Multiparameter Monitoring with a Wearable Cardioverter Defibrillator
Abstract
:1. Introduction
2. The Wearable Cardioverter Defibrillator (WCD)
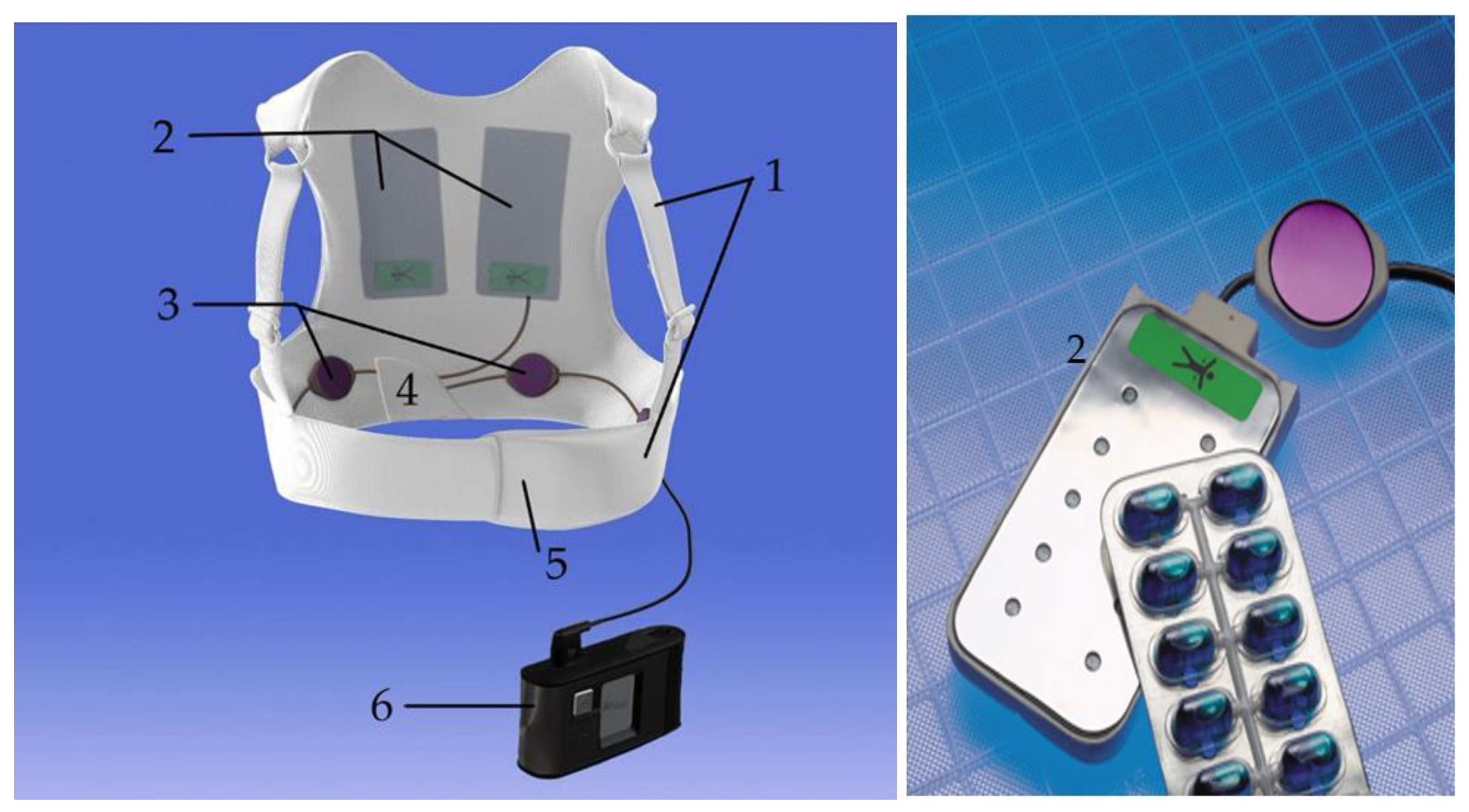
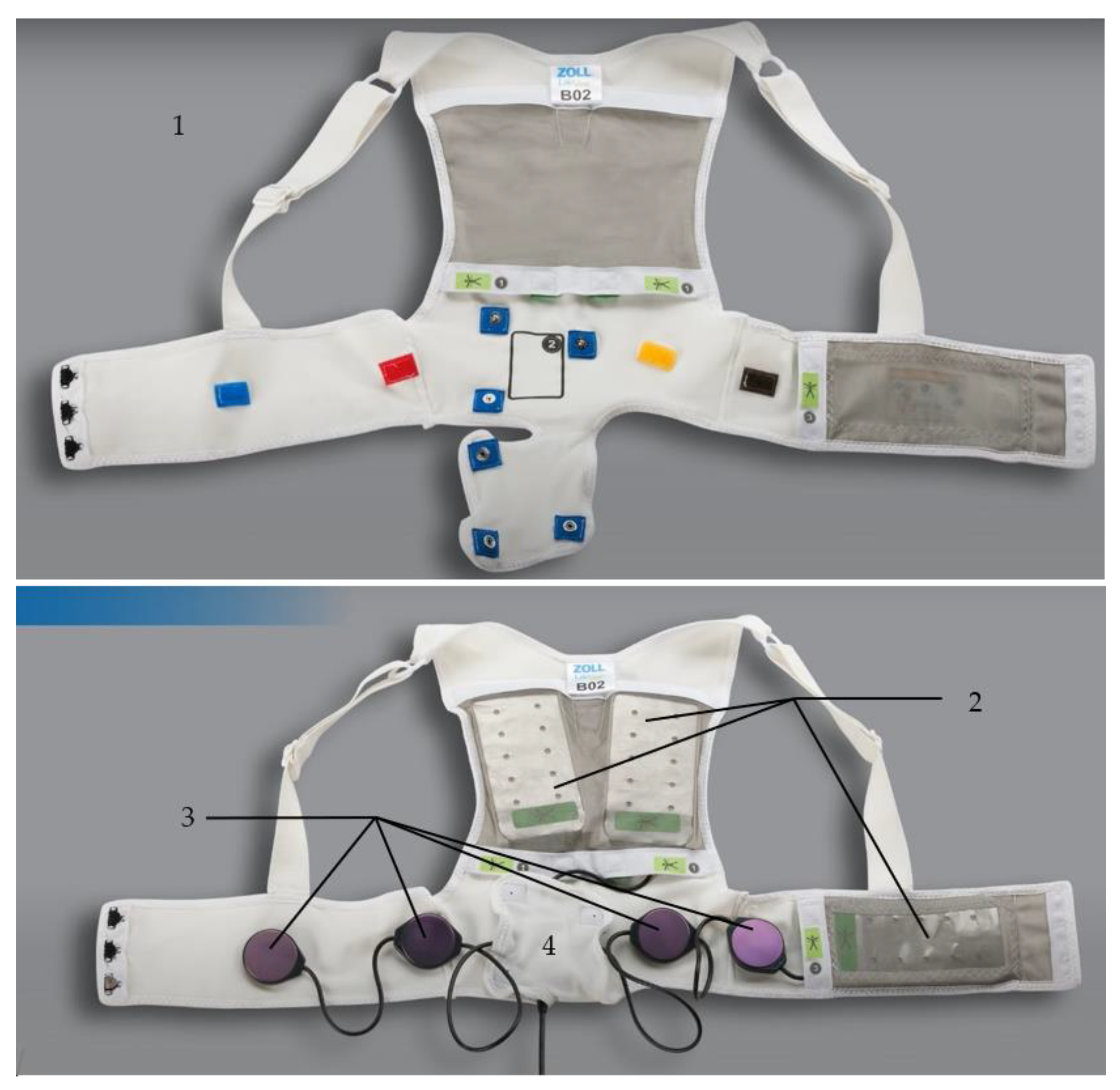
2.1. Composition of the Device
2.1.1. The Fabric Vest
2.1.2. The Defibrillation Pads
2.1.3. The ECG Electrodes
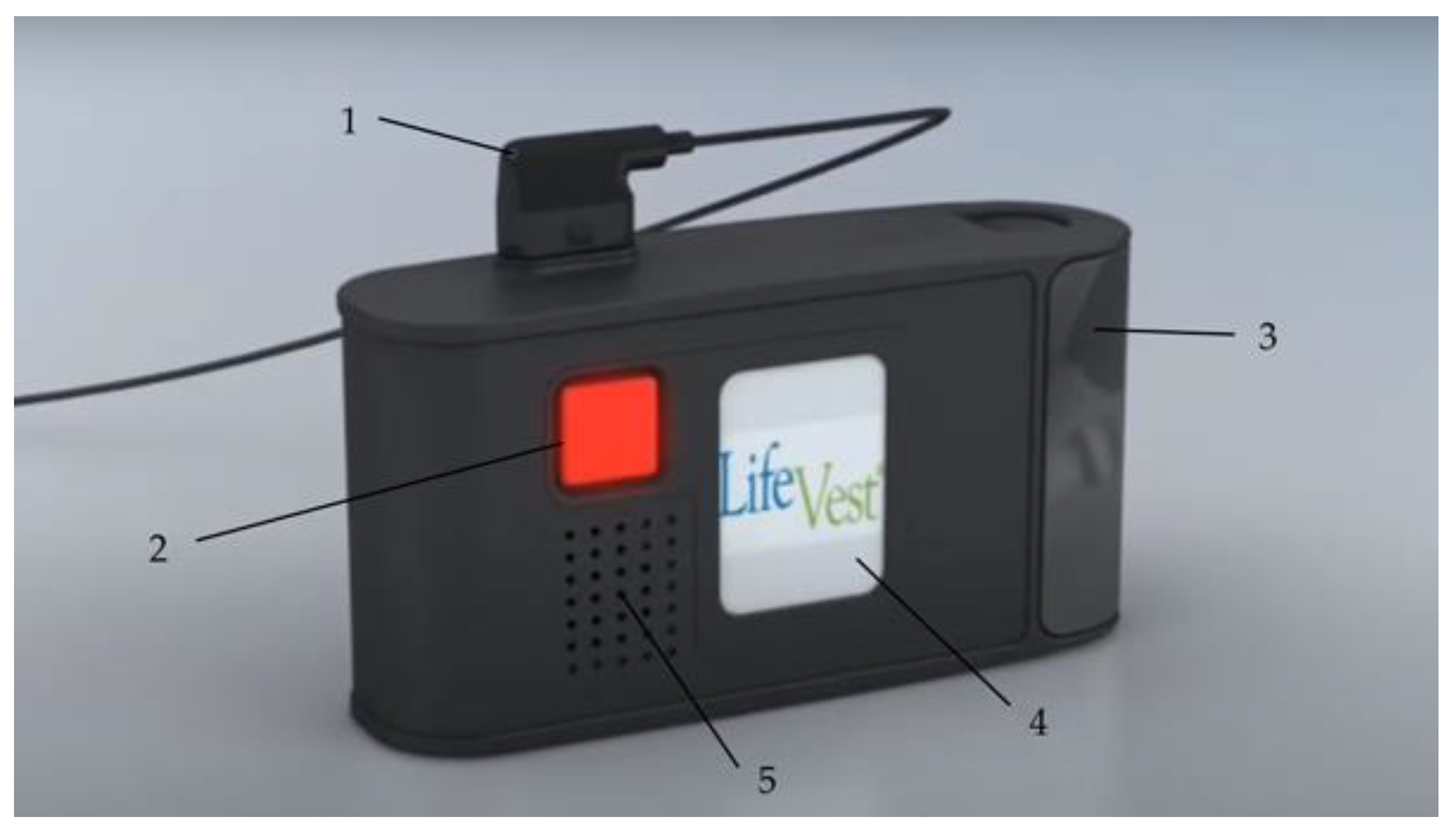
2.1.4. The Monitor
2.2. Clinical Application
3. Multiparameter Monitoring
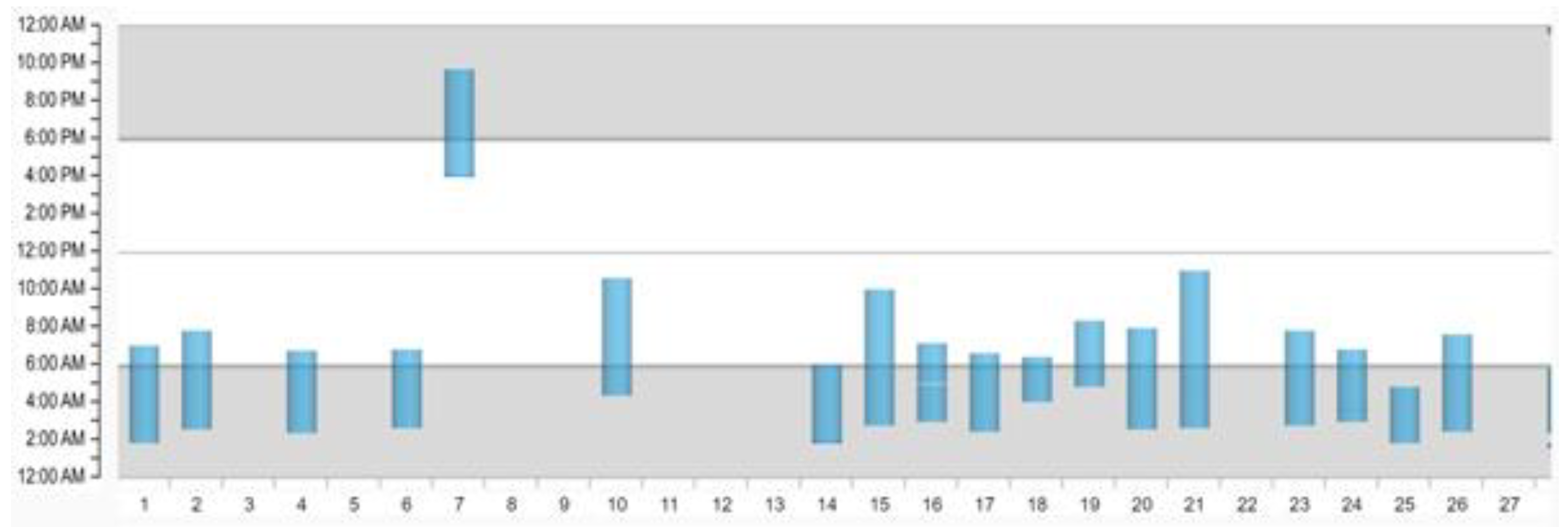
3.1. Monitoring Compliance
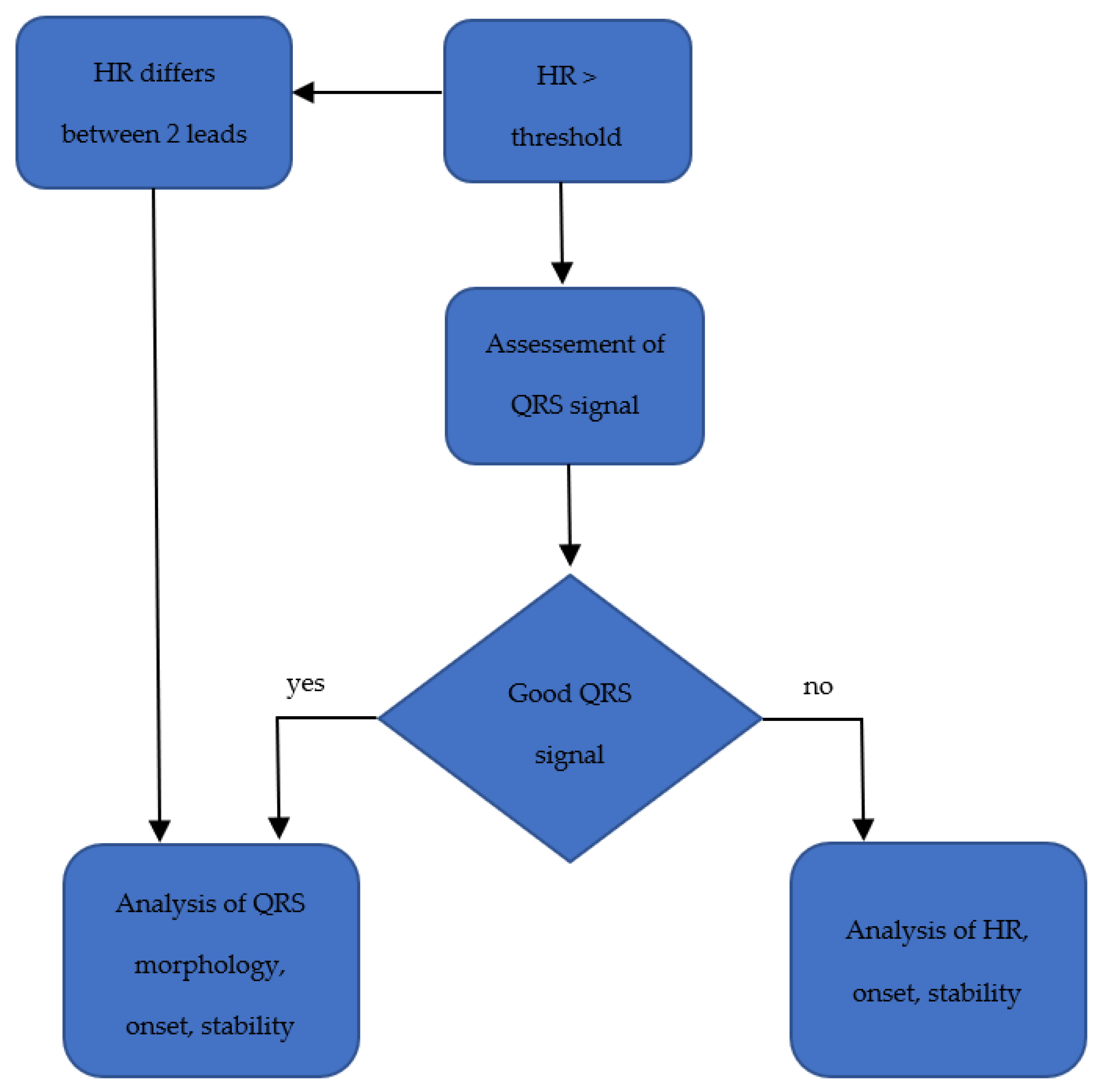
3.2. Monitoring Arrhythmias
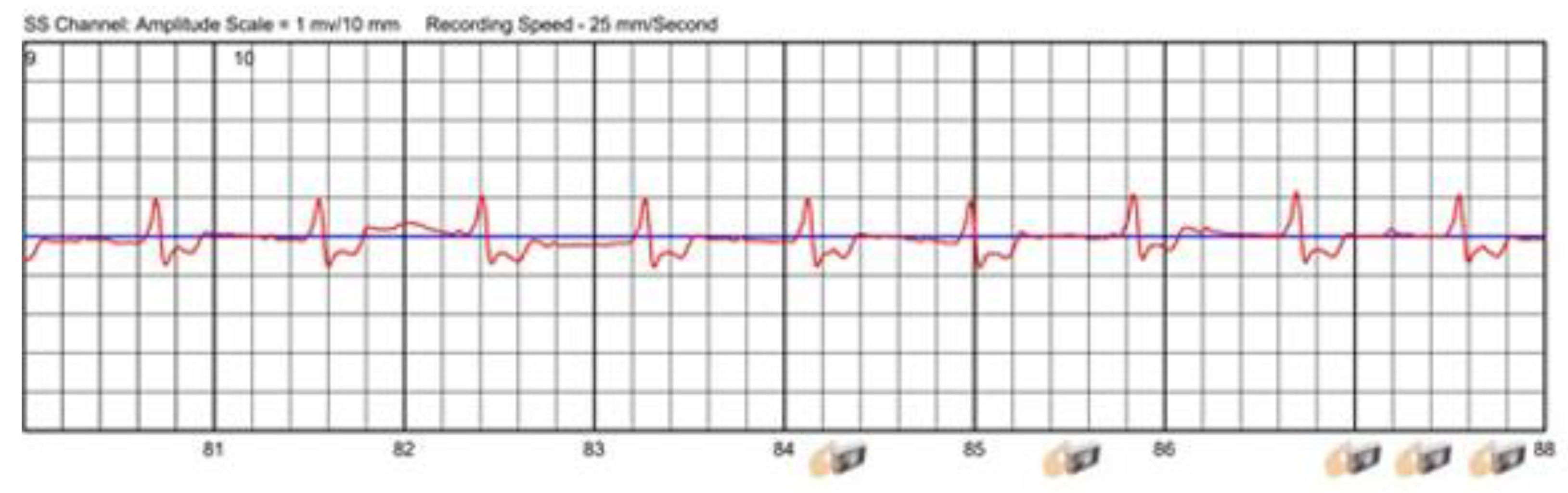
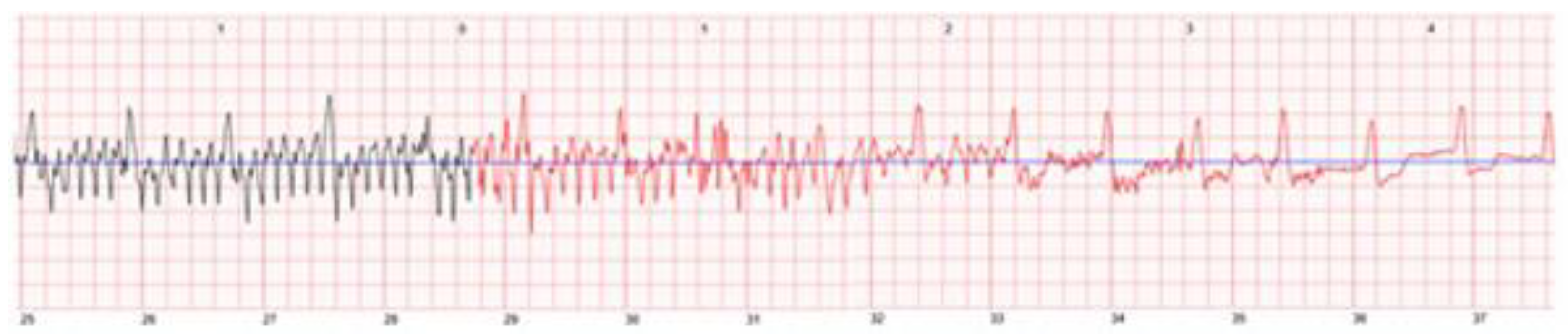
3.2.1. Sensor Interferences
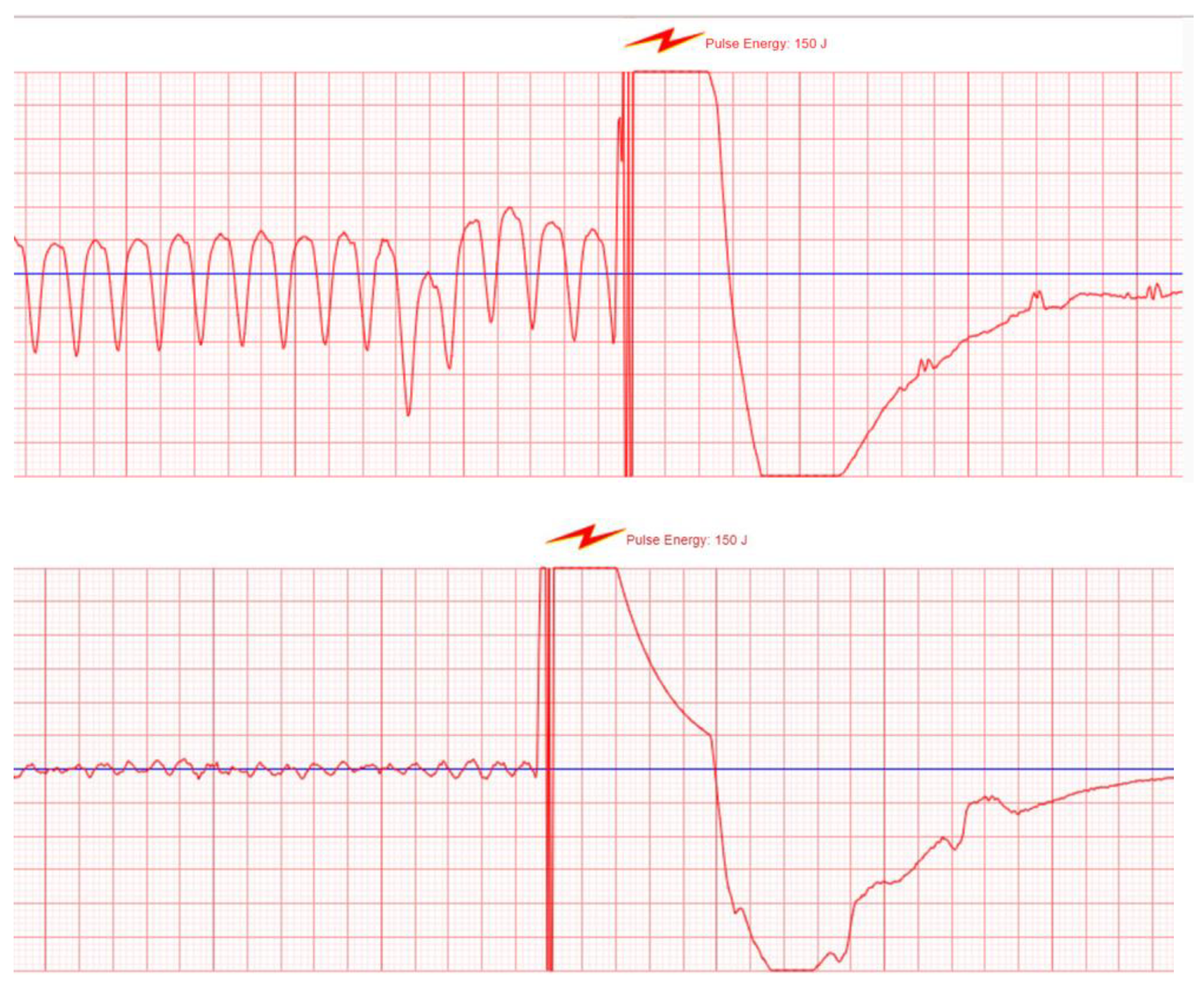
3.2.2. The WCD as a Shock Box
3.2.3. Manually Triggered Alarms
3.3. Monitoring Heart Failure
3.4. Monitor Therapy Success
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mendis, S.; Puska, P.; Norrving, B.; World Health Organization; World Heart Federation; World Stroke Organization. Global Atlas on Cardiovascular Disease Prevention and Control; Mendis, S., Puska, P., Norrving, B., World Health Organization, Eds.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Priori, S.G.; Blomstrom-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed] [Green Version]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017, 72, e91–e220. [Google Scholar]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 2008, 29, 2388–2442. [Google Scholar]
- Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N. Engl. J. Med. 1997, 337, 1576–1583. [Google Scholar] [CrossRef]
- Connolly, S.J.; Gent, M.; Roberts, R.S.; Dorian, P.; Green, M.S.; Klein, G.J.; Mitchell, L.B.; Sheldon, R.S.; Roy, D. Canadian Implantable Defibrillator Study (CIDS): Study design and organization. CIDS Co-Investigators. Am. J. Cardiol. 1993, 72, 103F–108F. [Google Scholar] [CrossRef]
