Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Yeast Preparation and Modification
2.3. Yeast Cell Viability Assays
2.4. Atomic-Force-Microscopy-Based Measurements
2.5. Electrochemical Measurements
2.6. Calculations
3. Results and Discussion
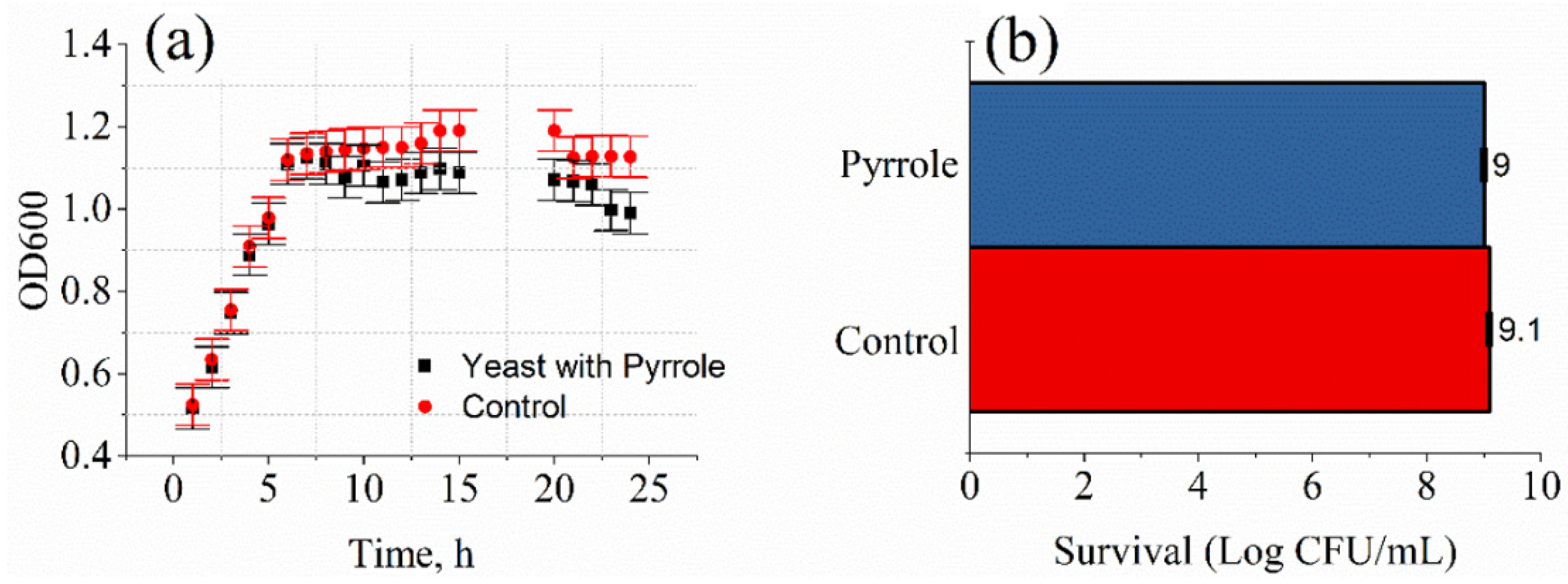
3.1. Evaluation of Yeast Cell Viability
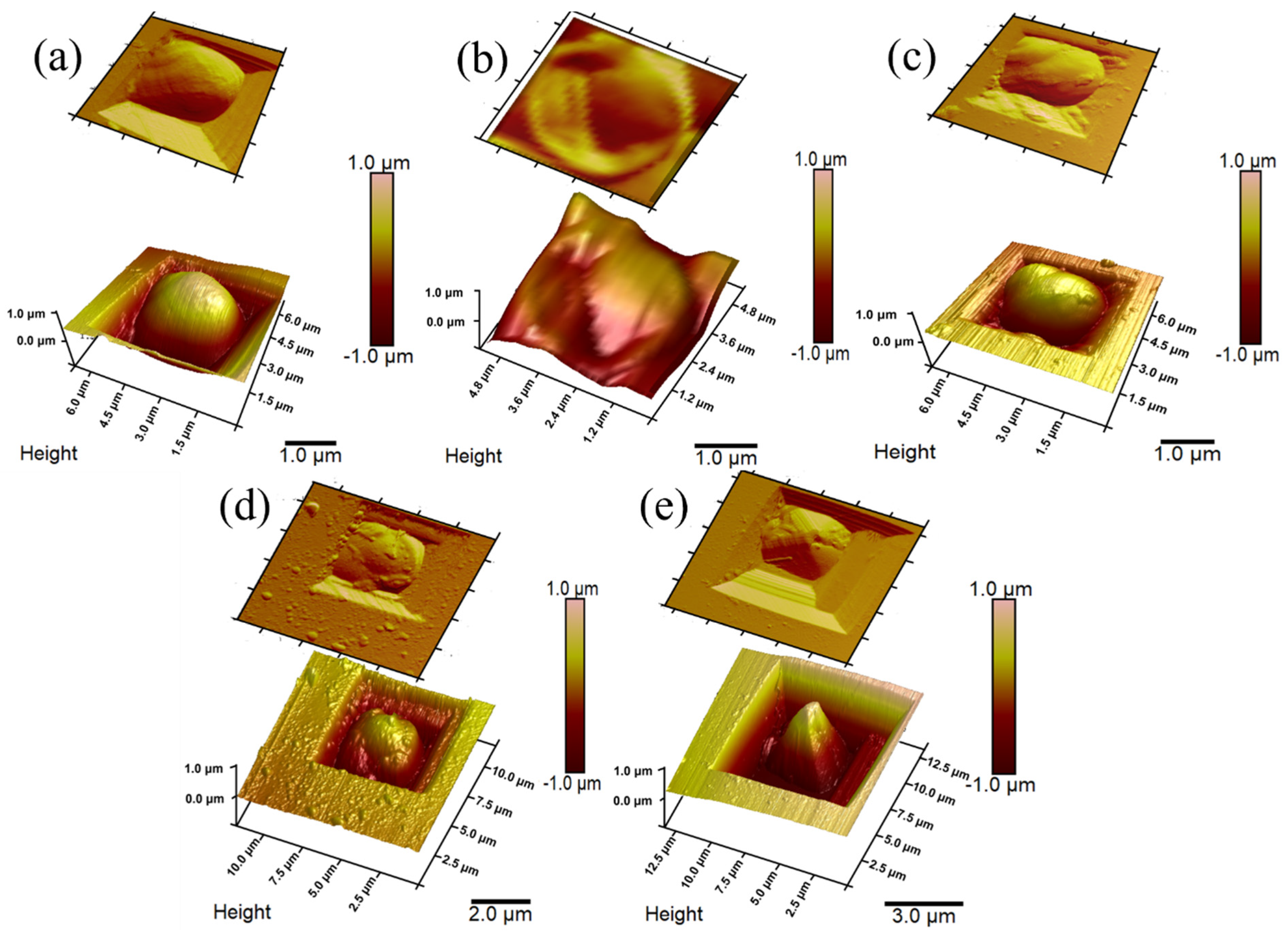
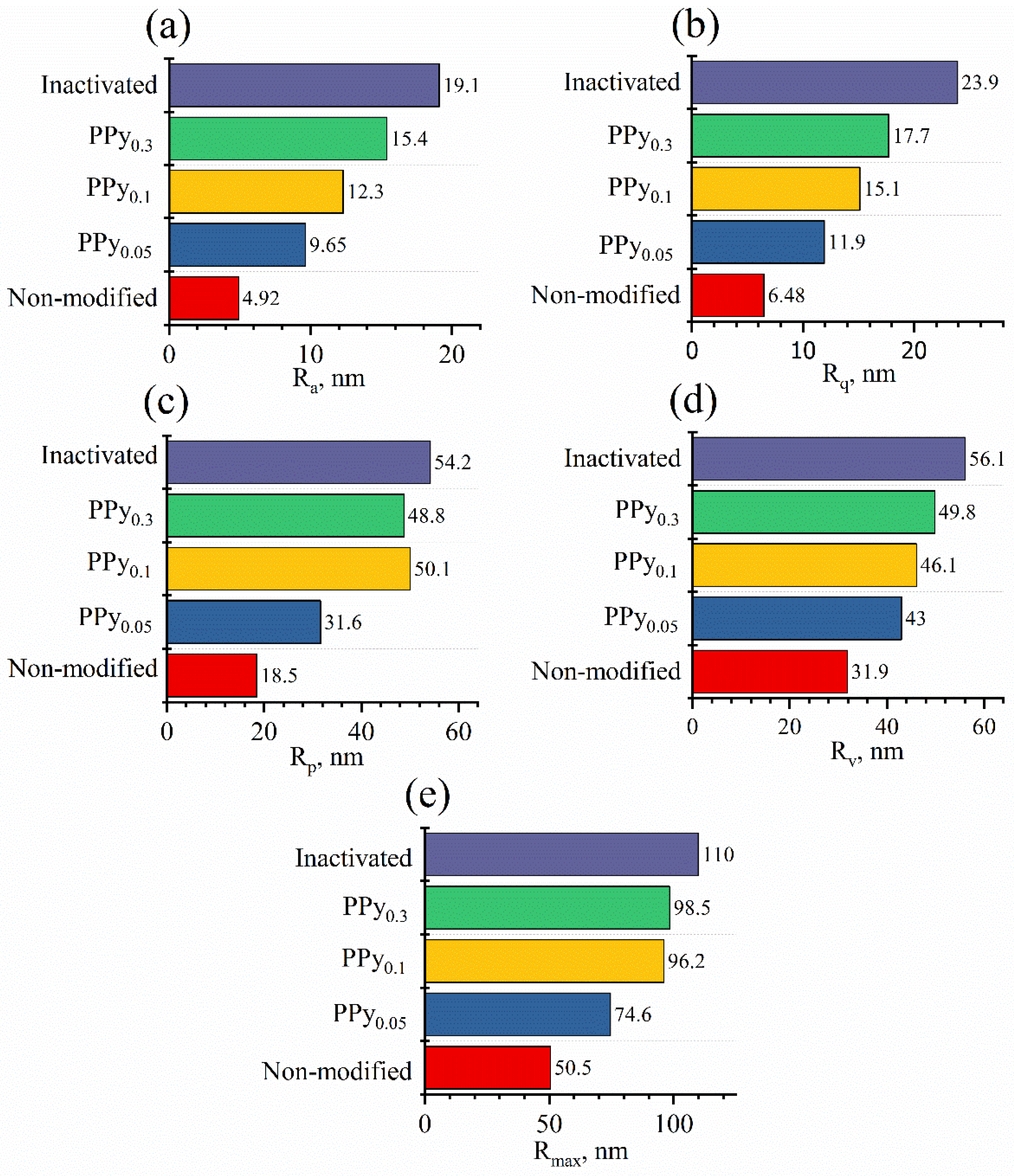
3.2. Evaluation of Yeast Cells’ Morphological Properties and Surface
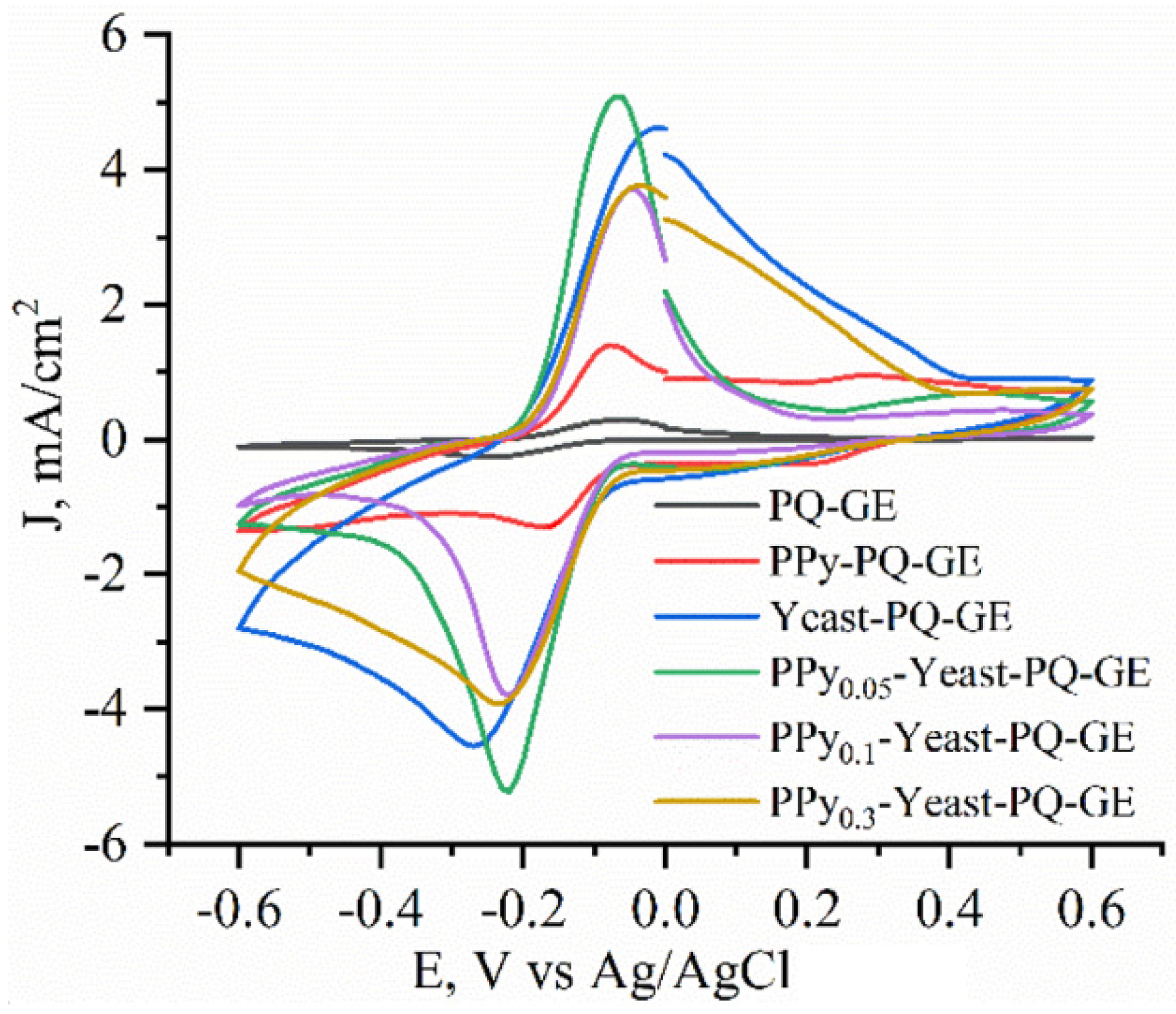
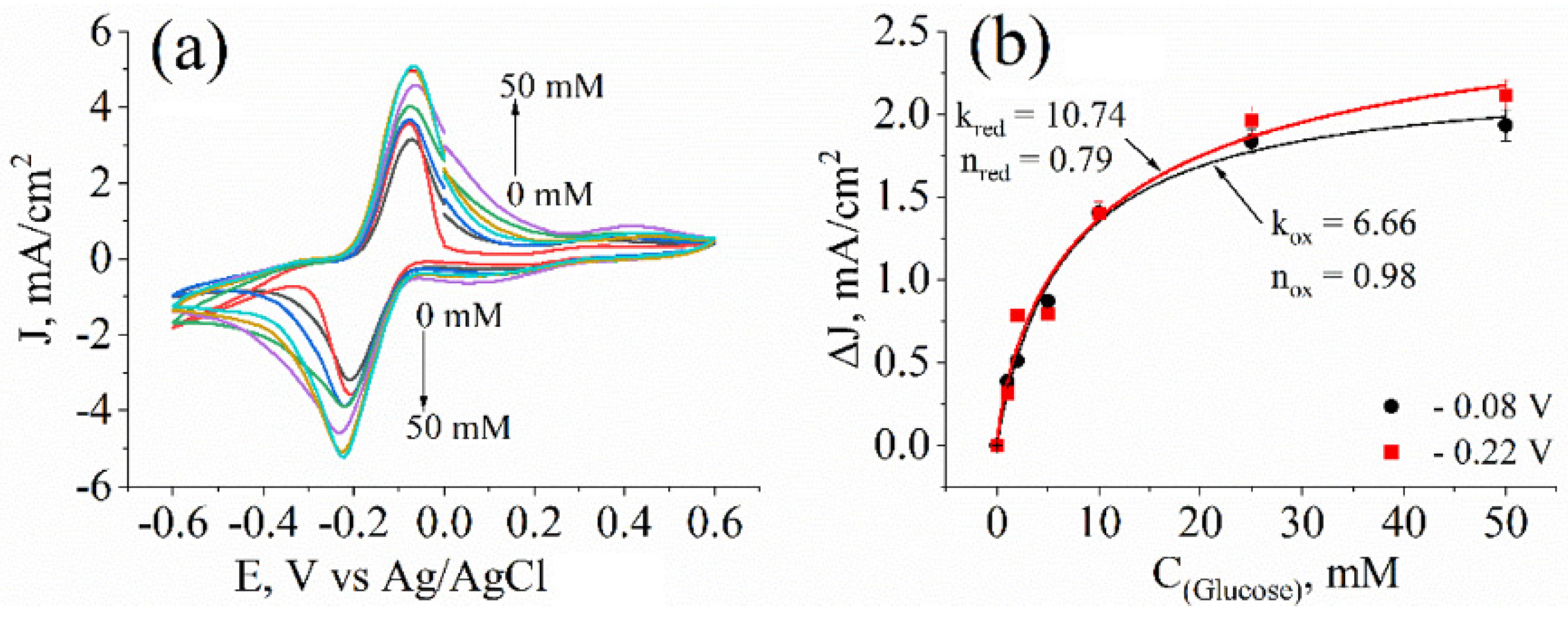
3.3. Evaluation of Yeast Cells’ Metabolic Activity by Cyclic Voltammetry
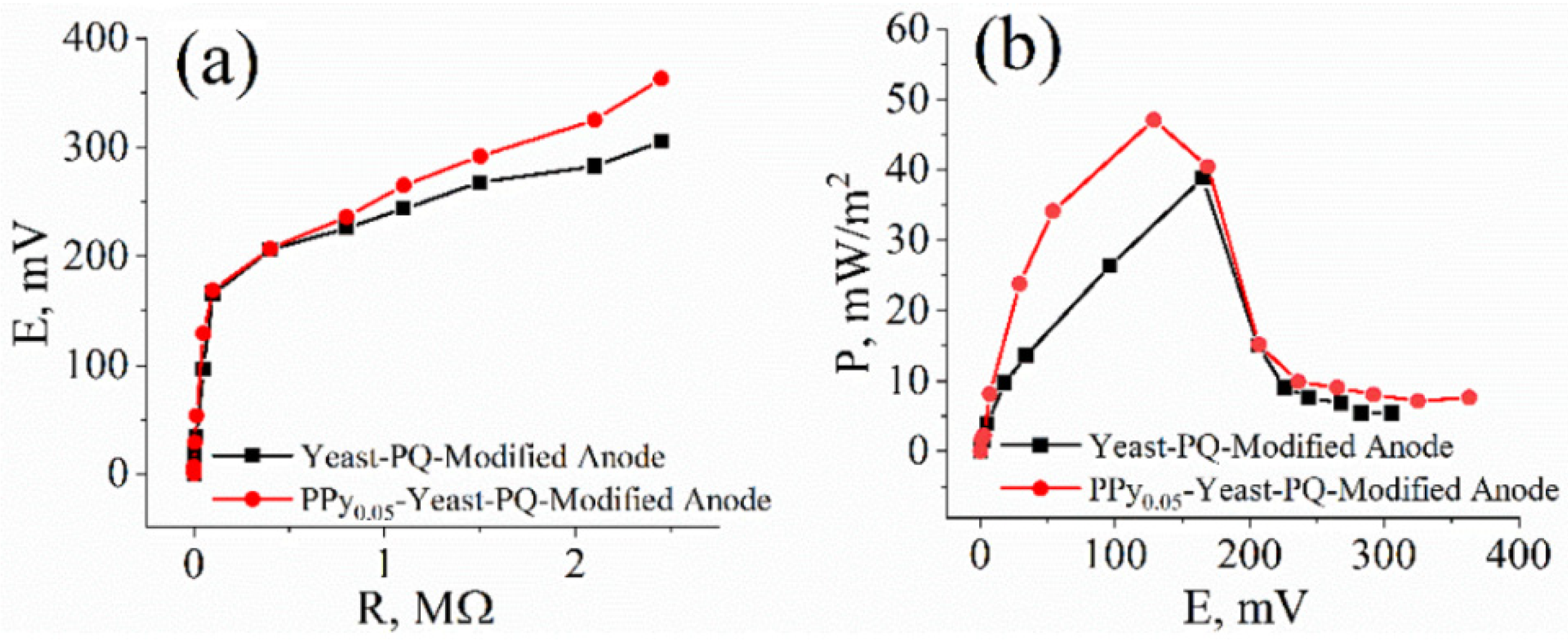
3.4. Assessment of Microbial Fuel Cell Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Andriukonis, E.; Celiesiute-Germaniene, R.; Ramanavicius, S.; Viter, R.; Ramanavicius, A. From Microorganism-Based Amperometric Biosensors towards Microbial Fuel Cells. Sensors 2021, 21, 2442. [Google Scholar] [CrossRef] [PubMed]
- Chansaenpak, K.; Kamkaew, A.; Lisnund, S.; Prachai, P.; Ratwirunkit, P.; Jingpho, T.; Blay, V.; Pinyou, P. Development of a Sensitive Self-Powered Glucose Biosensor Based on an Enzymatic Biofuel Cell. Biosensors 