Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review
Abstract
1. Introduction
2. Operating Principles of CNT-Based Chemical Sensors
2.1. Chemical Nanosensors
2.2. Carbon Nanotubes (CNTs) Sensors
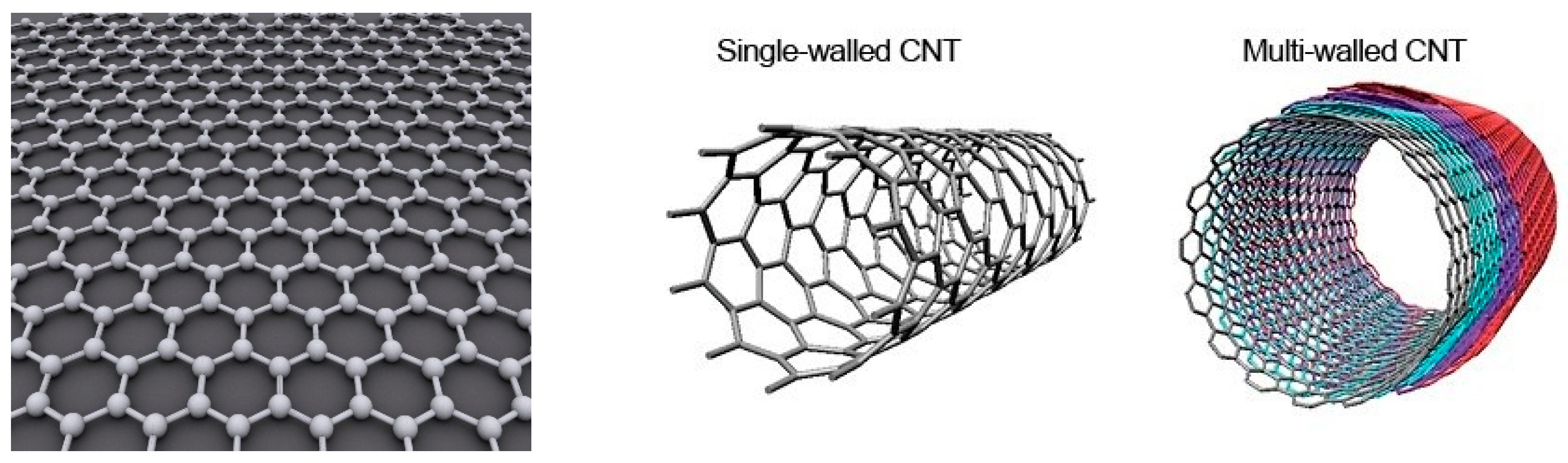
2.2.1. Carbon Nanotubes Structure
2.2.2. Functionalization of CNTs
2.2.3. CNT-Based Electronic Devices
2.3. CNT-Based Electrochemical Sensors
2.3.1. Electrochemical Cells
2.3.2. Electrochemical Transduction
2.3.3. Use of CNTs in Electrochemical Sensors
2.4. CNT-Based Chemistors
2.4.1. Chemistors
2.4.2. Use of CNT in Chemistors
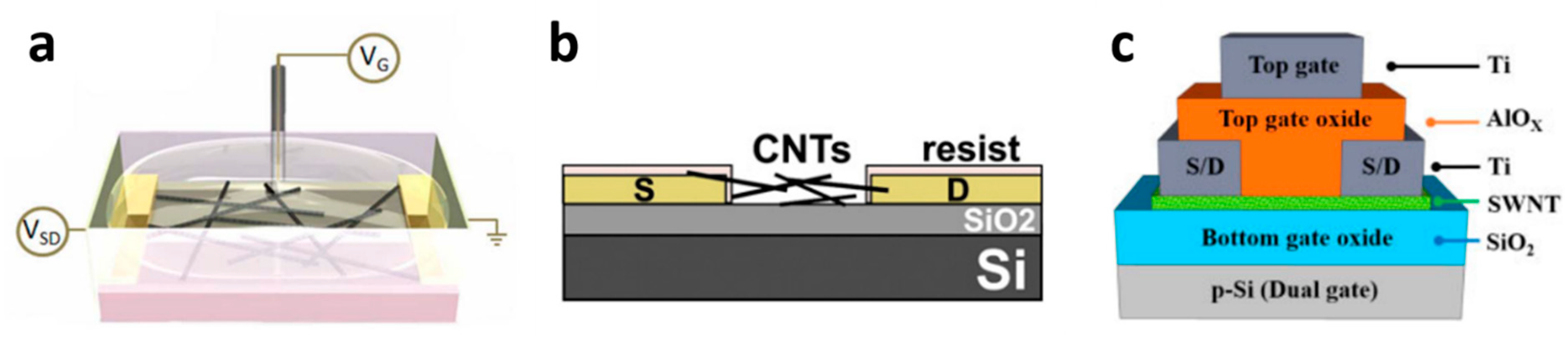
2.5. CNT-Based ChemFET
2.5.1. ChemFET
2.5.2. Use of CNT in ChemFET
2.6. Sensing Mechanisms in CNT-Based Chemistors and ChemFET
3. CNT-Based Sensors with Different Analytes in Water
- Materials: type of CNT (MWCNT or SWCNT), functional probe and type of functionalization (covalent or not),
- Device strategy: type of transduction (including type of electrochemical measurement and type of FET, gating), CNT deposition process, electrode material and configuration, choice of substrate
- Performances: limit of detection (LOD) (converted in the most used unit for the target analyte), sensitivity in the measured range of concentration (converted whenever possible in a common unit) and the results of interference study
3.1. pH
| Type of CNT | Functional Probe | Functionalization | Analyte | Detection Range | Detection Limit | Sensitivity Relative Sensitivity * | Transduction Method | CNT Deposition Method | Electrode Material Contact Configuration | Substrate | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWCNT | Polyaniline | Non covalent | pH | pH 2.1~12.8 | 2.74 nM | N/A | Chemistor | Drop-casting | Ti/Au | Si/SiO2 | [126] | |
| SWCNT | Nafion | Non covalent | pH | pH 1~12 | N.P. | 3.5%/pH | Chemistor | Screen printing | SWCNT | Polymide | [127] | |
| MWCNT | Ni NP * | Non covalent | pH | pH 2~10 | N.P. | 5.0%/pH | Chemistor | Continuous pulling of super-aligned, CVD grown MWCNTs | MWCNT | PDMS | [89] | |
| SWCNT | Pristine | Non functionalized | pH | pH 1~11 | <10 pM | 34 nS/pH 3.4%/pH (pH 1~6) 163 nS/pH 9.3%/pH (pH 7~11) | Chemistor | Spray-casting | Cr | Si/SiO2 | [57] | |
| SWCNT | COOH | Covalent | pH | pH 5~9 | N.P. | 75 Ω/pH 11%/pH | Chemistor | Dielectrophoresis (aligned CNTs) | Cr/Au | Si/SiO2 | Response time: 2 s at pH 5, 24 s at pH 9 | [88] |
| SWCNT | Pristine | Non functionalized | pH | pH 4~10 | N.P. | 5.2 kΩ/pH 14%/pH | Chemistor | Aerosol jet printing | Ag | Kapton | [58] | |
| MWCNT | Pristine | Non functionalized | pH | pH 5~9 | N.P. | 63 Ω/pH 18%/pH | Chemistor | Sucked by vacuum force | MWCNT | Filter paper | [123] | |
| SWCNT | Malt extract agar | Non covalent | pH | pH 3~5 | 100 mM | N/A | FET (hybrid top gate) | Dip coating | Ti/Au (10/30 nm) contacts | Si/SiO2(100 nm) | Multiplexed detection of Fungus (A. niger, A. versicolor) and Yeast (S. cerevisiae) * | [105] |
| SWCNT | ETH500 *, MDDA-Cl | Non covalent | pH | pH 2~7.5 | 10 mM | 71 nA/pH 7.5%/pH | FET (liquid gate) | Spray deposition | Aqueous electrolyte (gate) Cr/Au (5/50 nm) | Polymide (Kapton®) | Change from p-type to n-type transistor with the membrane layer | [112] |
| SWCNT | COOH | Covalent | pH | pH 3~8 | N.P. | 17 nA/pH 8.2%/pH | FET (top gate) | N.P. | Cr/Au (30/50 nm) source & drain electrodes, Ag/AgCl for reference electrode | Glass/APS(50–200 nm)/SWCNT /APS(500 nm)/TopGate | CNT placement controlled by location of APS (modified to immobilize the CNTs) | [106] |
| SWCNT | Pristine | Non functionalized | pH | pH 3.4~7.8 | 10 mM | 3.9 µA/pH 13%/pH | FET (bottom gate) | Spin coating | Cr/Au (5/40 nm) | Si/SiO2(65 nm) | [107] | |
| SWCNT | Poly(1-aminoanthracene) | Non covalent | pH | pH 3~11 | 1 μM | FET 19 µS/pH 14%/pH potentiometry 55 mV/pH | FET, potentiometry (liquid gate) | Dielectrophoresis (aligned CNTs) | Au contacts, Pt wire (Auxillary), Ag/AgCl electrode (Reference) | Si/SiO2(300 nm) | Multiplexed detection of Ca(II) and Na+ | [60] |
| SWCNT | Pristine | Non functionalized | pH | pH 3~10 | 1 mM | 7600 mV/pH 23%/pH (Dual-gate mode) 59.5 mV/pH (single-gate mode potentiometry) | FET (double gate) | Spin coating | 100 nm Ti contacts for source, drain and top gate | p-Si (substrate acting as bottom gate) | [54] | |
| SWCNT | Polyaniline | Non covalent | pH | pH 1~13 | N.P. | 56 mV/pH | potentiometry | Spray casting | Polyvinyl chloride-coated steel wire | PVC | Highly selective against Li+, Na+, K+ | [128] |
| MWCNT | COFTHi-TFPB * | Covalent | pH | pH 1~12 | N.P. | 54 mV/pH | Differential pulse voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | multiplexed detection of Ascorbic acid. | [125] |
| MWCNT | COOH | Covalent | pH | pH 4~9 | N.P. | 17 Ω/pH 23%/pH (Au), 16 Ω/pH 14%/pH (Al) | Impedance spectroscopy | Dip coating | Au and Al interdigitated electrodes | Kapton® | [59] |
3.2. Micronutrients and Heavy Metals
3.2.1. Detection of Pb(II)
3.2.2. Detection of Cd(II)
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity (Detection Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | Pristine | Non functionalized | Pb(II) (Cd(II), Zn(II), Cu(II)) | 0.3 ppb | 2.2 nA/ppb (210~830 ppb) | Stripping voltammetry | CNT thread | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | Pristine | Non functionalized | Pb(II) | 1.0 ppb | 1.5 nA/ppb (15~40 ppb) 3.5 nA/ppb (40~70 ppb) | Stripping voltammetry | Inkjet printing | Inkjet-printed silver ink | PEN * | Effects of copper and cadmium are reported. | [130] |
| MWCNT | Ionic liquid—dithizone based bucky-gel | Covalent | Pb(II) | 0.02 ppt | 0.024 µA/ppb (0.1ppt~210 ppb) | Stripping voltammetry | Drop-casting | Glassy carbon electrode | Glassy carbon | No interference of Cd(II) and Cu(II) ions with the detection of Pb(II) ion. | [132] |
| MWCNT | Thiacalixarene | Covalent | Pb(II) | 8 ppt | 3.8 µA/ppb (0.04–2.07 ppb) | Differential pulse anodic stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Detection of Pb(II) was clearly not affected by Zn(II), Cd(II), Ni(II) (100-fold excess) | [134] |
| MWCNT | Cysteine | Covalent | Pb(II) (Cu(II)) | 1 ppb | 0.23 * µA/ppb (25~750 ppb) | Differential pulse anodic stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon. | 40-fold Cl−, 30-fold SO42− and four fold CO32− did not have any significant effect on the stripping peak current of Pb(II) and Cu(II) | [74] |
| MWCNT | Poly(o-toluidine) Ce(III)tungstate | Covalent | Pb(II) | 210 ppb | 27 mV/decade (0.1 ppt–100 ppb) | Potentiometry | Liquid mixing and membrane formation through drying | Calomel electrode | Glass tube (araldite) | Strong selectivity (from 50 to 500 times) against Zn(II), Sr(II), Hg(II), Ca(II), Pd(II), Cu(II), Mg(II) | [133] |
| MWCNT | Nafion/Bismuth | Non covalent | Pb(II), (Cd(II)) | 25 ppt | 0.22 µA/ppb (0.05 to 5 ppb) 0.27 µA/ppb (5~100 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 500-fold of SCN−, Cl−, F−, PO43−, SO42−, NO3− and various cations such as Na+, Ca(II), Mg(II), Al(III), K+, Zn(II), Co(II) and Ni(II) had no influences on the signals of Pb(II) and Cd(II). | [73] |
| MWCNT | PSS-Bi * | Non covalent | Pb(II) (Cd(II)) | 0.04 ppb | 0.079 µA/ppb (0.5~90 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 20-fold amounts of Zn(II), 5-fold amounts of Sn(II) and 1-fold amounts of Cu(II) have influence on the determination of Cd(II) and Pb(II) with deviation of 10%. | [142] |
| MWCNT | Bismuth | Non covalent | Pb(II) (Cd(II)) | ~0.04 ppb | N/A | Stripping voltammetry | Plasma-enhanced CVD (vertically aligned MWCNTs in epoxy matrix) | Cr | Silicon | N.P. * | [143] |
| MWCNT | Fe3O4-LSG-CS-Bi * | Non covalent | Pb(II) (Cd(II)) | 0.07 ppb | 0.21 µA/ppb (1~20 ppb) 0.24 µA/ppb (20~200 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Slight changes in peak currents of Pb(II) and Cd(II) were observed in presence of interfering ions Na+, Cl−, SO42−, PO43−, Fe(II), Fe(III), Zn(II), As(III). Significant increase in response signals of Hg(II) was probably due to the formation of amalgam Dramatically decreased response signals of Cu(II) were ascribed to the formation of Pb-Cu inter-metallic compounds. | [144] |
| MWCNT | PPy-Bi NPs * | Non covalent | Pb(II) (Cd(II)) | 0.1 ppb | 1.1 µA/ppb (0.11~120 ppb) | Stripping voltammetry | Paste mixture with MWCNT, paraffin oil and graphite powder | Stainless steel rod | Teflon (PTFE *) tube | Good selectivity towards Fe(II), Al(III), Zn(II), Mg(II), SO42−, CO32−, Ca(II), K+, Na+. The absolute relative change of signal varied from 0.40 to 4.88%). High interference from Cu(II) (1-fold mass ratio was found as the tolerance ratios for the detection of Pb and Cd ions) | [145] |
| MWCNT | rGO-Bi * | Non covalent | Pb(II) (Cd(II)) | 0.2 ppb | 930 nA/ppb cm2 (20~200 ppb) | Stripping voltammetry | Spray coating | Cr(30 nm)/Au(200 nm) | Polymide (VTEC 1388) | 100-fold K+, Na+, Ca(II), Cl−, NO3− and a 30-fold Fe(III) increase had no significant effect on the signals of Cd and Pb ions. Cu ions were found to reduce the response of target metal ions due to the competition between electroplating Bi and Cu on the electrode surface (close reduction potential of Cu and Bi.) | [146] |
| MWCNT | Bismuth | Non covalent | Pb(II) (Cd(II),Zn(II)) | 0.2 ppb | 0.39 µA/ppb (2~18 ppb) 0.67 µA/ppb (20~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Ceramic substrates | N.P. | [138] |
| MWCNT | Bismuth | Non covalent | Pb(II) (Cd(II),Zn(II)) | 1.3 ppb | 1.2 µA/ppb (2~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Alumina plates | The addition of copper ions strongly influenced the stripping responses. Decrease of lead and cadmium pics by 65.5%. | [139] |
| MWCNT | Pristine | Non covalent | Pb(II) (Cd(II), Zn(II)) | 6.6 ppb | 0.47 * s/V/ppb (58~650 ppb) | Stripping potentiometry | Paste mixture of MWCNT and mineral oil | MWCNT paste electrode | Glass tube | Al(III), Mg(II), Fe(III), Ni(II), Co(II), Cr(III), Cu(II) and Sb(III) were investigated in the ratio analyte: Interferent 1:1 and 1:10. The interference was observed for the ratios analyte: interferent 1:1 and 1:10 for Co(II), 1:10 for Cr(III) and Cu(II). | [131] |
| MWCNT | Sb2O3 * | Non covalent | Pb(II) (Cd(II)) | 24 ppb | 2.7 µA/ppb (5–35 ppb) | Stripping voltammetry | Paste mixture of MWCNT, silicon oil, Sb2O3 powder and ionic liquid | Copper wire | PTFE tube | N.P. | [136] |
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity (Linear Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | Pristine | Non functionalized | Cd(II) (Pb(II) Zn(II), Cu(II)) | 0.23 ppb | 3.9 nA/ppb (170~500 ppb) | Stripping voltammetry | CNT thread | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | PSS-Bi * | Non covalent | Cd(II) (Pb(II)) | 0.02 ppb | 0.23 µA/ppb (0.5~50 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 20-fold amounts of Zn(II), 5-fold amounts of Sn(II) and 1-fold amounts of Cu(II) have influence on the determination of Cd(II) and Pb(II) with deviation of 10%. | [142] |
| MWCNT | Nafion/Bismuth | Non covalent | Cd(II) (Pb(II)) | 0.04 ppb | 0.18 µA/ppb (0.08~5 ppb) 0.16 µA/ppb (5~100 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 500-fold of SCN−, Cl−, F−, PO43−, SO42−, NO3− and various cations such as Na+, Ca(II), Mg(II), Al(III), K+, Zn(II), Co(II) and Ni(II) had no influences on the signals of Pb(II) and Cd(II). | [73] |
| MWCNT | Bismuth | Non covalent | Cd(II) (Pb(II)) | 0.04 ppb | 0.037 µA/ppb (0.5~8 ppb) | Stripping voltammetry | Plasma-enhanced CVD (vertically aligned MWCNTs in epoxy matrix) | Cr | Silicon | N.P. | [143] |
| MWCNT | Fe3O4-LSG-CS-Bi * | Non covalent | Cd(II) (Pb(II)) | 0.1 ppb | 0.097 µA/ppb (1~20 ppb) 0.32 µA/ppb (20~200 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Slight changes in peak currents of Pb(II) and Cd(II) were observed in presence of interfering ions Na+, Cl−, SO42−, PO43−, Fe(II), Fe(III), Zn(II), As(III). Significant increase in response signals of Hg(II) was probably due to the formation of amalgam Dramatically decreased response signals of Cu(II) was ascribed to the formation of Pb-Cu inter-metallic compounds. | [144] |