- Connolly, S.J.; Hallstrom, A.P.; Cappato, R.; Schron, E.B.; Kuck, K.H.; Zipes, D.P.; Greene, H.L.; Boczor, S.; Domanski, M.; Follmann, D.; et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs. Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur. Heart J. 2000, 21, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Klein, H.; Geller, C.J.; Reek, S.; Heilman, M.S.; Szymkiewicz, S.J. Clinical efficacy of the wearable cardioverter-defibrillator in acutely terminating episodes of ventricular fibrillation. Am. J. Cardiol. 1998, 81, 1253–1256. [Google Scholar] [CrossRef]
- Adler, A.; Halkin, A.; Viskin, S. Wearable cardioverter-defibrillators. Circulation 2013, 127, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018, 138, e272–e391. [Google Scholar]
- Dillon, K.A.; Szymkiewicz, S.J.; Kaib, T.E. Evaluation of the effectiveness of a wearable cardioverter defibrillator detection algorithm. J. Electrocardiol. 2010, 43, 63–67. [Google Scholar] [CrossRef]
- Reek, S.; Burri, H.; Roberts, P.R.; Perings, C.; Epstein, A.E.; Klein, H.U.; Committee, E.S.D.; Lip, G.; Gorenek, B.; Sticherling, C.; et al. The wearable cardioverter-defibrillator: Current technology and evolving indications. Europace 2017, 19, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch, A.E.; D’Souza, B.; Gimbel, J.R.; Rohrer, U.; Masuda, T.; Sears, S.; Scherr, D. Physical activity is reduced prior to ventricular arrhythmias in patients with a wearable cardioverter defibrillator. Clin. Cardiol. 2020, 43, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, M.; Narcisse, D.; Khouzam, N.; Khouzam, R.N. Wearable Cardioverter Defibrillator “The Lifevest”: Device Design, Limitations, and Areas of Improvement. Curr. Probl. Cardiol. 2018, 43, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.; Reek, S. Wearable cardioverter defibrillator: A life vest till the life boat (ICD) arrives. Indian Heart J. 2014, 66, 68–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, A.M.; Klein, H.; Tchou, P.; Murali, S.; Hall, W.J.; Mancini, D.; Boehmer, J.; Harvey, M.; Heilman, M.S.; Szymkiewicz, S.J.; et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: Results of the WEARIT/BIROAD. Pacing Clin. Electrophysiol. 2004, 27, 4–9. [Google Scholar] [CrossRef]