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Morkvenaite-Vilkonciene, I.; Kisieliute, A.; Petroniene, J.; Ramanaviciene, A. Scanning electrochemical microscopy based evaluation of influence of pH on bioelectrochemical activity of yeast cells—Saccharomyces cerevisiae. Colloids Surf. B Biointerfaces 2017, 149, 1–6. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Heydorn, R.L.; Engel, C.; Krull, R.; Dohnt, K. Strategies for the Targeted Improvement of Anodic Electron Transfer in Microbial Fuel Cells. ChemBioEng Rev. 2019, 7, 4–17. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Zhang, Y.; Lv, P.; Feng, Q.; Wei, Q. Encapsulation of enzyme by metal-organic framework for single-enzymatic biofuel cell-based self-powered biosensor. Nano Energy 2020, 68, 104308. [Google Scholar] [CrossRef]
- Beylier, M.R.; Balaguer, M.; Colprim, J.; Pellicer-Nàcher, C.; Ni, B.-J.; Smets, B.; Sun, S.-P.; Wang, R.-C. Biological Nitrogen Removal from Domestic Wastewater. Compr. Biotechnol. 2011, 6, 329–340. [Google Scholar]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Feng, Q.; Wang, Q.; Li, G.; Li, D.; Wei, Q. Biosynthesis of Bacterial Cellulose/Carboxylic Multi-Walled Carbon Nanotubes for Enzymatic Biofuel Cell Application. Materials 2016, 9, 183. [Google Scholar] [CrossRef] [Green Version]