| MWCNT | PPy-Bi * | Non covalent | Cd(II) (Pb(II)) | 0.16 ppb | 0.47 µA/ppb (0.16~120 ppb) | Stripping voltammetry | Paste mixture with MWCNT, paraffin oil and graphite powder | Stainless steel rod | Teflon (PTFE) tube | Good selectivity towards Fe(II), Al(III), Zn(II), Mg(II), SO42−, CO32−, Ca(II), K+, Na+. The absolute relative change of signal varied from 0.40 to 4.88%). High interference from Cu(II) (1-fold mass ratio was found as the tolerance ratios for the detection of Pb and Cd ions) | [145] |
| MWCNT | Poly(1,2-diaminobenzene) | Non covalent | Cd(II), (Cu(II)) | 0.25 ppb | 0.14 µA/ppb (5~100 ppb) | Stripping voltammetry | Multipulse potentiostatic method | Glassy carbon electrode | Glassy carbon | N.P. | [69] |
| MWCNT | rGO-Bi * | Non covalent | Cd(II) (Pb(II)) | 0.6 ppb | 26 nA/ppb cm2 (20~200 ppb) | Stripping voltammetry | Spray coating | Cr(30 nm)/Au(200 nm) | Polymide (VTEC 1388) | 100-fold K+, Na+, Ca(II), Cl−, NO3- and a 30-fold Fe(III) increase had no significant effect on the signals of Cd and Pb ions. Cu ions were found to reduce the response of target metal ions due to the competition between electroplating Bi and Cu on the electrode surface (close reduction potential of Cu and Bi.) | [146] |
| MWCNT | Bismuth | Non covalent | Cd(II) (Pb(II),Zn(II)) | 0.7 ppb | 0.22 µA/ppb (2~18 ppb) 1.5 µA/ppb (20~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Alumina plates | The addition of copper ions strongly influenced the stripping responses. Decrease of lead and cadmium pics by 65.5%. | [139] |
| MWCNT | Bismuth | Non Covalent | Cd(II) (Pb(II),Zn(II)) | 0.8 ppb | 0.59 µA/ppb (2~18 ppb) 0.80 µA/ppb (20~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Ceramic substrates | N.P. | [138] |
| MWCNT | Fe3O4/ eggshell | Non covalent | Cd(II) | 2.4 ppb | 19 µA/ppb (0.5~210 ppb) | Stripping voltammetry | Paste mixture of MWCNT, graphite powder, paraffin oil and Fe3O4-eggshell | Copper wire | Glass tube | 500-fold amounts of the following ions: Na+, Ca(II), Mg(II), Fe(III), Mn(II), Cr(III), Ba(II), Co(II), Hg(II), K+, NH4+, NO3−, SO42−, PO43− made no alteration of the peak currents of Cd(II). 100-fold amounts of Sn(II) and Cu(II) with deviation of 9%, 50 fold amounts of Ni(II) and Zn(II) with deviations of 8% and 6% respectively had influence on the determination of Cd(II). | [141] |
| MWCNT | Pristine | Non covalent | Cd(II) (Pb(II),Zn(II)) | 8.4 ppb | 0.36 * s/V/ppb (58~646 ppb) | Stripping potentiometry | Paste mixture of MWCNT and mineral oil | MWCNT paste electrode | Glass tube | Al (III), Mg (II), Fe (III), Ni (II), Co (II), Cr (III), Cu (II) and Sb (III) were investigated in the ratio analyte: Interferent 1:1 and 1:10. the interference was observed for the ratios analyte: interferent 1:1 and 1:10 for Co (II), 1:10 for Cr (III) and Cu (II). | [131] |
| MWCNT | Sb2O3 | Non covalent | Cd(II) (Pb(II)) | 17 ppb | 1.9 µA/ppb (80~150 ppb) | Stripping voltammetry | Paste mixture of MWCNT, silicon oil, Sb2O3 powder and ionic liquid | Copper wire | PTFE tube | N.P. | [136] |
3.2.3. Detection of Zn(II)
3.2.4. Detection of Hg(II)
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity (Linear Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | Pristine | Non functionalized | Zn(II) (Cd(II), Pb(II), Cu(II)) | 0.08 ppb | 3.4 pA/ppb (200~590 ppb) | Stripping voltammetry | CNT thread | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | Bismuth | Non covalent | Zn(II) (Pb(II), Cd(II)) | 11 ppb | 0.18 µA/ppb (12~18 ppb) 0.24 µA/ppb (20~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Ceramic substrates | N.P. | [138] |
| MWCNT | Bismuth | Non covalent | Zn(II) (Pb(II), Cd(II)) | 12 ppb | 0.38 µA/ppb (20~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Alumina plates | The addition of copper ions strongly influenced the stripping responses. Decrease of lead and cadmium pics by 65.5%. | [139] |
| MWCNT | Pristine | Non covalent | Zn(II) (Pb(II), Cd(II)) | 28 ppb | 0.11 * s/V/ppb (58~646 ppb) | Stripping potentiometry | Paste mixture of MWCNT and mineral oil | MWCNT paste electrode | Glass tube | Al(III), Mg(II), Fe(III), Ni(II), Co(II), Cr(III), Cu(II) and Sb(III) were investigated in the ratio analyte: Interferent 1:1 and 1:10. the interference was observed for the ratios analyte: interferent 1:1 and 1:10 for Co(II), 1:10 for Cr(III) and Cu(II). | [131] |
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity (Linear Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWCNT | Pristine | Non functionalized | Hg(II) | 0.6 ppm | 12 mV/ppm (1~30 ppm) | Chemistor | CVD | SWCNT | Glass | 1000 fold excess of Fe(II), Fe(III), Ni(II), Cu(II),Zn(II), Cr(III) and 500 folds of As(III), Sb(III), Se(IV) and Pb(II) had no interfering effect in the analysis of mercury solution. | [147] |
| SWCNT | Pristine | Non functionalized | Hg(II) | 2 ppb | 0.22/decade 0.2 ppb~201 ppm | FET (Liquid gate) | Dip coating with selective CNT placement | Pd/Au (10/30 nm) | Glass | Good selectivity towards interferent ions (only Hg(II) causes conductance increase.) | [110] |
| SWCNT | Thiophenol | Covalent | Hg(II) | 0.6 ppb | 0.14 µA/ppb (1~18 ppb) | Stripping voltammetry | Dip coating | Au | Au | The presence of 100-fold concentration of Cr(II), Mn(II), Co(II), Ni(II), Zn(II), 50-fold concentration of Fe(II),and 20-fold Cu(II), have no influence on the signals of 50 nM Hg(II) with deviation below 5%. | [150] |
| MWCNT | PANi-Bi NPs@GO * | Non covalent | Hg(II) (Cu(II)) | 2 ppt | 1.3 µA/ppb (2 ppt~1000 ppm) | Differential pulse voltammetry | Screen printing | (commercial) Carbon ink | PET | Not provided | [151] |
| MWCNT | Au NPs | Non covalent | Hg(II) | 0.06 ppb | 0.59 µA/ppb (0.1~1 ppb) 0.045 µA/ppb (1~250 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Not provided | [72] |
| MWCNT | * ENTZ | Non covalent | Hg(II) | 0.5 ppb | 29.3 mV/decade (1 ppb~20 ppm) | Potentiometry | Paste mixture of MWCNT, graphite powder, ENTZ ionophore and ionic liquid | Copper wire | Polypropylene syringe | The interfering ions (Ag+, Zn(II), Pb(II), Ni(II), Cd(II)and Cu(II)) do not have any effect on the response of proposed electrodes to Hg(II) | [79] |
| MWCNT | Thiol-functionalized chitosan | Non covalent | Hg(II) | 0.6 ppb | 1060 µA/ppb (2~28 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 100-fold Cd(II), 100-fold Pb(II), 50-fold Zn(II), 25-fold Cu(II), 10-fold Ag(II), 10-fold Fe(II) and 10-fold Mn(II) caused within ±5% changes of voltammetric signals for Hg(II). | [152] |
| MWCNT | Triazene (BEPT) | Non covalent | Hg(II) | 0.62 ppb | 29 mV/decade (0.8 ppb~440 ppm) | Potentiometry | Paste mixture of MWCNT, graphite powder, Triazene (BEPT) ionophore and paraffin oil | Copper wire | Polyethylene tube | The proposed electrode has a high performance to selective potentiometric assay of Hg(II) in aqueous samples containing some interfering ions (Cu(II), Ag(II), Cd(II), Co(II), Al(III), Pb(II), K+. | [153] |
3.2.5. Detection of As(III)
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity (Linear Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | COOH | Covalent | As(III) | N.A | 0.24 µA/ppb (0.3~50 ppb) | Stripping voltammetry | Dip coating | Au | Au electrode | Interference was significant when the Sb/As ratio is higher than 1. | [154] |
| MWCNT | Au-NP * | Non covalent | As(III) | 0.1 ppb | 26 µA/ppb (75 ppt–5.3 ppm) | Stripping voltammetry | Drop casting | Glassy carbon | Glassy carbon | Not provided | [157] |
| MWCNT | Leucine/Nafion | Non covalent | As(III) | 0.13 ppb | 0.27 µA/ppb (0.37~150 ppb) | Stripping voltammetry | Drop casting | Pt | Pt electrode | Zn(II) and Fe(II) could be tolerated up to at least 0.05 mM whereas commonly encountered matrix components such as Cd(II), Co(II), Mg(II), Ni(II) and Cu+ did not show high percentage of interference. | [155] |
| MWCNT | Pt-Fe NP | Non covalent | As(III) | 0.75 ppb | 64 nA/ppb (0.75~22 ppb) | Stripping voltammetry | Drop casting | Glassy carbon | Glassy carbon | No interference from copper ion | [158] |
| MWCNT | Au-NPs | Non covalent | As(III) | 0.75 ppb | 2.6 Q/mL/ppb * (0.75~750 ppb) | Stripping voltammetry | Vacuum filtration | MWCNT membrane | PTFE membrane | The presence of copper at 10 µM strongly affects the analytical response of As(III); The presence of Pb(II) caused a minor broadening of the peak of As(III) resulting in a slight reduction of the peak current; | [159] |
3.2.6. Detection of Cu(II)
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWCNT | PANI-GGHH * | Non covalent | Cu(II) | 3 ppt | N/A (3~29 ppt) | FET (liquid gate) | CVD | 300 nm Au | Si/SiO2 (120nm) | His6 shows higher chelation power for Ni(II) than to Cu(II). | [113] |
| MWCNT | C24H30N6 Schiff base | Non covalent | Cu(II) | 10 ppt | N/A (0.09~340 ppb) | Stripping voltammetry | Paste of MWCNT, Schiff base and mineral oil | Copper wire | Filter membrane | Not provided | [160] |
| MWCNT | Pristine | Non functionalized | Cu(II) (Cd(II), Zn(II), Pb(II)) | 17 ppt | 9.4 pA/ppb (32~220 ppb) | Stripping voltammetry | CNT thread aspirated into a glass capillary | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | 2-amino-4-thiazoleacetic acid | Non covalent | Cu(II) | 30 ppt | 0.02 µA/ppb * (44 ppb~3.2 ppm) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | At a concentration ratio below 10, the presence of Zn(II), Mn(II), Ni(II), Co(II) has led to lower than 6% decreasing of DPSV currents of Cu(II). | [161] |
| MWCNT | PANi-Bi NPs@GO * | Non covalent | Cu(II) (Hg(II)) | 32 ppt | 0.23 uA/ppb (32 ppt~320 ppm) | Differential pulse voltammetry | Screen printing | (commercial) Carbon ink | PET | Not provided | [151] |
| MWCNT | N-doped carbon spheres | Non covalent | Cu(II) | 92 ppt | 0.28 µA/ppb (0.5~200 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | EDTA can seriously affect the stripping peak current of Cu(II) with a decrease of 79%. | [163] |
| MWCNT | Poly(1,2-diaminobenzene) | Non covalent | Cu(II) (Cd(II)) | 0.33 ppb | 0.11 µA/ppb (5~100 ppb) | Stripping voltammetry | Multipulse potentiostatic method | Glassy carbon electrode | Glassy carbon | Not provided | [69] |
| MWCNT | SSA/MoS2* | Non covalent | Cu(II) | 3.6 ppb | 0.13 µA/ppb (6.4~−700 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 10-fold concentration of the metal ions (K+, Ca(II), Na+, Mg(II), Zn(II), Pb(II), Cd(II), Fe(III), Mn(II), Co(II), Cr(III), Cr6+, Ni(II) and Hg(II), has not any obvious effect on the Cu(II) peak current. | [164] |
| MWCNT | Cysteine | Covalent | Cu(II) (Pb(II)) | 15 ppb | 0.13 * µA/ppb (250~1500 ppb) | Differential pulse anodic stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 40-fold Cl−, 30-fold SO42− and four fold CO32− did not have any significant effect on the stripping peak current of Pb2+ and Cu2+ | [74] |
3.2.7. Detection of Other Metal Ions
3.2.8. Multiplexed Detection of Metal Ions
| Type of CNT | Functional Probe | Functionalization | Analyte (Add. Analytes) | Detection Limit | Sensitivity | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWCNT | Polypyrrole-Hisn * | Non covalent | Ni(II) | 2.8 ppt | 1.5 µS/decade (5%/decade) (0.59 ppt~59 ppb) | FET (liquid gate) | CVD | 300 nm Au Pt wire (Counter electrode), Ag/AgCl (Reference electrode) | Si/SiO2(120 nm) | His6 shows higher chelation power for Ni(II) than to Cu(II). | [113] |
| SWCNT | PAM * | Non covalent | Co(II) | 0.04 ppt | 0.014 */decade (0.04 ppt~440 ppm) | Chemistor | Spay-casting | Al tape Ag paint | Si/SiO2 | Selectivity to Co(II) was investigated in presence of Cu(II). The electrical response was higher with Co(II). | [165] |
| Type of CNT | Functional Probe | Functionalization | Cd(II) LOD | Pb(II) LOD (LOD Pb/Cd) | Zn(II) LOD (LOD Zn/Cd) | Cu(II) LOD (LOD Cu/Cd) | Hg(II) LOD | Ref. |
|---|---|---|---|---|---|---|---|---|
| MWCNT | Nafion/Bismuth | Non covalent | 0.04 ppb–0.4 nM | 0.025 ppb–0.12 nM 0.3 | [73] | |||
| MWCNT | Bismuth | Non covalent | 0.04 ppb –0.4 nM | ~0.04 ppb–0.2 nM 0.5 | [143] | |||
| MWCNT | PSS-Bi | Non covalent | 0.02 ppb–0.2 nM | 0.04 ppb–0.2 nM 1 | [142] | |||
| MWCNT | rGO-Bi | Non covalent | 0.6 ppb–50 nM | 0.2 ppb–1 nM 0.02 | [146] | |||
| MWCNT | PPy-Bi | Non covalent | 0.16 ppb–1.4 nM | 0.1 ppb–0.5 nM 0.4 | [145] | |||
| MWCNT | Fe3O4-LSG-CS-Bi | Non covalent | 0.1 ppb–0.9 nM | 0.07 ppb–0.3 nM 0.3 | [144] | |||
| MWCNT | Sb2O3 | Non covalent | 17 ppb–0.15 µM | 24 ppb–110 nM 0.7 | [136] | |||
