacterium |
| Rα:Rβ:Rγ (a) | ΔRα:ΔRβ:ΔRγ | New Bands/cm−1 | WBC/HPF (b) | |
|---|---|---|---|---|
| IRav/non | 100:45:30 | 0:0:0 | - | <5 |
| No. 1 (E. faec.) | 100:33:28 | 0:−12:−2 | 1771, 1396, 1310 | 6–10 |
| No. 2 (yeast) | 100:35:21 | 0:−10:−9 | 1772, 1398 | 20–29 |
| No. 3 (yeast) | 100:33:30 | 0:−12:0 | 1771, 1405 | 20–29 |
| No. 4 (yeast) | 100:37:28 | 0:−8:−2 | 1774, 1541, 1398, 1312 | >100 |
| No. 5 (E. coli) | 100:36:31 | 0:−9:+1 | 1396 | 20–29 |
| No. 6 (yeast) | 100:44:53 | 0:−1:+23 | 1033, 992 | 50–99 |
| No. 7 (P. aerug.) | 100:53:35 | 0:+8:+5 | 1772, 1397, 1311 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.-W.; Lu, H.-C.; Lo, J.-I.; Ho, L.-I.; Tseng, T.-R.; Ho, M.-L.; Cheng, B.-M. Using an ATR-FTIR Technique to Detect Pathogens in Patients with Urinary Tract Infections: A Pilot Study. Sensors 2022, 22, 3638. https://doi.org/10.3390/s22103638
Pan S-W, Lu H-C, Lo J-I, Ho L-I, Tseng T-R, Ho M-L, Cheng B-M. Using an ATR-FTIR Technique to Detect Pathogens in Patients with Urinary Tract Infections: A Pilot Study. Sensors. 2022; 22(10):3638. https://doi.org/10.3390/s22103638
Chicago/Turabian StylePan, Sheng-Wei, Hsiao-Chi Lu, Jen-Iu Lo, Li-Ing Ho, Ton-Rong Tseng, Mei-Lin Ho, and Bing-Ming Cheng. 2022. "Using an ATR-FTIR Technique to Detect Pathogens in Patients with Urinary Tract Infections: A Pilot Study" Sensors 22, no. 10: 3638. https://doi.org/10.3390/s22103638
APA StylePan, S.-W., Lu, H.-C., Lo, J.-I., Ho, L.-I., Tseng, T.-R., Ho, M.-L., & Cheng, B.-M. (2022). Using an ATR-FTIR Technique to Detect Pathogens in Patients with Urinary Tract Infections: A Pilot Study. Sensors, 22(10), 3638. https://doi.org/10.3390/s22103638