- Kutyifa, V.; Moss, A.J.; Klein, H.; Biton, Y.; McNitt, S.; MacKecknie, B.; Zareba, W.; Goldenberg, I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation 2015, 132, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Wassnig, N.K.; Gunther, M.; Quick, S.; Pfluecke, C.; Rottstadt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience with the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef]
- Chung, M.K.; Szymkiewicz, S.J.; Shao, M.; Zishiri, E.; Niebauer, M.J.; Lindsay, B.D.; Tchou, P.J. Aggregate national experience with the wearable cardioverter-defibrillator: Event rates, compliance, and survival. J. Am. Coll. Cardiol. 2010, 56, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Odeneg, T.; Ebner, C.; Mörtl, D.; Keller, H.; Dirninger, A.; Stix, G.; Föger, B.; Grimm, G.; Steinwender, C.; Gebetsberger, F.; et al. Indications for and outcome in patients with the wearable cardioverter-defibrillator in a nurse-based training programme: Results of the Austrian WCD Registry. Eur. J. Cardiovasc. Nurs. 2019, 18, 75–83. [Google Scholar] [CrossRef]
- Barsheshet, A.; Kutyifa, V.; Vamvouris, T.; Moss, A.J.; Biton, Y.; Chen, L.; Storozynsky, E.; Wan, C.; Szymkiewicz, S.J.; Goldenberg, I. Study of the wearable cardioverter defibrillator in advanced heart-failure patients (SWIFT). J. Cardiovasc. Electrophysiol. 2017, 28, 778–784. [Google Scholar] [CrossRef]
- Kao, A.C.; Krause, S.W.; Handa, R.; Karia, D.; Reyes, G. Bianco Wearable defibrillator use in heart failure (WIF): Results of a prospective registry. BMC Cardiovasc. Disord. 2012, 12, 123. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Wang, N.C.; Jain, S.; Voigt, A.H.; Saba, S.; Adelstein, E.C. Utility of the Wearable Cardioverter-Defibrillator in Patients with Newly Diagnosed Cardiomyopathy: A Decade-Long Single-Center Experience. J. Am. Coll. Cardiol. 