| MWCNT | Pristine | Non functionalized | 8.4 ppb–75 nM | 6.6 ppb–31 nM 0.4 | 28 ppb–0.43 µM 6 | [131] | ||
| MWCNT | Bismuth | Non covalent | 0.8 ppb–7 nM | 0.2 ppb–1 nM 0.14 | 11 ppb–0.17 µM 24 | [138] | ||
| MWCNT | Bismuth | Non covalent | 0.7 ppb–6 nM | 1.3 ppb–6.2 nM 1 | 12 ppb–0.18 µM 30 | [139] | ||
| MWCNT | Pristine | Non functionalized | 0.23 ppb–2 nM | 0.3 ppb–1 nM 0.5 | 0.08 ppb–1.2 nM 0.6 | 17 ppt–0.26 nM 0.13 | [129] | |
| MWCNT | Poly(1,2-diaminobenzene) | Non covalent | 0.25 ppb–0.22 nM | 0.33 ppb–5 nM 22 | [69] | |||
| MWCNT | Cysteine | Covalent | 1 ppb–4 nM | 15 ppb–0.23 µM | [74] | |||
| MWCNT | PANi-Bi NPs@GO | Non covalent | 32 ppt–0.5 nM | 2 ppt–0.01 nM | [151] |
3.2.9. Interference Studies
3.3. Nitrogen (Ammonia, Nitrite, Nitrate)
| Type of CNT | Functional Probe | Functionalization | Analyte | DETECTION LIMIT | Sensitivity (Detection Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | Co3O4− rGO * | Non covalent | Nitrite | 0.016 µM | 0.408 µA/µM/cm2 (0.1~8000 µM) | Voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 100-fold of alcohol, Na+, K+, Cl−, NO3−, N2H4, SO32−,SO42−, has no effect on sensor response. | [167] |
| MWCNT | PCMA * | Non covalent | Nitrite | 0.067 µM | −0.023 µA/µM (1~10 µM) −0.022 µA/µM (10~100 µM) −0.034 µA/µM\ (100~1000 µM) −0.026 µA/µM (1000~4000 µM) | Differential pulse voltammetry, Chronoamperometry | Drop cast of PCMA/MWCNT, then electrochemical crosslinking | Au | Au | Not provided | [169] |
| MWCNT | AuNPs/ PEI */ MWCNT-COOH | Non covalent | Nitrite | 0.2 µM | −0.500 µA/µM * (1~2000 µM) −58 µA/mM (1~1400 µM) | Voltammetry | Drop casting | Au | Au | Na+, Mg(II), Ca(II), Zn(II), Fe(II), Cl−, I− and SO42− did not have significant interference in the detection of nitrite. | [170] |
| SWCNT | Pd | Non covalent | Nitrite | 0.25 µM | 420 µA mM−1 cm−2 (2~240 µM ) 190 µA mM−1 cm−2 (280~1230 µM) | Differential pulse voltammetry | Vacuum filtration | SWCNT | PET | Negligible effect of K+, Na+, Cl−, PO43−, NH4+, CH3COO− and Zn(II) in concentration above500 mM and concentrations of Mg(II), Ca(II), Cd(II), CO32−, NO3−,and SO42− above 200 mM | [171] |
| MWCNT | Ni7S6 | Non covalent | Nitrite | 0.3 µM | 0.185 µA/µM (1~4200 µM) | Voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Results comparable to high-performance liquid chromatography for lake water, tap water and pickle water | [172] |
| MWCNT | GO-MWCNT-PMA-Au | Non covalent | Nitrite | 0.67 µM | 0.484 µA/µM (2~10,000 µM) | Differential pulse voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | No obvious response was observed when injection of 0.4 Mm of Na+, Ca(II), NO3−, CO32-, K+, Cl−, SO42−, IO3− | [173] |
| MWCNT | Au/TiO2 | Non covalent | Nitrite | 3 µM | N/A (4~225 µM) | Differential pulse voltammetry | Pulsed electrodeposition | Glassy carbon electrode | Glassy carbon | The presence of arginine, serine, tyrosine, cysteine, glucose, alanine (each of 0.1 mM) causes less than 5% variation on sensor response. | [166] |
| MWCNT | Thionine | Non covalent | Nitrite | 4 µM | 0.002 µA/µM (6 µM~15,000 µM) | Voltammetry | Transfer via abrasion from filter paper to heated GC electrode | Glassy carbon electrode | Glassy carbon | Not provided | [174] |
| MWCNT | PANI * | Non covalent | Nitrite | 6.1 µM | 0.684 µA/µM/cm2 (N/A) | Voltammetry | Electrodeposition | Glassy carbon electrode | Glassy carbon | Not provided | [175] |
| MWCNT | rGO * | Non covalent | Nitrite | 25 µM | 0.01 µA/µM (75~6060 µM) | Differential pulse voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 0.6 M Ca(II), Cu(II), K+, Na+, Zn(II),SO42−, l-cysteine, NO3− and Cl− did not interfere with the pick signals of 0.15 mM HQ, 0.15 mM CC, 0.15 mM PC and 0.15 mM NO2−. | [76] |
3.4. Water Hardness (Ca(II), Mg(II))
| Type of CNT | Functional Probe | Functionalization | Analyte | Detection Limit | Sensitivity (Detection Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SWCNT | Fluo-4 AM * | Non-covalent | Ca(II) | 100 pM | 69 nA/decade (100 nM~1 mM) | CNT-FET | Dip coating | Ti (10 nm)/Au (30 nm) (liquid, floating gate) | Glass (borosilicate glass capillary) | FET at the end of a nanoneedle for intracell monitoring | [178] |
| MWCNT | PDMS * | Non-covalent | Ca(II) (Mg(II)) | 25 µM | N/A (25 µM~5 mM (Not linear)) | Capacitive measurement | Mold injection and thermal curing | MWCNT | PDMS | Measured at 2.4 kHz frequency | [179] |
3.5. Dissolved Oxygen (DO)
3.6. Disinfectants (Hypochlorite, Hydrogen Peroxide, Chloramine, Peracetic Acid)
| Type of CNT | Functional Probe | Functionalization | Analyte | Detection Limit | Sensitivity (Linear Range) | Transduction Method | Deposition Method | Electrode | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MWCNT | Hemin | Non-covalent | O2 | N/A | N/A (N/A) | Cyclic voltammetry, Amperometry | In-place CVD (densely-packed, vertically aligned CNTs) | Glassy carbon electrode | [184] |
| MWCNT | Au NP * | Non-covalent | O2 | 0.1 ppm | N/A (0~50 ppm) | Cyclic voltammetry | Not provided | Glassy carbon electrode | [186] |
| Type of CNT | Functional Probe | Functionalization | Analyte | Detection Limit | Sensitivity (Detection Range) | Transduction | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | PVC, DBE * | Non covalent | Hydrogen peroxide | N/A | Not Provided | Amperometry, voltammetry | Screen Printing | CNT electrodes | Alumina | Not provided | [192] |
| MWCNT | Nitrogen doped Co-CNTs over graphene sheets | Non covalent | Hydrogen peroxide | 100 nM 3.4 ppb | −0.85 µA/ppm | Voltammetry, amperometry | Coating | Glassy carbon electrode | Glassy carbon electrode | No interference with uric acid, ascorbic acid and glucose | [188] |
| SWCNT | Cr-hcf * | Non covalent | Hydrogen peroxide | 0.5 µM 17 ppb | 1 µA/ppm (17 ppb~340 ppm) * | Amperometry, voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | No interference from ascorbic acid and uric acid | [75] |
| CNT (probably Multi-walled) | Fe-Ni | Non covalent | Hydrogen peroxide | 16 µM 540 ppb | 1.2 µA/ppm (34 ppm~510 ppm) | Voltammetry | Paste poured into electrode | Glassy carbon electrode | Glassy carbon electrode | Not provided | [189] |
| MWCNT | Chitosan/Cu/MWCNT-COOH | Non covalent | Hydrogen peroxide (pH) | <25 µM <850 ppb | 0.97 nA/ppb (500 µM~10 mM) | Amperometry | Potentiostatic polarization | Glassy carbon electrode | Chitosan-coated glassy carbon | No interference from ascorbic acid and uric acid | [190] |
| SWCNT | Phenyl capped aniline tetramer | Non covalent | Hydrogen peroxide | <3 ppm | 1%/ppm (3 ppm~8 ppm) Nonlinear <1%/100 ppm (48 ppm~1200 ppm) | Chemistor | Drop casting | Carbon ink | Glass | Not provided | [193] |
| MWCNT | Pristine | Non functionalized | Free chlorine in its hypochlorite ion form | <5 ppb | Logarithmic 39% /decade * (0.03~8 ppm) | Chemistor | Dielectrophoresis (aligned MWCNT) | Cr/Au | Glass | No information about selectivity, pH information not provided | [191] |
| MWCNT | Epoxy EpoTek H77A | Non covalent | Free chlorine under hypochlorous acid form (At pH 5.5) | 20 ppb | 0.15 µA/ppb (0.02~4 ppm) | Voltammetry | Paste poured into tube and thermally cured | Epoxy/MWCNT composite | Not provided (tube) | Validated in real water matrices (tap water and swimming pool) | [194] |
| SWCNT | Phenyl capped aniline tetramer | Covalent | Free chlorine | <60 ppb | 92 nA/decade (0.06~60 ppm (linear up to 6 ppm)) | Chemistor | Drop casting | Au | Glass | Non selective to different oxidants list of oxidants not provided Regeneration possible | [53] |
3.7. Sulfur (Sulfide, Sulfite, Sulfate)
| Type of CNTs | Functional Probe | Functionalization | Analyte | Detection Limit | Sensitivity (Detection Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCNT | Ferrocene-branched chitosan | Non covalent | Sulfite | 2.8 µM | 0.013 µA/µM (5 µM~1500 µM) | Amperometry | Drop casting | Glassy carbon electrode | Glassy carbon | 600-fold excess of Ca(II), Mg(II), Ba(II), PO43−, NO3−, CO32− and Cl− did not interfere in the determination of sulfite. | [68] |
| MWCNT | Ferrocene | Non covalent (Physical immobilization) | Sulfite | 0.1 µM | 3.3 µA/µM (0.4 µM~4 µM) 0.18 µA/µM (4 µM~120 µM) | Differential Pulse Voltammetry | Paste mixture with graphite powder blended with paraffin oil | MWCNT paste, Copper wire | Glass tube | Not provided | [78] |
| MWCNT | Hematoxylin | Non covalent | Sulfide | 0.2 µM | 103 nA/µM (0.5 µM~150 µM) | Amperometry | Paste mixture of MWCNT, mineral oil and graphite powder | Carbon paste | Teflon tube | No interference with Sn(II), Co(II), (II)Pb(II), (II)Zn(II), Cu(II), Ni(II), Mn(II), Fe(II) and Fe(III) | [197] |
| MWCNT | Platinum | Non covalent (plating) | Sulfide | 0.26 µM | 0.63 µA/µM (0.26 µM~40 µM and 40 µM~100 µM) | Amperometry Differential pulse voltammetry | Thermal CVD (vertically aligned CNTs) | Stainless steel | Stainless steel | Not provided | [198] |
| MWCNT | Pristine | Not functionalized | Sulfide | 0.3 µM (CVD *), 12.5 µM (ARC *) | 0.12 µA/µM (1.3 µM~113 µM) (CVD), 0.005 µA/µM (12.5 µM~87.5 µM) (ARC) | Hydrodynamic voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Not provided | [199] |
| MWCNT | Copper phenanthroline | Non covalent (Physical immobilization) | Sulfide | 1.2 µM | 34 nA/µM (5 µM~400 µM) | Amperometry | Drop casting | Glassy carbon electrode | Glassy carbon | No interference with SO32−, SO42−, S2 O32−, S4 O62−, Cysteine. | [200] |
3.8. Other Contaminants
| Type of CNT | Functional Probe | Functionalization | Analyte | Detection Limit | Sensitivity (Detection Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
| SWCNT | Pristine | Not functionalized | Glycerol | N/A | ~10 Ω/Glycerol by weight % in water (10~50%) | CNT-FET | Dielectrophoresis | Cr/Au | Si/SiO2 | Not provided | [101] |
| SWCNT | 1-phyrenemethylamine | Non-covalent | Trinitrotoluene | ~ppt | N/A (>0.01 ppb) | Chemistor with interdigitated electrodes (IDEs) | Dip coating | Cr/Au | Si/SiO2 | Relatively selective to 2,6-DNT *, 2,4-DNT, 1,3-DNB *, 1-NB *, Response time~30 s | [55] |
| SWCNT | Peptides, anti-BoNT/E-Lc * | Non-covalent | BoNT* | 60 pM (Peptide probe), 52 fM (Anti-BoNT/E-Lc probe) | 27.95 nS/nM (Peptide), 313 nS/pM (Anti-BoNT) | CNT-FET | CVD (vertically aligned SWCNTs) | Au foils Bottom gate | 120 nm SiO2 on PDMS film | Not provided | [109] |
| MWCNT | Nafion | Non covalent | p-aminophenol (Coliforms) | 10 cfu/mL | 10 to 104 cfu/mL | Cyclic voltammetry, amperometry | Drop casting | Glassy carbon electrode | Glassy carbon | Not provided | [202] |
| MWCNT | rGO* | Non covalent | Hydroquinone Catechol p-cresol (nitrite) | 2.6 µM 1.8 µM 1.6 µM | 0.19 µA/µM (8~391 µM) 0.07 µA/µM (5.5~540 µM) 0.04 µA/µM (5~430 µM) | Differential pulse voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 0.6 M Ca(II), Cu(II), K+, Na+, Zn(II),SO42−, l-cysteine, NO3− and Cl− did not interfere with the pic signals of 0.15 mM HQ, 0.15 mM CC, 0.15 mM PC | [76] |
| MWCNT | Fe-Co doped TNTs | Non-covalent | Sulpiride | 87 nM | 58.8 mV/decade (100 nM~10 mM) | Potentiometry | Paste mixture of graphite powder, MWCNT, Fe-CO-TNT, βCD ionophore, NaTPB anionic additive, DBP plasticizer | Carbon paste electrode | Syringe | No interference observed with K+, Na+, Ca(II), Mg(II), Cd(II), Co(II), Mn(II), Fe(II) | [203] |
| SWCNT | βCD * | Covalent | Bisphenol A | 1.0 nM | 1.3 mA/mM 11 nM–19 µM | Cyclic voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | No interference study, but tested on real plastic samples | [206] |
| MWCNT | βCD | Covalent | Bisphenol A | 14 nM | 7.2 µA/µM (125 nM~2 µM) 2.2 µA/µM (2 µM~30 µM) | Linear sweep voltammetry | Drop casting | Screen printed carbon electrode | Not provided | Selective to APAP *, BPA *, BPS | [204] |
| MWCNT | ZIF-67 * | Covalent | TBBPA * | 4.2 nM | 21.08 µA/µM (0.01~1.5 µM) | Differential pulse voltammetry, cyclic voltammetry | Paste mixture of paraffin oil, AB * and CNTs | Carbon paste electrode | Syringe | TBBME *, TBBDE *, BPAF *, BPA *, TCBPA *, TBBPS * did not show remarkable interference. | [205] |
4. Discussion
4.1. Summary of Best Performances
4.2. Discussion on Sensor Design Choices
4.2.1. Choice of Transduction Mode
- For pH, FET and impedance spectroscopy reach the same performance and are only slightly better than chemistor.