- Duarte, K.D.; Frattini, D.; Kwon, Y. High performance yeast-based microbial fuel cells by surfactant-mediated gold nanoparticles grown atop a carbon felt anode. Appl. Energy 2019, 256, 113912. [Google Scholar] [CrossRef]
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.; Mohan, S.V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour. Technol. 2011, 102, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Duarte, K.D.; Kwon, Y. In situ carbon felt anode modification via codeveloping Saccharomyces cerevisiae living-template titanium dioxide nanoclusters in a yeast-based microbial fuel cell. J. Power Sources 2020, 474, 228651. [Google Scholar] [CrossRef]
- Sarmin, S.; Tarek, M.; Roopan, S.M.; Cheng, C.K.; Khan, M.R. Significant improvement of power generation through effective substrate-inoculum interaction mechanism in microbial fuel cell. J. Power Sources 2020, 484, 229285. [Google Scholar] [CrossRef]
- Murakami, C.; Kaeberlein, M. Quantifying Yeast Chronological Life Span by Outgrowth of Aged Cells. J. Vis. Exp. 2009, e1156. [Google Scholar] [CrossRef] [Green Version]
- Nasheuer, H.-P.; Smith, R.; Bauerschmidt, C.; Grosse, F.; Weisshart, K. Initiation of eukaryotic DNA replication: Regulation and mechanisms. Prog. Nucleic Acid Res. Mol. Biol. 2002, 72, 41–94. [Google Scholar] [CrossRef]
- Van der Rest, M.E.; Kamminga, A.H.; Nakano, A.; Anraku, Y.; Poolman, B.; Konings, W.I.L.N. The Plasma Membrane of Saccharomyces cerevisiae: Structure, Function, and Biogenesis. Microbiol. Rev. 1995, 59, 304–322. [Google Scholar] [CrossRef]
- Rozene, J.; Morkvenaite-Vilkonciene, I.; Bruzaite, I.; Zinovicius, A.; Ramanavicius, A. Baker’s Yeast-Based Microbial Fuel Cell Mediated by 2-Methyl-1,4-Naphthoquinone. Membranes 2021, 11, 182. [Google Scholar] [CrossRef]
- Rozene, J.; Morkvenaite-Vilkonciene, I.; Bruzaite, I.; Dzedzickis, A.; Ramanavicius, A. Yeast-based microbial biofuel cell mediated by 9,10-phenantrenequinone. Electrochim. Acta 2021, 373, 137918. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—polypyrrole—for the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, R.-M.; Carac, G.; Bahrim, G.; Ramanaviciene, A.; Ramanavicius, A. Modification of Aspergillus niger by conducting polymer, Polypyrrole, and the evaluation of electrochemical properties of modified cells. Bioelectrochemistry 2018, 121, 46–55. [Google Scholar] [CrossRef]