2015, 66, 2607–2613. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.; Goldenberg, I.; Moss, A.J.; Klein, H.; Huang, D.T.; Bianco, N.R.; Szymkiewicz, S.J.; Zareba, W.; Brenyo, A.; Buber, J.; et al. Wearable defibrillator in congenital structural heart disease and inherited arrhythmias. Am. J. Cardiol. 2011, 108, 1632–1638. [Google Scholar] [CrossRef]
- Duncker, D.; Haghikia, A.; Konig, T.; Hohmann, S.; Gutleben, K.J.; Westenfeld, R.; Oswald, H.; Klein, H.; Bauersachs, J.; Hilfiker-Kleiner, D.; et al. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function-value of the wearable cardioverter/defibrillator. Eur. J. Heart Fail. 2014, 16, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Saltzberg, M.T.; Szymkiewicz, S.; Bianco, N.R. Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J. Card. Fail. 2012, 18, 21–27. [Google Scholar] [CrossRef]
- Zishiri, E.T.; Williams, S.; Cronin, E.M.; Blackstone, E.H.; Ellis, S.G.; Roselli, E.E.; Smedira, N.G.; Gillinov, A.M.; Glad, J.A.; Tchou, P.J.; et al. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ. Arrhythm. Electrophysiol. 2013, 6, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beiert, T.; Malotki, R.; Kraemer, N.; Stöckigt, F.; Linhart, M.; Nickenig, G.; Schrickel, J.W.; Andrié, R.P. A real world wearable cardioverter defibrillator experience—Very high appropriate shock rate in ischemic cardiomyopathy patients at a European single-center. J. Electrocardiol. 2017, 50, 603–609. [Google Scholar] [CrossRef]
- Epstein, A.E.; Abraham, W.T.; Bianco, N.R.; Kern, K.B.; Mirro, M.; Rao, S.V.; Rhee, E.K.; Solomon, S.D.; Szymkiewicz, S.J. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J. Am. Coll. Cardiol. 2013, 62, 2000–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Olgin, J.E.; Lee, B.K.; Vittinghoff, E.; Morin, D.P.; Zweibel, S.; Rashba, E.; Chung, E.H.; Borggrefe, M.; Hulley, S.; Lin, F.; et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020, 31, 1009–1018. [Google Scholar] [CrossRef]