- For Cu(II), the LOD achieved with FET is three times better than voltammetry.
- For Hg(II), the LOD achieved with voltammetry is three orders of magnitude better than that obtained with FET, the latter being two orders of magnitude better than with chemistor.
- For H2O2, the LOD achieved with voltammetry is three orders of magnitude better than with a chemistor.
- For Ca(II), the LOD achieved with FET is five orders of magnitude better than capacitive measurements (which can be seen as a derivative of impedance spectroscopy).
4.2.2. Functionalized versus Non-Functionalized CNT
| Analyte (Add. Analytes) | Type of CNT | Functional Probe | Functionalization | Detection Limit | Sensitivity (Detection Range) | Transduction Method | Deposition Method | Electrode Material Contact Configuration | Substrate | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | MWCNT | Pristine | Non functionalized | N.P. | 63 Ω/pH 18%/pH pH 5~9 | Chemistor | Sucked by vacuum force | MWCNT | Filter paper | Not provided | [123] |
| SWCNT | Pristine | Non functionalized | 1 mM | 7600 mV/pH 23%/pH (Dual-gate mode) pH 3~10 | FET, potentiometry (double gate) | Spin coating | 100 nm Ti contacts for source, drain and top gate | p-Si (substrate acting as bottom gate) | Not provided | [54] | |
| SWCNT | Poly(1-aminoanthracene) | Non covalent | 1 μM | FET 19 µS/pH 14%/pH | FET, potentiometry (liquid gate) | Dielectrophoresis (aligned CNTs) | Au contacts, Pt wire (Auxillary), Ag/AgCl electrode (Reference) | Si/SiO2 (300nm) | Multiplexed detection of Ca(II) and Na+ | [60] | |
| MWCNT | COOH | Covalent | N.P. | 17 Ω/pH 23%/pH (Au) pH 4~9 | Impedance spectroscopy | Dip coating | Au and Al interdigitated electrodes | Kapton® | Not provided | [59] | |
| Pb(II) | MWCNT | Pristine | Non functionalized | 0.3 ppb | 2.2 nA/ppb (210~830 ppb) | Stripping voltammetry | CNT thread | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | Ionic liquid—dithizone based bucky-gel | Covalent | 0.02 ppt | 0.024 µA/ppb (0.1ppt~210 ppb) | Stripping voltammetry | Drop-casting | Glassy carbon electrode | Glassy carbon | No interference of Cd(II) and Cu(II) ions with the detection of Pb(II) ion. | [132] | |
| MWCNT | Nafion/Bismuth | Non covalent | 25 ppt | 0.22 µA/ppb (0.05 to 5 ppb) 0.27 µA/ppb (5~100 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 500-fold of SCN−, Cl−, F−, PO4 3−, SO42−, NO3− and various cations such as Na+, Ca(II), Mg(II), Al(III), K+, Zn(II), Co(II) and Ni(II) had no influences on the signals of Pb(II) and Cd(II). | [73] | |
| MWCNT | PSS-Bi | Non covalent | 0.04 ppb | 0.079 µA/ppb (0.5~90 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 20-fold amounts of Zn(II), 5-fold amounts of Sn(II) and 1-fold amounts of Cu(II) have influence on the determination of Cd(II) and Pb(II) with deviation of 10%. | [142] | |
| Cd(II) | MWCNT | Pristine | Non functionalized | 0.23 ppb | 3.9 nA/ppb (170~500 ppb) | Stripping voltammetry | CNT thread | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | PSS-Bi | Non covalent | 0.02 ppb | 0.23 µA/ppb (0.5~50 ppb) | Stripping voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 20-fold amounts of Zn(II), 5-fold amounts of Sn(II) and 1-fold amounts of Cu(II) have influence on the determination of Cd(II) and Pb(II) with deviation of 10%. | [142] | |
| Zn(II) | MWCNT | Pristine | Non functionalized | 0.08 ppb | 3.4 pA/ppb (200~590 ppb) | Stripping voltammetry | CNT thread | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] |
| MWCNT | Bismuth | Non covalent | 11 ppb | 0.18 µA/ppb (12~18 ppb) 0.24 µA/ppb (20~100 ppb) | Stripping voltammetry | Screen printing | Screen printed MWCNT based electrode | Ceramic substrates | N.P. | [138] | |
| Hg(II) | SWCNT | Pristine | Non functionalized | 0.6 ppm | 12 mV/ppm (1~30 ppm) | Chemistor | CVD | SWCNT | Glass | 1000 fold excess of Fe(II), Fe(III), Ni(II), Cu(II),Zn(II), Cr(III) and 500 folds of As(III), Sb(III), Se(IV) and Pb(II) had no interfering effect in the analysis of mercury solution. | [147] |
| SWCNT | Pristine | Non functionalized | 2 ppb | 0.22/decade 0.2 ppb~201 ppm | FET (Liquid gate) | Dip coating with selective CNT placement | Pd/Au (10/30 nm) | Glass | Good selectivity towards interferent ions | [110] | |
| SWCNT | Thiophenol | Covalent | 0.6 ppb | 0.14 µA/ppb (1~18 ppb) | Stripping voltammetry | Dip coating | Au | Au | The presence of 100-fold concentration of Cr(II), Mn(II), Co(II), Ni(II), Zn(II), 50-fold concentration of Fe(II),and 20-fold Cu(II), have no influence on the signals of 50 nM Hg(II) with deviation below 5%. | [150] | |
| MWCNT | PANi-Bi NPs@GO | Non covalent | 2 ppt | 1.3 µA/ppb (2 ppt~1000 ppm) | Differential pulse voltammetry | Screen printing | (commercial) Carbon ink | PET | Not provided | [151] | |
| As(III) | MWCNT | COOH | Covalent | N.A | 0.24 µA/ppb (0.3~50 ppb) | Stripping voltammetry | Dip coating | Au | Au electrode | Interference was significant when the Sb/As ratio is higher than 1. | [154] |
| MWCNT | Au-NP | Non covalent | 0.1 ppb | 26 µA/ppb (75 ppt–5.3 ppm) | Stripping voltammetry | Drop casting | Glassy carbon | Glassy carbon | Not provided | [157] | |
| Cu(II) | SWCNT | PANI-GGHH | Non covalent | 3 ppt | N/A (3~29 ppt) | FET (liquid gate) | CVD | 300 nm Au | Si/SiO2 (120nm) | His6 shows higher chelation power for Ni(II) than to Cu(II). | [113] |
| MWCNT | C24H30N6 Schiff base | Non covalent | 10 ppt | N/A (0.09~340 ppb) | Stripping voltammetry | Paste of MWCNT, Schiff base and mineral oil | Copper wire | Filter membrane | Not provided | [160] | |
| MWCNT | Pristine | Non functionalized | 17 ppt | 9.4 pA/ppb (32~220 ppb) | Stripping voltammetry | CNT thread aspirated into a glass capillary | Metal wire and silver conductive epoxy | Glass capillary | Simultaneous determination of Cd(II), Cu(II), Pb(II) and Zn(II) demonstrated The presence of Dissolved Oxygen changes the calibration law for Cd(II) | [129] | |
| Nitrite | MWCNT | Co3O4-rGO | Non covalent | 0.016 µM | 0.408 µA/µM/cm2 (0.1~8000 µM) | Voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | 100-fold of alcohol, Na+, K+, Cl−, NO3−, N2H4, SO32−, SO42−, has no effect on sensor response. | [167] |
| Ca(II) | SWCNT | Fluo-4 AM | Non-covalent | 100 pM | 69 nA/decade (100 nM~1 mM) | FET | Dip coating | Ti (10 nm)/Au (30 nm) (liquid, floating gate) | Glass (borosilicate glass capillary) | FET at the end of a nanoneedle for intracell monitoring | [178] |
| MWCNT | PDMS | Non-covalent | 25 µM | N/A (25 µM~5 mM (Not linear)) | Capacitive measurement | Mold injection and thermal curing | MWCNT | PDMS | Measured at 2.4 kHz frequency | [179] | |
| O2 | MWCNT | Au NP | Non-covalent | 0.1 ppm | N/A (0~50 ppm) | Cyclic voltammetry | Not provided | Glassy carbon electrode | Glassy carbon | Not provided | [186] |
| Hydrogen peroxide | MWCNT | nitrogen doped Co-CNTs over graphene sheets | Non covalent | 100nM 3.4 ppb | −0.85 µA/ppm | Voltammetry, amperometry | Coating | Glassy carbon electrode | Glassy carbon electrode | No interference with uric acid, ascorbic acid and glucose | [188] |
| SWCNT | Phenyl capped aniline tetramer | Non covalent | <3 ppm | 1%/ppm (3 ppm~8 ppm) Nonlinear <1%/100 ppm (48 ppm~1200 ppm) | Chemistor | Drop casting | Carbon ink | Glass | Not provided | [193] | |
| Free chlorine | MWCNT | Pristine | Non functionalized | <5 ppb (ClO−) | Logarithmic 39% /decade * (0.03~8 ppm) | Chemistor | Dielectrophoresis (aligned MWCNT) | Cr/Au | Glass | No information about selectivity, pH information not provided | [191] |
| MWCNT | Epoxy EpoTek H77A | Non covalent | 20 ppb (HClO) | 0.15 µA/ppb (0.02~4 ppm) | Voltammetry | Paste poured into tube and thermally cured | Epoxy/MWCNT composite | Not provided (tube) | Validated in real water matrices (tap water and swimming pool) | [194] | |
| SWCNT | Phenyl capped aniline tetramer | Covalent | <60 ppb | 92 nA/decade (0.06~60 ppm (linear up to 6 ppm)) | Chemistor | Drop casting | Au | Glass | Non selective to different oxidants—list of oxidants not provided Regeneration possible | [53] | |
| Sulfite | MWCNT | Ferrocene | Non covalent (Physical immobilization) | 0.1 µM | 3.3 µA/µM (0.4 µM~4 µM) 0.18 µA/µM (4 µM~120 µM) | Differential Pulse Voltammetry | Paste mixture with graphite powder blended with paraffin oil | MWCNT paste, Copper wire | Glass tube | Not provided | [78] |
| Sulfide | MWCNT | Hematoxylin | Non covalent | 0.2 µM | 103 nA/µM(0.5 µM~150 µM) | Amperometry | Paste mixture of MWCNT, mineral oil and graphite powder | Carbon paste | Teflon tube | No interference with Sn(II), Co(II), Pb(II), Zn(II), Cu(II), Ni(II), Mn(II), Fe(II) and Fe(III) | [197] |
| MWCNT | Pristine | Not functionalized | 0.3 µM (CVD), 12.5 µM (ARC) | 0.12 µA/µM (1.3 µM~113 µM) (CVD), 0.005 µA/µM (12.5 µM~87.5 µM) (ARC) | Hydrodynamic voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | Not provided | [199] | |
| Bisphenol A | SWCNT | βCD | Covalent | 1.0 nM | 1.3 mA/mM 11 nM–19 µM | Cyclic voltammetry | Drop casting | Glassy carbon electrode | Glassy carbon | No interference study, but tested on real plastic samples | [206] |
4.2.3. Covalent versus Non-Covalent Functionalization
4.2.4. On the Diversity of Functional Probes
4.2.5. Type of CNT and CNT Alignment
4.3. Challenges and Perspectives
4.3.1. Optimal Sensing and Sensing Mechanisms—The Role of Modelling
4.3.2. Covering the Extreme Diversity of Analytes
4.3.3. Managing the Complexity of the Water Matrices through e-Tongue Strategy
4.3.4. Toward Real Applications: The Need for Ageing Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hinrichsen, D.; Tacio, H. The Coming Freshwater Crisis Is Already Here; ESCP Publication: Washington, DC, USA, 2002; pp. 1–26. [Google Scholar]
- Solomon, A.; Ahmed, Z.; Biruktawit, K.; Amare, D.; Solomon, A.; Endalew, Z.; Abera, S.; Zeyinudin, A.; Kebede, B.; Deribew, A.; et al. Bacteriological analysis of drinking water sources. Afr. J. Microbiol. Res. 2011, 5, 2638–2641. [Google Scholar] [CrossRef]
- Aydin, A. The microbiological and physico-chemical quality of groundwater in West Thrace, Turkey. Pol. J. Environ. Stud. 2007, 16, 377–383. [Google Scholar]
- Gyamfi, E.; Ackah, M.; Anim, A.; Hanson, J.; Kpattah, L.; Enti-Brown, S.; Adjei-Kyereme, Y.; Nyarko, E. Chemical analysis of potable water samples from selected suburbs of Accra, Ghana. Proc. Int. Acad. Ecol. Environ. Sci. 2012, 2, 118–127. [Google Scholar]
- Clark, R.M.; Coyle, J.A. Measuring and Modeling Variations in Distribution System Water Quality. J.-Am. Water Work. Assoc. 1990, 82, 46–53. [Google Scholar] [CrossRef]
- Raich, J. Review of sensors to monitor water quality. Eur. Ref. Netw. Crit. Infrastruct. Prot. 2013. [Google Scholar] [CrossRef]
- Kruse, P. Review on water quality sensors. J. Phys. D Appl. Phys. 2018, 51, 203002. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Gupta, K.K.; Gupta, R. A review of emerging trends on water quality measurement sensors. In Proceedings of the 2015 International Conference on Technologies for Sustainable Development (ICTSD), Mumbai, India, 4–6 February 2015; pp. 1–6. [Google Scholar] [CrossRef]
- Mao, S.; Chang, J.; Zhou, G.; Chen, J. Nanomaterial-enabled Rapid Detection of Water Contaminants. Small 2015, 11, 5336–5359. [Google Scholar] [CrossRef]
- Soni, R.; Soni, M.; Shukla, D.P. Emerging Techniques and Materials for Water Pollutants Detection. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Singapore, 2019; pp. 277–297. [Google Scholar] [CrossRef]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Calvo, J.Q.; Palleschi, G.; Moscone, D.; Amine, A. Bismuth-modified electrodes for lead detection. TrAC Trends Anal. Chem. 2010, 29, 1295–1304. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-Enabled Wearable Sensors for Healthcare. Adv. Healthc. Mater. 2017, 7, 1700889. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Gupta, K.K.; Gupta, R.K. Raspberry Pi-based smart sensing platform for drinking-water quality monitoring system: A Python framework approach. Drink. Water Eng. Sci. 2019, 12, 31–37. [Google Scholar] [CrossRef]
- Subramanian, B.; Chen, S.S.; Reddy, K.R. Emerging Technologies for Agriculture and Environment: Select Proceedings of ITsFEW 2018; Springer: Singapore, 2020. [Google Scholar]
- Luo, X.; Yang, J. A Survey on Pollution Monitoring Using Sensor Networks in Environment Protection. J. Sens. 2019, 2019, 6271206. [Google Scholar] [CrossRef]
- Arash, B.; Wang, Q.; Varadan, V.K. Carbon Nanotube-Based Sensors for Detection of Gas Atoms. J. Nanotechnol. Eng. Med. 2011, 2, 021010. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Carbon nanotube-based fluorescence sensors. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 20–34. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Arif, K.M. A comprehensive review of micro fluidic water quality monitoring sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Wigginton, K.R. Nanomaterial Enabled Biosensors for Pathogen Monitoring—A Review. Environ. Sci. Technol. 2010, 44, 3656–3669. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, J. Chapter 2—Structure and Properties of Carbon Nanotubes. In Industrial Applications of Carbon Nanotubes; Peng, H., Li, Q., Chen, T., Eds.; Elsevier: Boston, MA, USA, 2017; pp. 47–69. [Google Scholar]
- Soni, S.K.; Thomas, B.; Kar, V.R. A Comprehensive Review on CNTs and CNT-Reinforced Composites: Syntheses, Characteristics and Applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar] [CrossRef]
- Aius, A. Graphene Honeycomb Lattice. CC BY-SA 3.0.