- Gniadek, M.; Wichowska, A.; Antos-Bielska, M.; Orlowski, P.; Krzyzowska, M.; Donten, M. Synthesis and characterization of polypyrrole and its composites coatings on flexible surface and its antibacterial properties. Synth. Met. 2020, 266, 116430. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrolein vitro andin vivo. J. Biomed. Mater. Res. 2003, 68, 411–422. [Google Scholar] [CrossRef]
- Ferraz, N.; Strømme, M.; Fellström, B.; Pradhan, S.; Nyholm, L.; Mihranyan, A. In vitro and in vivo toxicity of rinsed and aged nanocellulose-polypyrrole composites. J. Biomed. Mater. Res. Part A 2012, 100A, 2128–2138. [Google Scholar] [CrossRef]
- Zha, Z.; Deng, Z.; Li, Y.; Li, C.; Wang, J.; Wang, S.; Qu, E.; Dai, Z. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, J.; Bagchi, R.; Howe, J.; Lian, K. Redox Active Organic-Carbon Composites for Capacitive Electrodes: A Review. Sustain. Chem. 2021, 2, 407–440. [Google Scholar] [CrossRef]
- Arlyapov, V.; Kharkova, A.; Kurbanaliyeva, S.; Kuznetsova, L.; Machulin, A.; Tarasov, S.; Melnikov, P.; Ponamoreva, O.; Alferov, V.; Reshetilov, A. Use of biocompatible redox-active polymers based on carbon nanotubes and modified organic matrices for development of a highly sensitive BOD biosensor. Enzym. Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Daigaku, O.; Daigaku, O. Covalent functionalization of carbon materials with redox-active. Nanoscale 2020, 13, 36–50. [Google Scholar]
- Kisieliute, A.; Popov, A.; Apetrei, R.-M.; Cârâc, G.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Ramanavicius, A. Towards microbial biofuel cells: Improvement of charge transfer by self-modification of microoganisms with conducting polymer—Polypyrrole. Chem. Eng. J. 2019, 356, 1014–1021. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of polypyrrole within the cell wall of yeast by redox-cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzym. Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Makdasi, E.; Laskar, O.; Milrot, E.; Schuster, O.; Shmaya, S.; Yitzhaki, S. Whole-Cell Multiparameter Assay for Ricin and Abrin Activity-Based Digital Holographic Microscopy. Toxins 2019, 11, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibayrenc, P.; Preziosi-Belloy, L.; Roger, J.-M.; Ghommidh, C. Assessing yeast viability from cell size measurements? J. Biotechnol. 2010, 149, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef] [PubMed]
- Andriukonis, E.; Stirke, A.; Garbaras, A.; Mikoliunaite, L.; Ramanaviciene, A.; Remeikis, V.; Thornton, B.; Ramanavicius, A. Yeast-assisted synthesis of polypyrrole: Quantification and influence on the mechanical properties of the cell wall. Colloids Surf. B Biointerfaces 2018, 164, 224–231. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Processing Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Qi, Q.; Li, T.; Scott, P.; Jiang, X. A Correlational Study of Areal Surface Texture Parameters on Some Typical Machined Surfaces. Procedia CIRP 2015, 27, 149–154. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, R.R.L.; Albuquerque, D.A.C.; Cruz, T.G.S.; Yamaji, F.M.; Leite, F.L. Measurement of the Nanoscale Roughness by Atomic Force Microscopy: Basic Principles and Applications. In Atomic Force Microscopy—Imaging, Measuring and Manipulating Surfaces at the Atomic Scale; Bellitto, V., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Viktor, P. Astakhov Surface Integrity in Machining; Davim, J.P., Ed.; Springer: London, UK, 2010; ISBN 9781848828735. [Google Scholar]
- Mardiana, U.; Innocent, C.; Jarrar, H.; Cretin, M.; Buchari; Gandasasmita, S. Electropolymerized neutral red as redox mediator for yeast fuel cell. Int. J. Electrochem. Sci. 2015, 10, 8886–8898. [Google Scholar]