- Mitacchione, G.; Schiavone, M.; Gasperetti, A.; Viecca, M.; Curnis, A.; Forleo, G.B. Neglected lead tip erosion: An unusual case of S-ICD inappropriate shock. J. Cardiovasc. Electrophysiol. 2020, 31, 3322–3325. [Google Scholar] [CrossRef]
- Piccini, J.P.; Allen, L.A.; Kudenchuk, P.J.; Page, R.L.; Patel, M.R.; Turakhia, M.P. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Science Advisory from the American Heart Association. Circulation 2016, 133, 1715–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deneke, T.; Bosch, R.; Eckardt, L.; Nowak, B.; Schwab, J.O.; Sommer, P.; Veltmann, C.; Helms, T.M. Der tragbare Kardioverter/Defibrillator (WCD)—Indikationen und Einsatz. Kardiologe 2019, 13, 292–304. [Google Scholar] [CrossRef]
- Klein, H.U.; Meltendorf, U.; Reek, S.; Smid, J.; Kuss, S.; Cygankiewicz, I.; Jons, C.; Szymkiewicz, S.; Buhtz, F.; Wollbrueck, A.; et al. Bridging a temporary high risk of sudden arrhythmic death. Experience with the wearable cardioverter defibrillator (WCD). Pacing Clin. Electrophysiol. 2010, 33, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Zylla, M.M.; Hillmann, H.A.K.; Proctor, T.; Kieser, M.; Scholz, E.; Zitron, E.; Katus, H.A.; Thomas, D. Use of the wearable cardioverter-defibrillator (WCD) and WCD-based remote rhythm monitoring in a real-life patient cohort. Heart Vessels 2018, 33, 1390–1402. [Google Scholar] [CrossRef]
- Schmitt, J.; Abaci, G.; Johnson, V.; Erkapic, D.; Gemein, C.; Chasan, R.; Weipert, K.A.Y.; Hamm, C.W.; Klein, H.U. Safety of the Wearable Cardioverter Defibrillator (WCD) in Patients with Implanted Pacemakers. Pacing Clin. Electrophysiol. 2017, 40, 271–277. [Google Scholar] [CrossRef]
- Manninger, M.; Odeneg, T.; Fruhwald, F.; Brussee, H.; Scherr, D. Oversensing of the wearable cardioverter defibrillator during bipolar ventricular stimulation. Wien. Klin. Wochenschr. 2017, 129, 910–912. [Google Scholar] [CrossRef] [Green Version]