- Zhang, Y.; Bai, Y.; Yan, B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov. Today 2010, 15, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mubeen, S.; Myung, N.V.; Deshusses, M.A. Recent progress in carbon nanotube-based gas sensors. Nanotechnology 2008, 19, 332001. [Google Scholar] [CrossRef]
- Andringa, A.-M.; Meijboom, J.R.; Smits, E.C.P.; Mathijssen, S.G.J.; Blom, P.W.M.; de Leeuw, D.M. Gate-Bias Controlled Charge Trapping as a Mechanism for NO2 Detection with Field-Effect Transistors. Adv. Funct. Mater. 2010, 21, 100–107. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Banerjee, S.; Hemraj-Benny, T.; Wong, S.S. Covalent Surface Chemistry of Single-Walled Carbon Nanotubes. Adv. Mater. 2005, 17, 17–29. [Google Scholar] [CrossRef]
- Star, A.; Stoddart, J.F.; Steuerman, D.; Diehl, M.; Boukai, A.; Wong, E.W.; Yang, X.; Chung, S.-W.; Choi, H.; Heath, J.R. Preparation and Properties of Polymer-Wrapped Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2001, 40, 1721–1725. [Google Scholar] [CrossRef]
- Britz, D.A.; Khlobystov, A.N. Noncovalent interactions of molecules with single walled carbon nanotubes. Chem. Soc. Rev. 2006, 35, 637–659. [Google Scholar] [CrossRef] [PubMed]
- Bondavalli, P.; Gorintin, L.; Feugnet, G.; Lehoucq, G.; Pribat, D. Selective gas detection using CNTFET arrays fabricated using air-brush technique, with different metal as electrodes. Sens. Actuators B Chem. 2014, 202, 1290–1297. [Google Scholar] [CrossRef]
- Cardenas-Benitez, B.; Djordjevic, I.; Hosseini, S.; Madou, M.J.; Martinez-Chapa, S.O. Review—Covalent Functionalization of Carbon Nanomaterials for Biosensor Applications: An Update. J. Electrochem. Soc. 2018, 165, B103–B117. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed]
- Toshimitsu, F.; Nakashima, N. Semiconducting single-walled carbon nanotubes sorting with a removable solubilizer based on dynamic supramolecular coordination chemistry. Nat. Commun. 2014, 5, 5041. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, G.; Lebental, B.; Loisel, L.; Ramachandran, S.; Gutiérrez, A.F.; Wang, X.; Godumala, M.; Bodeleot, L. Chemical Sensors Based on Carbon Nanotubes Functionalised by Conjugated Polymers for Analysis in Aqueous Medium. Patent 3609881A1, 10 April 2018. [Google Scholar]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suárez, G. Surface modification of multiwall carbon nanotubes by sulfonitric treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Gong, H.; Kim, S.-T.; Lee, J.D.; Yim, S. Simple quantification of surface carboxylic acids on chemically oxidized multi-walled carbon nanotubes. Appl. Surf. Sci. 2013, 266, 219–224. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Anantram, M.P.; Léonard, F. Physics of carbon nanotube electronic devices. Rep. Prog. Phys. 2006, 69, 507–561. [Google Scholar] [CrossRef]
- Tans, S.J.; Verschueren, A.R.M.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar] [CrossRef]
- Snow, E.S.; Novak, J.P.; Campbell, P.M.; Park, D. Random networks of carbon nanotubes as an electronic material. Appl. Phys. Lett. 2003, 82, 2145–2147. [Google Scholar] [CrossRef]
- Zhou, X.; Boey, F.; Zhang, H. Controlled growth of single-walled carbon nanotubes on patterned substrates. Chem. Soc. Rev. 2011, 40, 5221–5231. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low temperature growth of carbon nanotubes—A review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Seidel, R.; Graham, A.P.; Unger, E.; Duesberg, G.S.; Liebau, M.; Steinhoegl, W.; Kreupl, F.; Hoenlein, W.; Pompe, W. High-Current Nanotube Transistors. Nano Lett. 2004, 4, 831–834. [Google Scholar] [CrossRef]
- Zhang, G.; Qi, P.; Wang, X.; Lu, Y.; Li, X.; Tu, R.; Bangsaruntip, S.; Mann, D.; Zhang, L.; Dai, H. Selective Etching of Metallic Carbon Nanotubes by Gas-Phase Reaction. Science 2006, 314, 974–977. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xian, X.; Zhang, J.; Liu, Z. Sorting out Semiconducting Single-Walled Carbon Nanotube Arrays by Preferential Destruction of Metallic Tubes Using Xenon-Lamp Irradiation. J. Phys. Chem. C 2008, 112, 3849–3856. [Google Scholar] [CrossRef]
- Zhao, Q.; Yao, F.; Wang, Z.; Deng, S.; Tong, L.; Liu, K.; Zhang, J. Real-Time Observation of Carbon Nanotube Etching Process Using Polarized Optical Microscope. Adv. Mater. 2017, 29, 1701959. [Google Scholar] [CrossRef]
- Lei, T.; Pochorovski, I.; Bao, Z. Separation of Semiconducting Carbon Nanotubes for Flexible and Stretchable Electronics Using Polymer Removable Method. Acc. Chem. Res. 2017, 50, 1096–1104. [Google Scholar] [CrossRef]
- Hsu, L.H.H.; Hoque, E.; Kruse, P.; Selvaganapathy, P.R. A carbon nanotube based resettable sensor for measuring free chlorine in drinking water. Appl. Phys. Lett. 2015, 106, 063102. [Google Scholar] [CrossRef]
- Pyo, J.-Y.; Cho, W.-J. High-sensitivity pH sensor using separative extended-gate field-effect transistors with single-walled carbon-nanotube networks. Jpn. J. Appl. Phys. 2018, 57, 04FP02. [Google Scholar] [CrossRef]
- Wei, L.; Lu, D.; Wang, J.; Wei, H.; Zhao, J.; Geng, H.; Zhang, Y. Highly sensitive detection of trinitrotoluene in water by chemiresistive sensor based on noncovalently amino functionalized single-walled carbon nanotube. Sens. Actuators B Chem. 2013, 190, 529–534. [Google Scholar] [CrossRef]
- Eshkalak, S.K.; Chinnappan, A.; Jayathilaka, D.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. A review on inkjet printing of CNT composites for smart applications. Appl. Mater. Today 2017, 9, 372–386. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Lee, K.-S.; Lee, Y.-H.; Ju, B.-K. Single-Wall Carbon Nanotube-Based pH Sensor Fabricated by the Spray Method. Electrochem. Solid-State Lett. 2006, 9, H85–H87. [Google Scholar] [CrossRef]
- Goh, G.L.; Agarwala, S.; Tan, Y.J.; Yeong, W.Y. A low cost and flexible carbon nanotube pH sensor fabricated using aerosol jet technology for live cell applications. Sens. Actuators B Chem. 2018, 260, 227–235. [Google Scholar] [CrossRef]
- Stojanović, G.; Kojić, T.; Radovanović, M.; Vasiljević, D.; Panić, S.; Srdić, V.; Cvejić, J. Flexible sensors based on two conductive electrodes and MWCNTs coating for efficient pH value measurement. J. Alloys Compd. 2019, 794, 76–83. [Google Scholar] [CrossRef]
- Gou, P.; Kraut, N.D.; Feigel, I.M.; Bai, H.; Morgan, G.J.; Chen, Y.; Tang, Y.; Bocan, K.; Stachel, J.; Berger, L.; et al. Carbon Nanotube Chemiresistor for Wireless pH Sensing. Sci. Rep. 2014, 4, 4468. [Google Scholar] [CrossRef]
- Endo, M.; Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Terrones, M.; Dresselhaus, M.S. ‘Buckypaper’ from coaxial nanotubes. Nature 2005, 433, 476. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, R.L. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001; Volume 38, pp. 1364–1365. [Google Scholar]
- Biesheuvel, P.M.; Porada, S.; Dykstra, J.E. The difference between Faradaic and non-Faradaic electrode processes. arXiv 2021, arXiv:1809.02930. [Google Scholar]
- Bakker, E.; Pretsch, E. Potentiometric sensors for trace-level analysis. TrAC Trends Anal. Chem. 2005, 24, 199–207. [Google Scholar] [CrossRef]
- Hanrahan, G.; Patil, D.G.; Wang, J. Electrochemical sensors for environmental monitoring: Design, development and applications. J. Environ. Monit. 2004, 6, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Simões, F.R.; Xavier, M.G. 6—Electrochemical Sensors. In Nanoscience and Its Applications; Róz, A.L.D., Ferreira, M., Leite, F.L., Osvaldo, N., Oliveira, A., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 155–178. [Google Scholar]
- March, G.; Nguyen, T.D.; Piro, B. Modified Electrodes Used for Electrochemical Detection of Metal Ions in Environmental Analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.; Sun, C. Amperometric sulfite sensor based on multiwalled carbon nanotubes/ferrocene-branched chitosan composites. Talanta 2008, 77, 366–371. [Google Scholar] [CrossRef]
- Gao, X.; Wei, W.; Yang, L.; Guo, M. Carbon Nanotubes/Poly(1,2-diaminobenzene) Nanoporous Composite Film Electrode Prepared by Multipulse Potentiostatic Electropolymerisation and Its Application to Determination of Trace Heavy Metal Ions. Electroanalysis 2006, 18, 485–492. [Google Scholar] [CrossRef]
- Farid, M.U.; Khanzada, N.K.; An, A.K. Understanding fouling dynamics on functionalized CNT-based membranes: Mechanisms and reversibility. Desalination 2019, 456, 74–84. [Google Scholar] [CrossRef]
- Britto, P.; Santhanam, K.; Ajayan, P. Carbon nanotube electrode for oxidation of dopamine. Bioelectrochem. Bioenerg. 1996, 41, 121–125. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, L.; Xing, S.; Shi, G.; Xian, Y.; Jin, L. Microwave-radiated synthesis of gold nanoparticles/carbon nanotubes composites and its application to voltammetric detection of trace mercury(II). Electrochem. Commun. 2008, 10, 1839–1843. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, L.; Xing, S.; Xian, Y.; Shi, G.; Jin, L. Ultrasensitive Voltammetric Detection of Trace Lead(II) and Cadmium(II) Using MWCNTs-Nafion/Bismuth Composite Electrodes. Electroanalysis 2008, 20, 2655–2662. [Google Scholar] [CrossRef]
- Morton, J.; Havens, N.; Mugweru, A.; Wanekaya, A.K. Detection of Trace Heavy Metal Ions Using Carbon Nanotube- Modified Electrodes. Electroanalysis 2009, 21, 1597–1603. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Zhang, X.; Fang, B. Amperometric Detection of Hydrogen Peroxide Using Glassy Carbon Electrodes Modified with Chromium Hexacyanoferrate/Single-Walled Carbon Nanotube Nanocomposites. Electroanalysis 2009, 21, 179–183. [Google Scholar] [CrossRef]
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D.; Wang, C. Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal. Chim. Acta 2012, 724, 40–46. [Google Scholar] [CrossRef]
- Hu, C.; Hu, S. Carbon Nanotube-Based Electrochemical Sensors: Principles and Applications in Biomedical Systems. J. Sens. 2009, 2009, 187615. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ensafi, A.A.; Beitollahi, H.; Nasiri, V.; Khalilzadeh, M.A.; Biparva, P. Electrocatalytic determination of sulfite using a modified carbon nanotubes paste electrode: Application for determination of sulfite in real samples. Ionics 2012, 18, 687–694. [Google Scholar] [CrossRef]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef]
- Gong, K.; Yan, Y.; Zhang, M.; Su, L.; Xiong, S.; Mao, L. Electrochemistry and Electroanalytical Applications of Carbon Nanotubes: A Review. Anal. Sci. 2005, 21, 1383–1393. [Google Scholar] [CrossRef][Green Version]
- He, Y.; Chen, W.; Gao, C.; Zhou, J.; Li, X.; Xie, E. An overview of carbon materials for flexible electrochemical capacitors. Nanoscale 2013, 5, 8799–8820. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, A.X.; Du, Z. Effect of Chemical Oxidation on the Structure of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Luo, H.; Shi, Z.; Li, N.; Gu, Z.; Zhuang, Q. Investigation of the Electrochemical and Electrocatalytic Behavior of Single-Wall Carbon Nanotube Film on a Glassy Carbon Electrode. Anal. Chem. 2001, 73, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zahab, A.; Spina, L.; Poncharal, P.; Marlière, C. Water-vapor effect on the electrical conductivity of a single-walled carbon nanotube mat. Phys. Rev. B 2000, 62, 10000–10003. [Google Scholar] [CrossRef]
- Van der Pauw, L.J. A method of measuring specific resistivity and hall effect of discs of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon nanotube-based chemiresistive sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef]
- Li, P.; Martin, C.M.; Yeung, K.K.; Xue, W. Dielectrophoresis aligned single-walled carbon nanotubes as pH sensors. Biosensors 2011, 1, 23–35. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Lee, G.S. pH-sensing characteristics of multi-walled carbon nanotube sheet. Mater. Lett. 2014, 122, 281–284. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Delzeit, L.; Meyyappan, M. A Gas Sensor Array Using Carbon Nanotubes and Microfabrication Technology. Electrochem. Solid-State Lett. 2005, 8, H100–H102. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Michelis, F.; Bodelot, L.; Bonnassieux, Y.; Lebental, B. Highly reproducible, hysteresis-free, flexible strain sensors by inkjet printing of carbon nanotubes. Carbon 2015, 95, 1020–1026. [Google Scholar] [CrossRef]
- Naderi, A.; Tahne, B.A. Review—Methods in Improving the Performance of Carbon Nanotube Field Effect Transistors. ECS J. Solid State Sci. Technol. 2016, 5, M131–M140. [Google Scholar] [CrossRef]
- Imam, S.-A.; Kalam, N.; Abdullah, S. Comparative Analysis of Control Coefficients on the Performance of CNTFET Under Different Parameters. Int. J. Nanosci. 2016, 15, 1640005. [Google Scholar] [CrossRef]