- Sumisha, A.; Haribabu, K. Modification of graphite felt using nano polypyrrole and polythiophene for microbial fuel cell applications-a comparative study. Int. J. Hydrogen Energy 2018, 43, 3308–3316. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinovicius, A.; Rozene, J.; Merkelis, T.; Bruzaite, I.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells. Sensors 2022, 22, 327. https://doi.org/10.3390/s22010327
Zinovicius A, Rozene J, Merkelis T, Bruzaite I, Ramanavicius A, Morkvenaite-Vilkonciene I. Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells. Sensors. 2022; 22(1):327. https://doi.org/10.3390/s22010327
Chicago/Turabian StyleZinovicius, Antanas, Juste Rozene, Timas Merkelis, Ingrida Bruzaite, Arunas Ramanavicius, and Inga Morkvenaite-Vilkonciene. 2022. "Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells" Sensors 22, no. 1: 327. https://doi.org/10.3390/s22010327
APA StyleZinovicius, A., Rozene, J., Merkelis, T., Bruzaite, I., Ramanavicius, A., & Morkvenaite-Vilkonciene, I. (2022). Evaluation of a Yeast–Polypyrrole Biocomposite Used in Microbial Fuel Cells. Sensors, 22(1), 327. https://doi.org/10.3390/s22010327