- Rohrer UM, M.; Odeneg, T.; Ebner, C.; Moertl, D.; Dirninger, A.; Keller, H.; Stix, G.; Alber, H.; Steinwender, C.; Binder, R.; et al. Incidence and predictors of alarms in patients with wearable cardioverter defibrillator (WCD)—Results of the Austrian WCD Registry. Eur. Heart J. 2019, 40, 1346. [Google Scholar] [CrossRef]
- Erath, J.W.; Vamos, M.; Sirat, A.S.; Hohnloser, S.H. The wearable cardioverter-defibrillator in a real-world clinical setting: Experience in 102 consecutive patients. Clin. Res. Cardiol. 2017, 106, 300–306. [Google Scholar] [CrossRef]
- Schuger, C.; Daubert, J.P.; Brown, M.W.; Cannom, D.; Estes, N.A., 3rd; Hall, W.; Kayser, T.; Klein, H.; Olshansky, B.; Power, K.A.; et al. Multicenter automatic defibrillator implantation trial: Reduce inappropriate therapy (MADIT-RIT): Background, rationale, and clinical protocol. Ann. Noninvasive Electrocardiol. 2012, 17, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Reiss, N.; Schmidt, T.; Hoffmann, J.D.; Cannom, D.; Estes, N.A., 3rd; Hall, W.J.; Kayser, T.; Klein, H.; Olshansky, B.; Power, K.A.; et al. Telemedical Concepts for Heart Failure Patients Treated with a Wearable Cardioverter Defibrillator. Stud. Health Technol. Inform. 2020, 271, 93–100. [Google Scholar]
- Ross, J.S.; Chen, J.; Lin, Z.; Bueno, H.; Curtis, J.P.; Keenan, P.S.; Normand, S.L.; Schreiner, G.; Spertus, J.A.; Vidán, M.T. Recent national trends in readmission rates after heart failure hospitalization. Circ. Heart Fail. 2010, 3, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Negishi, K.; Marwick, T.H. Meta-Analysis of Risks for Short-Term Readmission in Patients with Heart Failure. Am. J. Cardiol. 2016, 117, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Bennett, T.D.; St John Sutton, M.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): A randomised controlled trial. Lancet 2014, 384, 583–590. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart. J. 2016, 37, 2129–2200. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Erath, J.W.; Wanczura, P.; Wranicz, J.; Linke, A.; Rohrer, U.; Scherr, D. Influence of decompensated heart failure on cardiac acoustic biomarkers: Impact on early readmissions. ESC Heart Fail. 2020, 7, 4198–4205. [Google Scholar] [CrossRef]
- Mueller-Leisse, J.; Brunn, J.; Zormpas, C.; Hohmann, S.; Hillmann, H.A.K.; Eiringhaus, J.; Bauersachs, J.; Veltmann, C.; Duncker, D. Extended follow-up after wearable cardioverter-defibrillator period: The PROLONG-II study. ESC Heart Fail. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, H.A.K.; Hohmann, S.; Mueller-Leisse, J.; Zormpas, C.; Eiringhaus, J.; Bauersachs, J.; Veltmann, C.; Duncker, D. Feasibility and First Results of Heart Failure Monitoring Using the Wearable Cardioverter–Defibrillator in Newly Diagnosed Heart Failure with Reduced Ejection Fraction. Sensors 2021, 21, 7798. [Google Scholar] [CrossRef]
- Ekblom, O.; Ek, A.; Cider, Å.; Hambraeus, K.; Börjesson, M. Increased Physical Activity Post-Myocardial Infarction Is Related to Reduced Mortality: Results from the SWEDEHEART Registry. J. Am. Heart Assoc. 2018, 7, e010108. [Google Scholar] [CrossRef] [Green Version]
- Vegh, E.M.; Kandala, J.; Orencole, M.; Upadhyay, G.A.; Sharma, A.; Miller, A.; Merkely, B.; Parks, K.A.; Singh, J.P. Device-measured physical activity versus six-minute walk test as a predictor of reverse remodeling and outcome after cardiac resynchronization therapy for heart failure. Am. J. Cardiol. 2014, 113, 1523–1528. [Google Scholar] [CrossRef]
- Conraads, V.M.; Spruit, M.A.; Braunschweig, F.; Cowie, M.R.; Tavazzi, L.; Borggrefe, M.; Hill, M.R.; Jacobs, S.; Gerritse, B.; van Veldhuisen, D.J. Physical activity measured with implanted devices predicts patient outcome in chronic heart failure. Circ. Heart Fail. 2014, 7, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Burch, A.E.; Erath, J.W.; Kutyifa, V.; Aßmus, B.; Bonderman, D.; Russo, A.M. Decline in physical activity in the weeks preceding sustained ventricular arrhythmia in women. Heart Rhythm. O2 2020, 1, 283–287. [Google Scholar] [CrossRef]