- Karimi, N.V.; Pourasad, Y. Investigating the effect of some parameters of the channel on the characteristics of tunneling carbon nanotube field-effect transistor. Int. Nano Lett. 2016, 6, 215–221. [Google Scholar] [CrossRef]
- Kim, W.; Javey, A.; Vermesh, O.; Wang, Q.; Li, A.Y.; Dai, H. Hysteresis Caused by Water Molecules in Carbon Nanotube Field-Effect Transistors. Nano Lett. 2003, 3, 193–198. [Google Scholar] [CrossRef]
- Joshi, S.; Bhatt, V.D.; Rani, H.; Becherer, M.; Lugli, P. Understanding the influence of in-plane gate electrode design on electrolyte gated transistor. Microelectron. Eng. 2018, 199, 87–91. [Google Scholar] [CrossRef]
- Saito, R.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 1992, 60, 2204–2206. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Rinzan, M.; Boyd, A.K.; Yang, Y.; Guadagno, L.; Giubileo, F.; Barbara, P. Electrical properties and memory effects of field-effect transistors from networks of single- and double-walled carbon nanotubes. Nanotechnology 2010, 21, 115204. [Google Scholar] [CrossRef]
- Derycke, V.; Martel, R.; Appenzeller, J.; Avouris, P. Carbon Nanotube Inter- and Intramolecular Logic Gates. Nano Lett. 2001, 1, 453–456. [Google Scholar] [CrossRef]
- Zhao, J.; Hashmi, A.; Xu, J.; Xue, W. A compact lab-on-a-chip nanosensor for glycerol detection. Appl. Phys. Lett. 2012, 100, 243109. [Google Scholar] [CrossRef]
- Pimparkar, N.; Kocabas, C.; Kang, S.J.; Rogers, J.; Alam, M. Limits of Performance Gain of Aligned CNT Over Randomized Network: Theoretical Predictions and Experimental Validation. IEEE Electron Device Lett. 2007, 28, 593–595. [Google Scholar] [CrossRef]
- Barman, S.N.; Lemieux, M.C.; Baek, J.; Rivera, R.; Bao, Z. Effects of Dispersion Conditions of Single-Walled Carbon Nanotubes on the Electrical Characteristics of Thin Film Network Transistors. ACS Appl. Mater. Interfaces 2010, 2, 2672–2678. [Google Scholar] [CrossRef]
- Ruiz-Soria, G.; Paz, A.P.; Sauer, M.; Mowbray, D.J.; Lacovig, P.; Dalmiglio, M.; Lizzit, S.; Yanagi, K.; Rubio, A.; Goldoni, A.; et al. Revealing the adsorption mechanisms of nitroxides on ultrapure, metallicity-sorted carbon nanotubes. ACS Nano 2014, 8, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, H.S.; Kim, T.; Kim, J.; Seo, S.; Lee, B.Y. Real-time monitoring of microbial activity using hydrogel-hybridized carbon nanotube transistors. Sens. Actuators B Chem. 2018, 263, 486–492. [Google Scholar] [CrossRef]
- Takeda, S.; Nakamura, M.; Ishii, A.; Subagyo, A.; Hosoi, H.; Sueoka, K.; Mukasa, K. A pH sensor based on electric properties of nanotubes on a glass substrate. Nanoscale Res. Lett. 2007, 2, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Münzer, A.; Melzer, K.; Heimgreiter, M.; Scarpa, G. Random CNT network and regioregular poly(3-hexylthiophen) FETs for pH sensing applications: A comparison. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.I.; Eremina, V.A.; Shook, J.; Collins, A.; Walker, P.; Fedotov, P.V.; Zakhidov, A.A.; Obraztsova, E.D. Field Effect Transistor Based on Solely Semiconducting Single-Walled Carbon Nanotubes for the Detection of 2-Chlorophenol. Phys. Status Solidi B 2017, 255, 1700139. [Google Scholar] [CrossRef]
- Lee, N.H.; Nahm, S.-H.; Choi, I.S. Real-Time Monitoring of a Botulinum Neurotoxin Using All-Carbon Nanotube-Based Field-Effect Transistor Devices. Sensors 2018, 18, 4235. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.; Hong, S. Highly Selective Environmental Nanosensors Based on Anomalous Response of Carbon Nanotube Conductance to Mercury Ions. J. Phys. Chem. C 2009, 113, 19393–19396. [Google Scholar] [CrossRef]
- Lee, C.-S.; Ju, Y.; Kim, J.; Kim, T.H. Electrochemical functionalization of single-walled carbon nanotubes with amine-terminated dendrimers encapsulating Pt nanoparticles: Toward facile field-effect transistor-based sensing platforms. Sens. Actuators B Chem. 2018, 275, 367–372. [Google Scholar] [CrossRef]
- Joshi, S.; Bhatt, V.D.; Jaworska, E.; Michalska, A.; Maksymiuk, K.; Becherer, M.; Gagliardi, A.; Lugli, P. Ambient Processed, Water-Stable, Aqueous-Gated sub 1 V n-type Carbon Nanotube Field Effect Transistor. Sci. Rep. 2018, 8, 11386. [Google Scholar] [CrossRef]
- Forzani, E.S.; Li, X.; Zhang, P.; Tao, N.; Zhang, R.; Amlani, I.; Tsui, R.; Nagahara, L.A. Tuning the Chemical Selectivity of SWNT-FETs for Detection of Heavy-Metal Ions. Small 2006, 2, 1283–1291. [Google Scholar] [CrossRef]
- Star, A.; Bradley, K.; Gabriel, J.-C.; Gruner, G. Nano-Electronic Sensors: Chemical Detection Using Carbon Nanotubes. Polym. Mater. Sci. Eng. 2003, 89, 204. [Google Scholar]
- Rosenblatt, S.; Yaish, Y.; Park, J.; Gore, J.; Sazonova, V.; McEuen, P.L. High Performance Electrolyte Gated Carbon Nanotube Transistors. Nano Lett. 2002, 2, 869–872. [Google Scholar] [CrossRef]
- Xu, X.; Tang, Z.; Shao, J.; Fan, Z. The study on the interface adhesion comparison of MgF2, Al2O3, SiO2, and Ag thin films. Appl. Surf. Sci. 2005, 245, 11–15. [Google Scholar] [CrossRef]
- Boyd, A.; Dube, I.; Fedorov, G.; Paranjape, M.; Barbara, P. Gas sensing mechanism of carbon nanotubes: From single tubes to high-density networks. Carbon 2014, 69, 417–423. [Google Scholar] [CrossRef]
- Benda, R.; Cancès, E.; Lebental, B. Effective resistance of random percolating networks of stick nanowires: Functional dependence on elementary physical parameters. J. Appl. Phys 2019, 126, 44306. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors 2020, 20, 2221. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cheng, N.; Luo, Y.; Lin, Y.; Xu, W.; Du, D. Recent advances in nanomaterials-based electrochemical (bio)sensors for pesticides detection. TrAC Trends Anal. Chem. 2020, 132, 116041. [Google Scholar] [CrossRef]
- Covington, A.K.; Bates, R.G.; Durst, R.A. Definition of pH scales, standard reference values, measurement of pH and related terminology (Recommendations 1984). Pure Appl. Chem. 1985, 57, 531–542. [Google Scholar] [CrossRef]
- World Health Organization. pH in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Lei, K.F.; Lee, K.-F.; Yang, S.-I. Fabrication of carbon nanotube-based pH sensor for paper-based microfluidics. Microelectron. Eng. 2012, 100, 1–5. [Google Scholar] [CrossRef]
- Jasti, R.; Bhattacharjee, J.; Neaton, J.B.; Bertozzi, C.R. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]-Cycloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Yang, Y.; Liang, H.; Wang, L.; Song, Y. Electroactive Covalent Organic Frameworks/Carbon Nanotubes Composites for Electrochemical Sensing. ACS Appl. Nano Mater. 2020, 3, 1412–1419. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, C.; Zhang, Y.; Strong, V.; Tang, J.; Li, X.-G.; Kalantar-Zadeh, K.; Hoek, E.M.V.; Wang, K.L.; Kaner, R.B. Carbon Nanotube/Polyaniline Composite Nanofibers: Facile Synthesis and Chemosensors. Nano Lett. 2011, 11, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-Y.; Kang, B.-C.; Ha, T.-J. Flexible pH sensors based on printed nanocomposites of single-wall carbon nanotubes and Nafion. Appl. Surf. Sci. 2020, 514, 145956. [Google Scholar] [CrossRef]
- Kaempgen, M.; Roth, S. Transparent and Flexible Carbon Nanotube/Polyaniline pH Sensor. J. Electroanal. Chem. 2005, 586, 72–76. [Google Scholar] [CrossRef]
- Zhao, D.; Guo, X.; Wang, T.; Alvarez, N.; Shanov, V.N.; Heineman, W.R. Simultaneous Detection of Heavy Metals by Anodic Stripping Voltammetry Using Carbon Nanotube Thread. Electroanalysis 2014, 26, 488–496. [Google Scholar] [CrossRef]
- Rahm, C.E.; Torres-Canas, F.; Gupta, P.; Poulin, P.; Alvarez, N.T. Inkjet Printed Multi-walled Carbon Nanotube Sensor for the Detection of Lead in Drinking Water. Electroanalysis 2020, 32, 1533–1545. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Santos, V.; Baeta, B.; Pereira, A.; Kubota, L.T. Simultaneous determination of zinc, cadmium and lead in environmental water samples by potentiometric stripping analysis (PSA) using multiwalled carbon nanotube electrode. J. Hazard. Mater. 2009, 169, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Yu, F.; Zhu, L.; Wang, X.; Yang, N. Bucky-gel coated glassy carbon electrodes, for voltammetric detection of femtomolar leveled lead ions. Talanta 2010, 82, 1820–1825. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Shi, G.; Peng, C.; Ding, Y. Thiacalixarene Covalently Functionalized Multiwalled Carbon Nanotubes as Chemically Modified Electrode Material for Detection of Ultratrace Pb2+ Ions. Anal. Chem. 2012, 84, 10560–10567. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, N.E.; Khan, A. Design and Preparation of a New and Novel Nanocomposite with CNTs and Its Sensor Applications. J. Mater. Res. Technol. 2019, 8, 2238–2246. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular Ordering of Organic Molten Salts Triggered by Single-Walled Carbon Nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Hai, T.L.; Hung, L.C.; Phuong, T.T.B.; Ha, B.T.T.; Nguyen, B.-S.; Hai, T.D.; Nguyen, V.-H. Multiwall carbon nanotube modified by antimony oxide (Sb2O3/MWCNTs) paste electrode for the simultaneous electrochemical detection of cadmium and lead ions. Microchem. J. 2020, 153, 104456. [Google Scholar] [CrossRef]
- Wang, J. Stripping Analysis at Bismuth Electrodes: A Review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Injang, U.; Noyrod, P.; Siangproh, W.; Dungchai, W.; Motomizu, S.; Chailapakul, O. Determination of trace heavy metals in herbs by sequential injection analysis-anodic stripping voltammetry using screen-printed carbon nanotubes electrodes. Anal. Chim. Acta 2010, 668, 54–60. [Google Scholar] [CrossRef]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar] [CrossRef]
- Ivaska, A.; Kubiak, W.W. Application of sequential injection analysis to anodic stripping voltammetry. Talanta 1997, 44, 713–723. [Google Scholar] [CrossRef]
- Mohammadi, S.; Taher, M.A.; Beitollahi, H.; Naghizadeh, M. Sensitive voltammetric determination of cadmium at a carbon nanotubes/Fe3O4/eggshell composites modified carbon paste electrode. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100241. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Wang, E. High-Sensitivity Determination of Lead(II) and Cadmium(II) Based on the CNTs-PSS/Bi Composite Film Electrode. Electroanalysis 2010, 22, 1682–1687. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y.; Tu, Y.; Ren, Z. Ultrasensitive voltammetric detection of trace heavy metal ions using carbon nanotube nanoelectrode array. Analyst 2005, 130, 1098–1101. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, X.; Ma, Q.; Tang, B.; Lu, Z.; Zhang, J.; Mo, G.; Ye, J.; Ye, J. A sensitive electrochemical sensor for simultaneous voltammetric sensing of cadmium and lead based on Fe3O4/multiwalled carbon nanotube/laser scribed graphene composites functionalized with chitosan modified electrode. Mater. Chem. Phys. 2019, 238, 121877. [Google Scholar] [CrossRef]
- Oularbi, L.; Turmine, M.; El Rhazi, M. Preparation of novel nanocomposite consisting of bismuth particles, polypyrrole and multi-walled carbon nanotubes for simultaneous voltammetric determination of cadmium(II) and lead(II). Synth. Met. 2019, 253, 1–8. [Google Scholar] [CrossRef]
- Xuan, X.; Park, J.Y. A miniaturized and flexible cadmium and lead ion detection sensor based on micro-patterned reduced graphene oxide/carbon nanotube/bismuth composite electrodes. Sen. Actuators Chem. 2018, 255, 1220–1227. [Google Scholar] [CrossRef]
- Safavi, A.; Maleki, N.; Doroodmand, M.M. Fabrication of a selective mercury sensor based on the adsorption of cold vapor of mercury on carbon nanotubes: Determination of mercury in industrial wastewater. J. Hazard. Mater. 2010, 173, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guerin, D.; Lmimouni, K. Improving performance of OFET by tuning occurrence of charge transport based on pentacene interaction with SAM functionalized contacts. Microelectron. Eng. 2018, 195, 62–67. [Google Scholar] [CrossRef]
- Bassyouni, M.; Mansi, A.E.; ElGabry, A.; Ibrahim, B.A.; Kassem, O.A.; Alhebeshy, R. Utilization of carbon nanotubes in removal of heavy metals from wastewater: A review of the CNTs’ potential and current challenges. Appl. Phys. A 2019, 126, 38. [Google Scholar] [CrossRef]
- Wei, J.; Yang, D.; Chen, H.; Gao, Y.; Li, H. Stripping voltammetric determination of mercury(II) based on SWCNT-PhSH modified gold electrode. Sens. Actuators B Chem. 2014, 190, 968–974. [Google Scholar] [CrossRef]
- Bao, Q.; Li, G.; Yang, Z.; Pan, P.; Liu, J.; Chang, J.; Wei, J.; Lin, L. Electrochemical performance of a three-layer electrode based on Bi nanoparticles, multi-walled carbon nanotube composites for simultaneous Hg(II) and Cu(II) detection. Chin. Chem. Lett. 2020, 31, 2752–2756. [Google Scholar] [CrossRef]
- Deng, W.; Tan, Y.; Li, Y.; Wen, Y.; Su, Z.; Huang, Z.; Huang, S.; Meng, Y.; Xie, Q.; Luo, Y.; et al. Square wave voltammetric determination of Hg(II) using thiol functionalized chitosan-multiwalled carbon nanotubes nanocomposite film electrode. Microchim. Acta 2010, 169, 367–373. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Ramezani, S.; Rofouei, M.K. Development of a novel MWCNTs–triazene-modified carbon paste electrode for potentiometric assessment of Hg(II) in the aquatic environments. Mater. Sci. Eng. C 2015, 47, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Profumo, A.; Fagnoni, M.; Merli, D.; Quartarone, E.; Protti, S.; Dondi, D.; Albini, A. Multiwalled Carbon Nanotube Chemically Modified Gold Electrode for Inorganic As Speciation and Bi(III) Determination. Anal. Chem. 2006, 78, 4194–4199. [Google Scholar] [CrossRef]
- Daud, N.; Yusof, N.A.; Tee, T.W.; Abdullah, A.H. Electrochemical sensor for As(III) utilizing CNTs/Leucine/Nafion modified electrode. Int. J. Electrochem. Sci. 2012, 7, 175–185. [Google Scholar]
- Kempahanumakkagari, S.; Deep, A.; Kim, K.-H.; Kailasa, S.K.; Yoon, H.-O. Nanomaterial-based electrochemical sensors for arsenic—A review. Biosens. Bioelectron. 2017, 95, 106–116. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive electrochemical detection of arsenic (III) using gold nanoparticle modified carbon nanotubes via anodic stripping voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Hong, H.-G. Anodic Stripping Voltammetric Detection of Arsenic(III) at Platinum-Iron(III) Nanoparticle Modified Carbon Nanotube on Glassy Carbon Electrode. Bull. Korean Chem. Soc. 2010, 31, 3077–3083. [Google Scholar] [CrossRef]
- Buffa, A.; Mandler, D. Arsenic(III) detection in water by flow-through carbon nanotube membrane decorated by gold nanoparticles. Electrochim. Acta 2019, 318, 496–503. [Google Scholar] [CrossRef]
- Izadkhah, V.; Farmany, A.; Mortazavi, S. Voltammetric determination of copper in water samples using a Schiff base/carbon nanotube-modified carbon paste electrode. J. Ind. Eng. Chem. 2015, 21, 994–996. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Y.; Ma, Y.; Wu, Z.; Cao, Q.; He, Y.; Li, X.; Yuan, Z. Poly(2-amino-4-thiazoleacetic acid)/multiwalled carbon nanotubes modified glassy carbon electrodes for the electrochemical detection of copper(II). Electrochim. Acta 2010, 55, 2518–2521. [Google Scholar] [CrossRef]
- Cabaniss, S.E. Forward Modeling of Metal Complexation by NOM: II. Prediction of Binding Site Properties. Environ. Sci. Technol. 2011, 45, 3202–3209. [Google Scholar] [CrossRef]
- Qin, D.; Mamat, X.; Li, Y.; Hu, X.; Cheng, H.; Hu, G. A composite with botryoidal texture prepared from nitrogen-doped carbon spheres and carbon nanotubes for voltammetric sensing of copper(II). Microchem. J. 2019, 153, 104299. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Qiu, Y.; Zhuang, X.; Wu, X.; Jiang, J. Facile synthesis of oxidized multi-walled carbon nanotubes functionalized with 5-sulfosalicylic acid/MoS2 nanosheets nanocomposites for electrochemical detection of copper ions. Appl. Surf. Sci. 2019, 487, 766–772. [Google Scholar] [CrossRef]
- Gou, P.; Kraut, N.D.; Feigel, I.M.; Star, A. Rigid versus Flexible Ligands on Carbon Nanotubes for the Enhanced Sensitivity of Cobalt Ions. Macromolecules 2013, 46, 1376–1383. [Google Scholar] [CrossRef]
- Li, P.; Sun, L.; Li, M.; Yang, B.; Li, H.; Li, C. Simultaneous Determination of Small Biomolecules and Nitrite Using an Au/TiO 2 /Carbon Nanotube Composite-Modified Electrode. J. Electrochem. Soc. 2016, 163, 567–572. [Google Scholar]
- Zhao, Z.; Zhang, J.; Wang, W.; Sun, Y.; Li, P.; Hu, J.; Chen, L.; Gong, W. Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl. Surf. Sci. 2019, 485, 274–282. [Google Scholar] [CrossRef]
- Thamae, M.; Nyokong, T. Cobalt(II) porphyrazine catalysed reduction of nitrite. J. Electroanal. Chem. 1999, 470, 126–135. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, X.; Hong, H.; Hu, K.; Yang, R.; Wang, X.; Li, X.; Liu, X. An electro-active amphiphilic copolymer to functionalize carbon nanotubes for highly sensitive determination of nitrite in water. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 123–129. [Google Scholar] [CrossRef]
- Azadbakht, A.; Abbasi, A.R.; Derikvand, Z.; Amraei, S. Immobilized organoruthenium(II) complexes onto polyethyleneimine-wrapped carbon nanotubes/in situ formed gold nanoparticles as a novel electrochemical sensing platform. Mater. Sci. Eng. C 2015, 48, 270–278. [Google Scholar] [CrossRef]
- Pham, X.-H.; Li, C.A.; Han, K.N.; Huynh-Nguyen, B.-C.; Le, T.-H.; Ko, E.; Kim, J.H.; Seong, G.H. Electrochemical detection of nitrite using urchin-like palladium nanostructures on carbon nanotube thin film electrodes. Sens. Actuators B Chem. 2014, 193, 815–822. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Jin, J.; Wu, H.; Wang, S.; Ding, Y.; Ou, J. Sensing nitrite with a glassy carbon electrode modified with a three-dimensional network consisting of Ni7S6 and multi-walled carbon nanotubes. Microchim. Acta 2016, 183, 3159–3166. [Google Scholar] [CrossRef]
- Rao, D.; Sheng, Q.; Zheng, J. Self-assembly preparation of gold nanoparticle decorated 1-pyrenemethylamine functionalized graphene oxide–carbon nanotube composites for highly sensitive detection of nitrite. Anal. Methods 2016, 8, 4926–4933. [Google Scholar] [CrossRef]
- Salimi, A.; Noorbakhash, A.; Karonian, F.S. Amperometric detection of nitrite, iodate and periodate on glassy carbon electrode modified with thionin and multi-wall carbon nanotubes. Int. J. Electrochem. Sci. 2006, 1, 435–446. [Google Scholar]
- Hui, N.; Chai, F.; Lin, P.; Song, Z.; Sun, X.; Li, Y.; Niu, S.; Luo, X. Electrodeposited Conducting Polyaniline Nanowire Arrays Aligned on Carbon Nanotubes Network for High Performance Supercapacitors and Sensors. Electrochim. Acta 2016, 199, 234–241. [Google Scholar] [CrossRef]
- Lerga, T.M.; O’Sullivan, C.K. Rapid determination of total hardness in water using fluorescent molecular aptamer beacon. Anal. Chim. Acta 2008, 610, 105–111. [Google Scholar] [CrossRef]
- Gee, K.; Brown, K.; Chen, W.-N.; Bishop-Stewart, J.; Gray, D.; Johnson, I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium 2000, 27, 97–106. [Google Scholar] [CrossRef]
- Son, D.; Park, S.Y.; Kim, B.; Koh, J.T.; Kim, T.H.; An, S.; Jang, D.; Kim, G.T.; Jhe, W.; Hong, S. Nanoneedle Transistor-Based Sensors for the Selective Detection of Intracellular Calcium Ions. ACS Nano 2011, 5, 3888–3895. [Google Scholar] [CrossRef]
- Akhter, F.; Nag, A.; Alahi, E.E.; Liu, H.; Mukhopadhyay, S. Electrochemical detection of calcium and magnesium in water bodies. Sens. Actuators A Phys. 2020, 305, 111949. [Google Scholar] [CrossRef]
- Mulholland, P.J.; Houser, J.N.; Maloney, K.O. Stream diurnal dissolved oxygen profiles as indicators of in-stream metabolism and disturbance effects: Fort Benning as a case study. Ecol. Indic. 2005, 5, 243–252. [Google Scholar] [CrossRef]
- Sánchez, E.; Colmenarejo, M.F.; Vicente, J.; Rubio, A.; García, M.G.; Travieso, L.; Borja, R. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol. Indic. 2007, 7, 315–328. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Moonoosawmy, K.R.; Kruse, P. Cause and Consequence of Carbon Nanotube Doping in Water and Aqueous Media. J. Am. Chem. Soc. 2010, 132, 1572–1577. [Google Scholar] [CrossRef]
- Ye, J.-S.; Wen, Y.; De Zhang, W.; Cui, H.-F.; Gan, L.M.; Xu, G.Q.; Sheu, F.-S. Application of multi-walled carbon nanotubes functionalized with hemin for oxygen detection in neutral solution. J. Electroanal. Chem. 2004, 562, 241–246. [Google Scholar] [CrossRef]
- Shi, C.; Anson, F.C. Catalytic pathways for the electroreduction of oxygen by iron tetrakis(4-N-methylpyridyl)porphyrin or iron tetraphenylporphyrin adsorbed on edge plane pyrolytic graphite electrodes. Inorg. Chem. 1990, 29, 4298–4305. [Google Scholar] [CrossRef]
- Tsai, T.H.; Yang, C.Y.; Chen, S.M. Development of a dissolved oxygen sensor for commercial applications. Int. J. Electrochem. Sci. 2013, 8, 5250–5261. [Google Scholar]
- Sosna, M.; Denuault, G.; Pascal, R.W.; Prien, R.D.; Mowlem, M. Development of a reliable microelectrode dissolved oxygen sensor. Sens. Actuators B Chem. 2007, 123, 344–351. [Google Scholar] [CrossRef]
- Balamurugan, J.; Thanh, T.D.; Karthikeyan, G.; Kim, N.H.; Lee, J.H. A novel hierarchical 3D N-Co-CNT@NG nanocomposite electrode for non-enzymatic glucose and hydrogen peroxide sensing applications. Biosens. Bioelectron. 2017, 89, 970–977. [Google Scholar] [CrossRef]
- Naqvi, S.T.R.; Shirinfar, B.; Hussain, D.; Majeed, S.; Ashiq, M.N.; Aslam, Y.; Ahmed, N. Electrochemical Sensing of Ascorbic Acid, Hydrogen Peroxide and Glucose by Bimetallic (Fe, Ni)−CNTs Composite Modified Electrode. Electroanalysis 2019, 31, 851–857. [Google Scholar] [CrossRef]
- Annamalai, S.K.; Palani, B.; Pillai, K.C. Highly stable and redox active nano copper species stabilized functionalized-multiwalled carbon nanotube/chitosan modified electrode for efficient hydrogen peroxide detection. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 207–216. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Qu, Y.; Dong, Z.; Li, W.J. Carbon nanotube-sensor-integrated microfluidic platform for real-time chemical concentration detection. Electrophoresis 2009, 30, 3198–3205. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon nanotube screen-printed electrochemical sensors. Analyst 2003, 129, 1–2. [Google Scholar] [CrossRef]
- Patel, V.; Kruse, P.; Selvaganapathy, P.R. Hydrogen peroxide chemiresistive detection platform with wide range of detection. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Olivé-Monllau, R.; Pereira, A.; Bartrolí, J.; Baeza, M.; Céspedes, F. Highly sensitive CNT composite amperometric sensors integrated in an automated flow system for the determination of free chlorine in waters. Talanta 2010, 81, 1593–1598. [Google Scholar] [CrossRef]
- Isaac, A.; Davis, J.; Livingstone, C.; Wain, A.J.; Compton, R.G. Electroanalytical methods for the determination of sulfite in food and beverages. TrAC Trends Anal. Chem. 2006, 25, 589–598. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Karimi-maleh, H. Electrocatalytic Determination of Sulfite at the Surface of a New Ferrocene Derivative-Modified Carbon Paste Electrode. Int. J. Electrochem. Sci. 2007, 2, 257–269. [Google Scholar]
- Vu, L.; Cervenka, L. Determination of Sulfide by Hematoxylin Multiwalled Carbon Nanotubes Modified Carbon Paste Electrode. Electroanalysis 2013, 25, 1967–1973. [Google Scholar]
- Mohajeri, S.; Dolati, A.; Salmani-Rezaie, S. Electrochemical sensors based on functionalized carbon nanotubes modified with platinum nanoparticles for the detection of sulfide ions in aqueous media. J. Chem. Sci. 2019, 131, 21. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Deo, R.P.; Wang, J. Electrochemical determination of hydrogen sulfide at carbon nanotube modified electrodes. Anal. Chim. Acta 2004, 517, 131–137. [Google Scholar] [CrossRef]
- Shamsipur, M.; Roushani, M.; Pourmortazavi, S.M.; Shahabadi, N. Amperometric determination of sulfide ion by glassy carbon electrode modified with multiwall carbon nanotubes and copper (II) phenanthroline complex. Open Chem. 2014, 12, 1091–1099. [Google Scholar] [CrossRef]
- Perales, E.; García, C.; Lomba, L.; García, J.I.; Pires, E.; Sancho, M.C.; Navarro, E.; Giner, B. Comparative ecotoxicity study of glycerol-biobased solvents. Environ. Chem. 2017, 14, 370–377. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Huang, J.; Xian, Y.; Zhang, W.; Zhang, Z.; Jin, L. Rapid amperometric detection of coliforms based on MWNTs/Nafion composite film modified glass carbon electrode. Talanta 2008, 75, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.; Farghali, A.A.; El Rouby, W.M.A.; Abd-Elgawad, I.H. Preparation and characterization of novel MWCNTs/Fe-Co doped TNTs nanocomposite for potentiometric determination of sulpiride in real water samples. Sci. Rep. 2020, 10, 8607. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Alam, A.U.; Howlader, M.M. Fabrication of highly sensitive Bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B Chem. 2020, 320, 128319. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, X.; Xu, Y.; Tao, Y.; Luo, D.; Hu, L.; Jing, T.; Zhou, Y.; Wang, P.; Mei, S. Electrochemical determination of tetrabromobisphenol A in water samples based on a carbon nanotubes@zeolitic imidazole framework-67 modified electrode. RSC Adv. 2020, 10, 2123–2132. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Yang, D.; Luo, X.; Tang, Y.; Li, H. Sensitivity and selectivity determination of bisphenol A using SWCNT–CD conjugate modified glassy carbon electrode. J. Hazard. Mater. 2012, 199–200, 111–118. [Google Scholar] [CrossRef]
- Dey, S.; Bano, F.; Malik, A. 1—Pharmaceuticals and personal care product (PPCP) contamination—A global discharge inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Butterworth-Heinemann: Oxford, UK; pp. 1–26.