- Tripp, C.; Burch, A.E.; Erath, J.W.; Hain, A.; Sears, S.F. Physical Activity in Adults with Wearable Cardioverter Defibrillators in the Post-Myocardial Infarction Period. J. Cardiopulm. Rehabil. Prev. 2020, 40, 164–166. [Google Scholar] [CrossRef]
- Pulickal, T.; Helms, T.M.; Perings, C.A. Der Wearable Kardioverter-Defibrillator als Diagnostikum. Herzschrittmachertherapie Elektrophysiologie 2021, 32, 264–268. [Google Scholar] [CrossRef]
- Duncker, D.; König, T.; Hohmann, S.; Bauersachs, J.; Veltmann, C. Avoiding Untimely Implantable Cardioverter/Defibrillator Implantation by Intensified Heart Failure Therapy Optimization Supported by the Wearable Cardioverter/Defibrillator-The PROLONG Study. J. Am. Heart Assoc. 2017, 6, e004512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.C.; Olgin, J.E.; Lee, B.K. Wearable cardioverter-defibrillators: A review of evidence and indications. Trends Cardiovasc. Med. 2021, 31, 196–201. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohrer, U.; Manninger, M.; Zirlik, A.; Scherr, D. Multiparameter Monitoring with a Wearable Cardioverter Defibrillator. Sensors 2022, 22, 22. https://doi.org/10.3390/s22010022
Rohrer U, Manninger M, Zirlik A, Scherr D. Multiparameter Monitoring with a Wearable Cardioverter Defibrillator. Sensors. 2022; 22(1):22. https://doi.org/10.3390/s22010022
Chicago/Turabian StyleRohrer, Ursula, Martin Manninger, Andreas Zirlik, and Daniel Scherr. 2022. "Multiparameter Monitoring with a Wearable Cardioverter Defibrillator" Sensors 22, no. 1: 22. https://doi.org/10.3390/s22010022
APA StyleRohrer, U., Manninger, M., Zirlik, A., & Scherr, D. (2022). Multiparameter Monitoring with a Wearable Cardioverter Defibrillator. Sensors, 22(1), 22. https://doi.org/10.3390/s22010022