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Benda, R.; Vezin, T.; Zucchi, G.; Cancès, E.; Lebental, B. Prediction of the interaction strength of an urea-based probe towards ions in water by means of DFT/PCM calculations. J. Comput.-Aided Mol. Des. 2021. Preprint. [Google Scholar]
- Star, A.; Joshi, V.; Skarupo, S.; Thomas, D.; Gabriel, J.-C.P. Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Fenniri, H. Cutting Edge Methods for Non-Invasive Disease Diagnosis Using E-Tongue and E-Nose Devices. Biosensors 2017, 7, 59. [Google Scholar] [CrossRef]
- Daikuzono, C.M.; Dantas, C.A.R.; Volpati, D.; Constantino, C.J.L.; Piazzetta, M.H.O.; Gobbi, A.L.; Taylor, D.M.; Oliveira, O.N.; Riul, A. Microfluidic electronic tongue. Sens. Actuators B Chem. 2015, 207, 1129–1135. [Google Scholar] [CrossRef]
- Lebental, B.; Benda, R.; Bodelot, L.; Florea, I.; Godumala, M.; Gusarov, B.; Gutierrez, A.; Loisel, L.; Merliot, E.; Ramachandran, S.; et al. Carbon nanotube sensor array for water monitoring with conjugated polymers. In Proceedings of the C’NANO 2017, The Nanoscience Meeting, Lyon, France, 5–7 December 2017; p. 18. [Google Scholar]
- Shaun, F.; Pellegrino, M.; Cesar, W.; Marty, F.; Xu, Z.; Capo-Chichi, M.; Basset, P.; Lebental, B.; Bourouina, T. Robust multi-parameter sensing probe for water monitoring based on ALD-coated metallic micro-patterns. In Proceedings of the 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, 17–20 April 2016; pp. 342–345. [Google Scholar] [CrossRef]
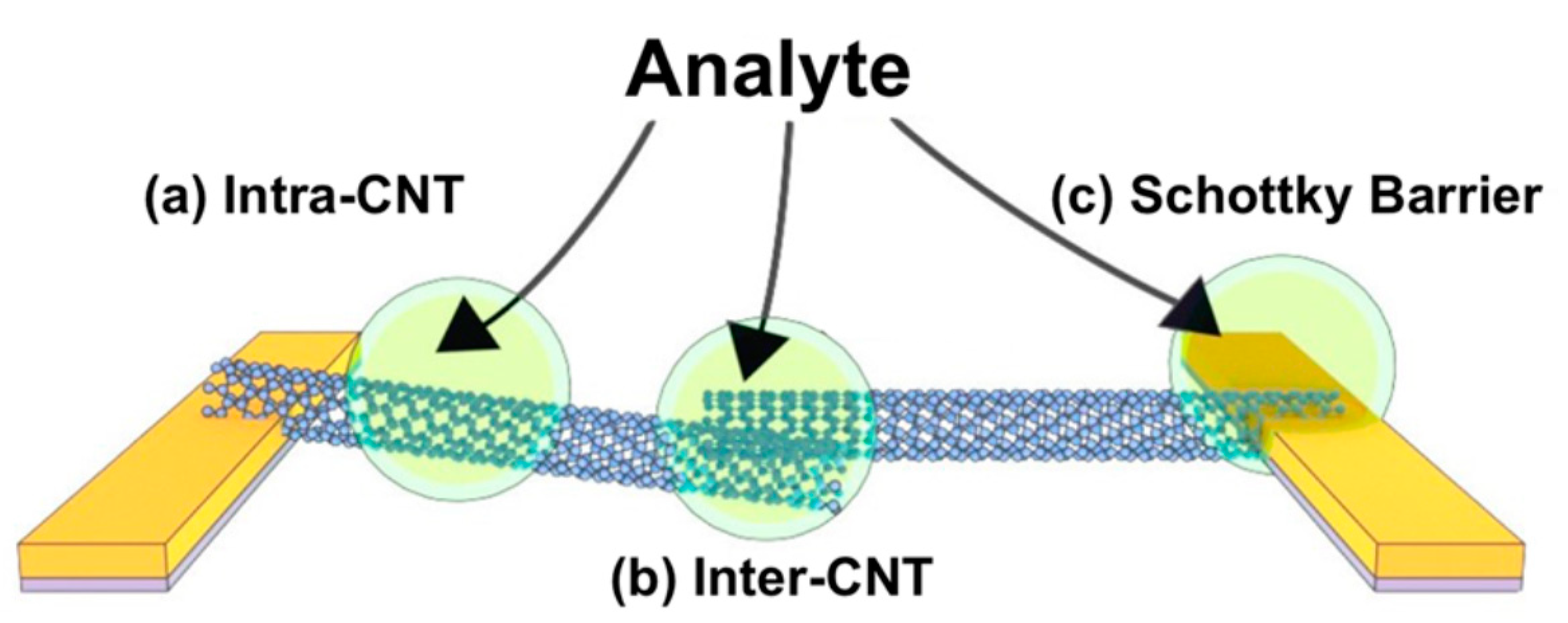
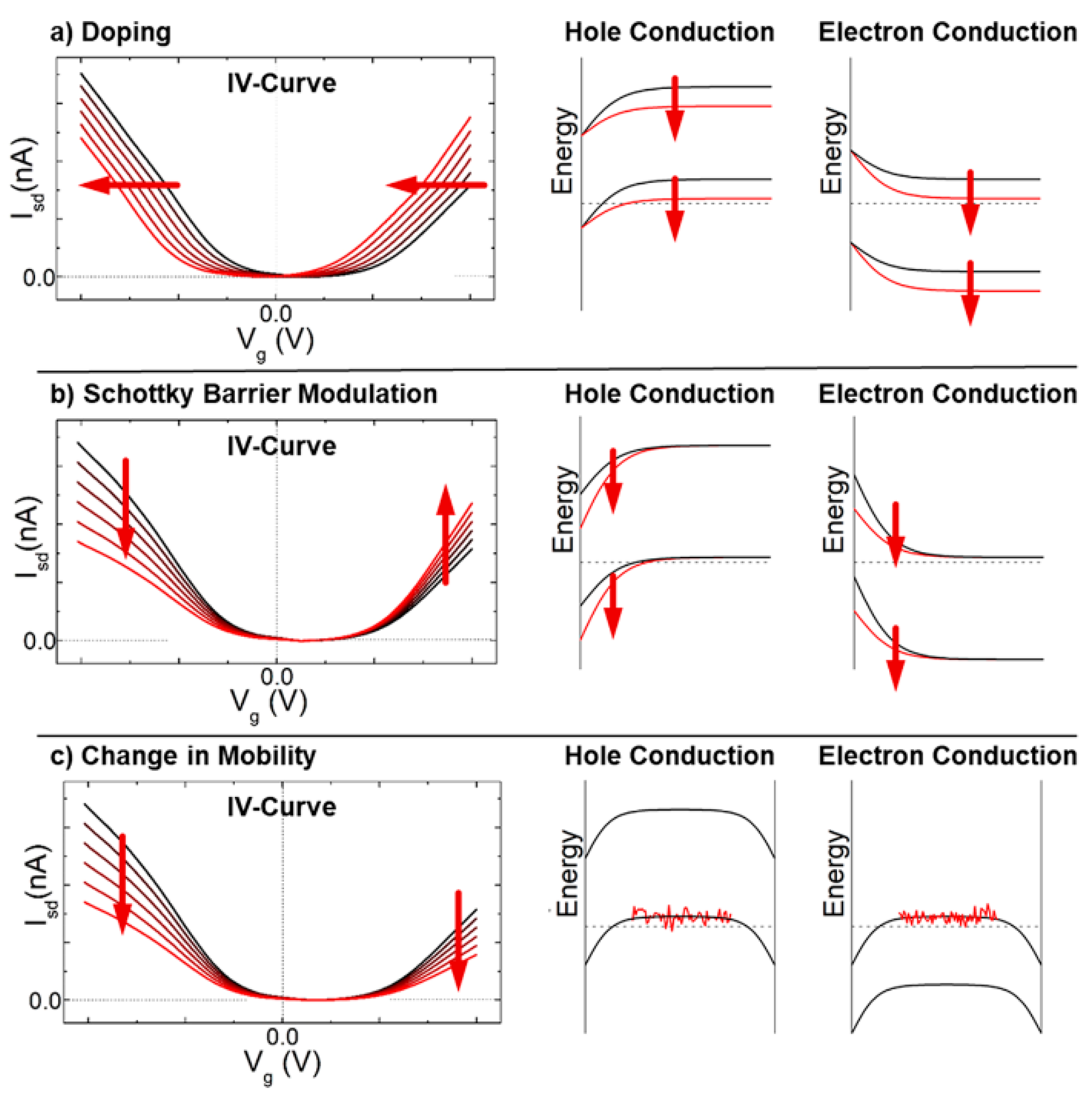
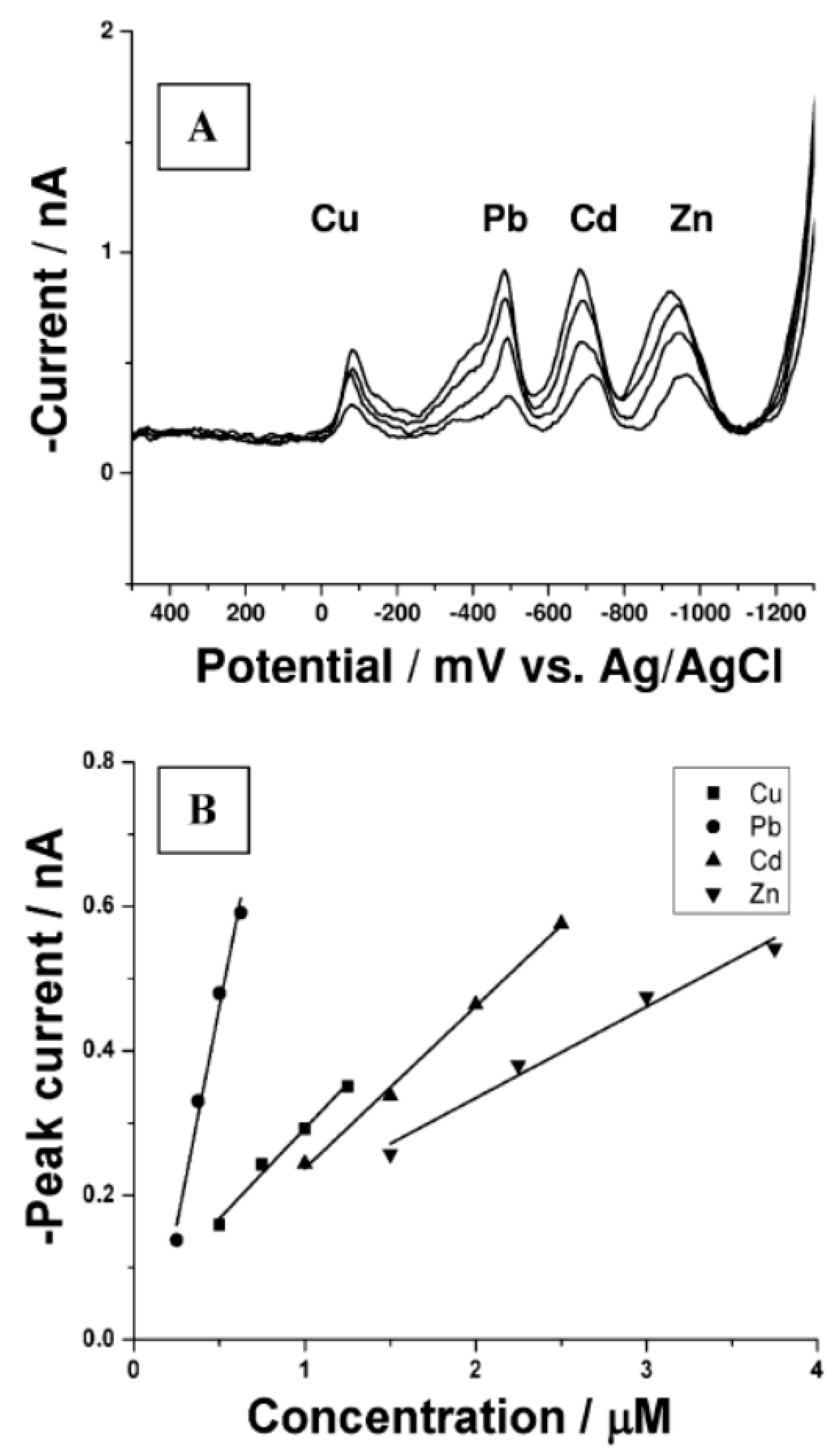
| Type of Analytes | Numbers of Refs. | Ref. SWCNT | Ref. MWCNT | Ref. CNTFET | Ref. Chemistors | Ref. EC | Ref. Functionalized (COOH Excluded) | |
|---|---|---|---|---|---|---|---|---|
| All Analytes | 90 | 26 (29%) | 64 (71%) | 11 (12%) | 13 (14%) | 66 (73%) | 74 (82%) | |
| pH | 16 (18%) | 12 | 4 | 6 | 7 | 5 (2 with CNTFET) | 8 | |
| Micronutrients and toxic metals (total) | All included | 36 (40%) | 5 | 32 | 2 | 2 | 33 | 31 |
| Pb(II) | 16 (18%) | 0 | 16 | 0 | 0 | 16 | 12 | |
| Cd (II) | 13 (14%) | 0 | 13 | 0 | 0 | 13 | 11 | |
| Cu(II) | 9 (10%) | 1 | 9 | 1 | 0 | 8 | 8 | |
| Hg(II) | 8 (9%) | 3 | 5 | 1 | 1 | 6 | 6 | |
| As(III) | 5 (6%) | 0 | 5 | 0 | 0 | 5 | 5 | |
| Zn(II) | 4 (4%) | 0 | 4 | 0 | 0 | 4 | 2 | |
| Miscellaneous | 2 (2%) | 2 | 0 | 1 | 1 | 0 | 2 | |
| Nitrite | 10 (11%) | 1 | 9 | 0 | 0 | 10 | 10 | |
| Water hardness | 2 (2%) | 1 | 1 | 1 | 0 | 1 | 2 | |
| DO | 2 (2%) | 0 | 2 | 0 | 0 | 2 | 2 | |
| Disinfectants | Free chlorine | 3 (3%) | 1 | 2 | 0 | 2 | 1 | 2 |
| Hydrogen peroxide | 6 (7%) | 1 | 5 | 0 | 1 | 5 | 6 | |
| Sulfur | Sulfide | 4 (4%) | 0 | 4 | 0 | 0 | 4 | 4 |
| Sulfite | 2 (2%) | 0 | 2 | 0 | 0 | 2 | 2 | |
| Miscellaneous | 9 (10%) | 4 | 5 | 2 | 1 | 5 | 8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors 2022, 22, 218. https://doi.org/10.3390/s22010218
Cho G, Azzouzi S, Zucchi G, Lebental B. Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors. 2022; 22(1):218. https://doi.org/10.3390/s22010218
Chicago/Turabian StyleCho, Gookbin, Sawsen Azzouzi, Gaël Zucchi, and Bérengère Lebental. 2022. "Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review" Sensors 22, no. 1: 218. https://doi.org/10.3390/s22010218
APA StyleCho, G., Azzouzi, S., Zucchi, G., & Lebental, B. (2022). Electrical and Electrochemical Sensors Based on Carbon Nanotubes for the Monitoring of Chemicals in Water—A Review. Sensors, 22(1), 218. https://doi.org/10.3390/s22010218