Objective Assessment of Regional Stiffness in Vastus Lateralis with Different Measurement Methods: A Reliability Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Inclusion Procedure
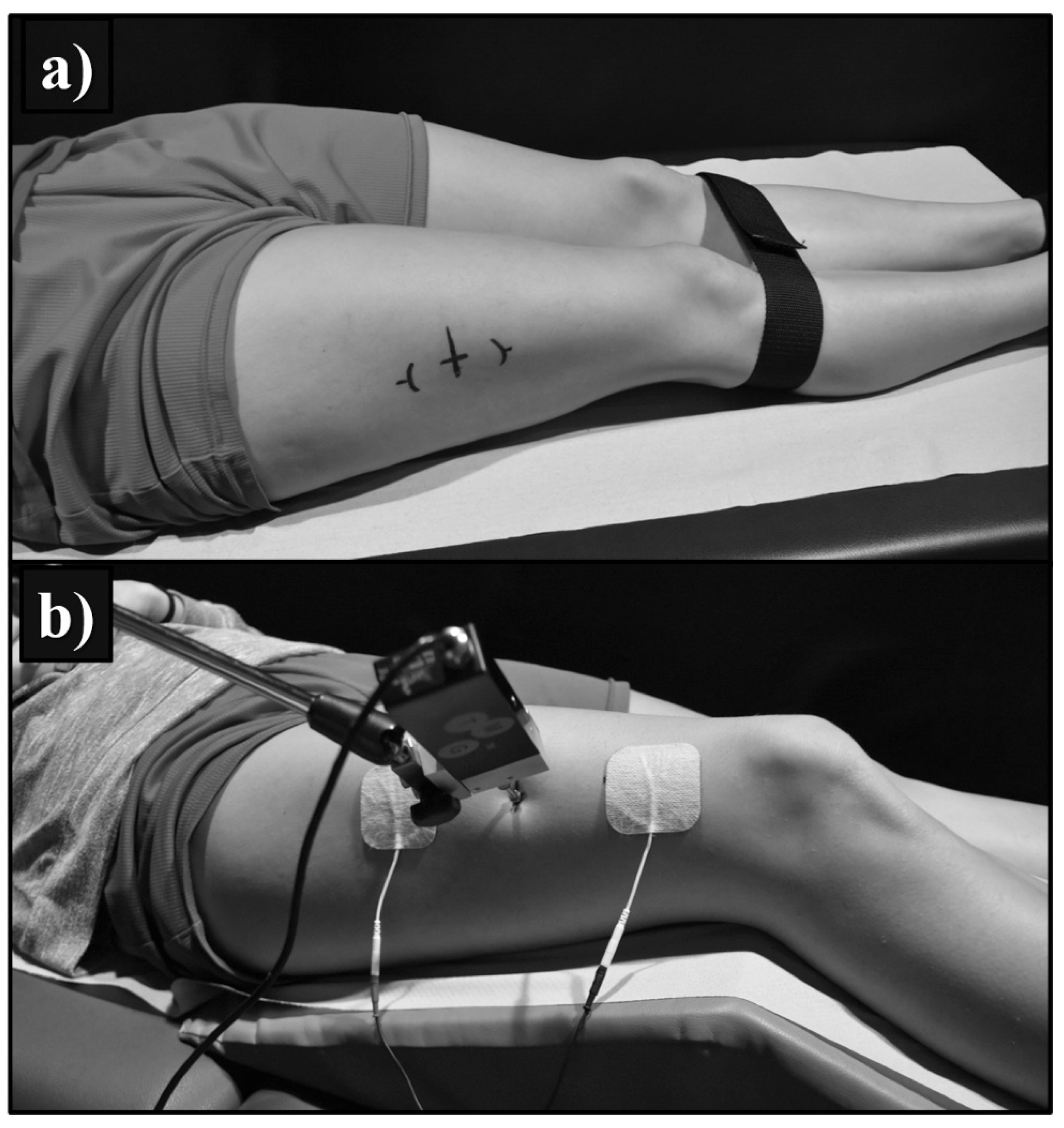
2.2. Design and Procedure
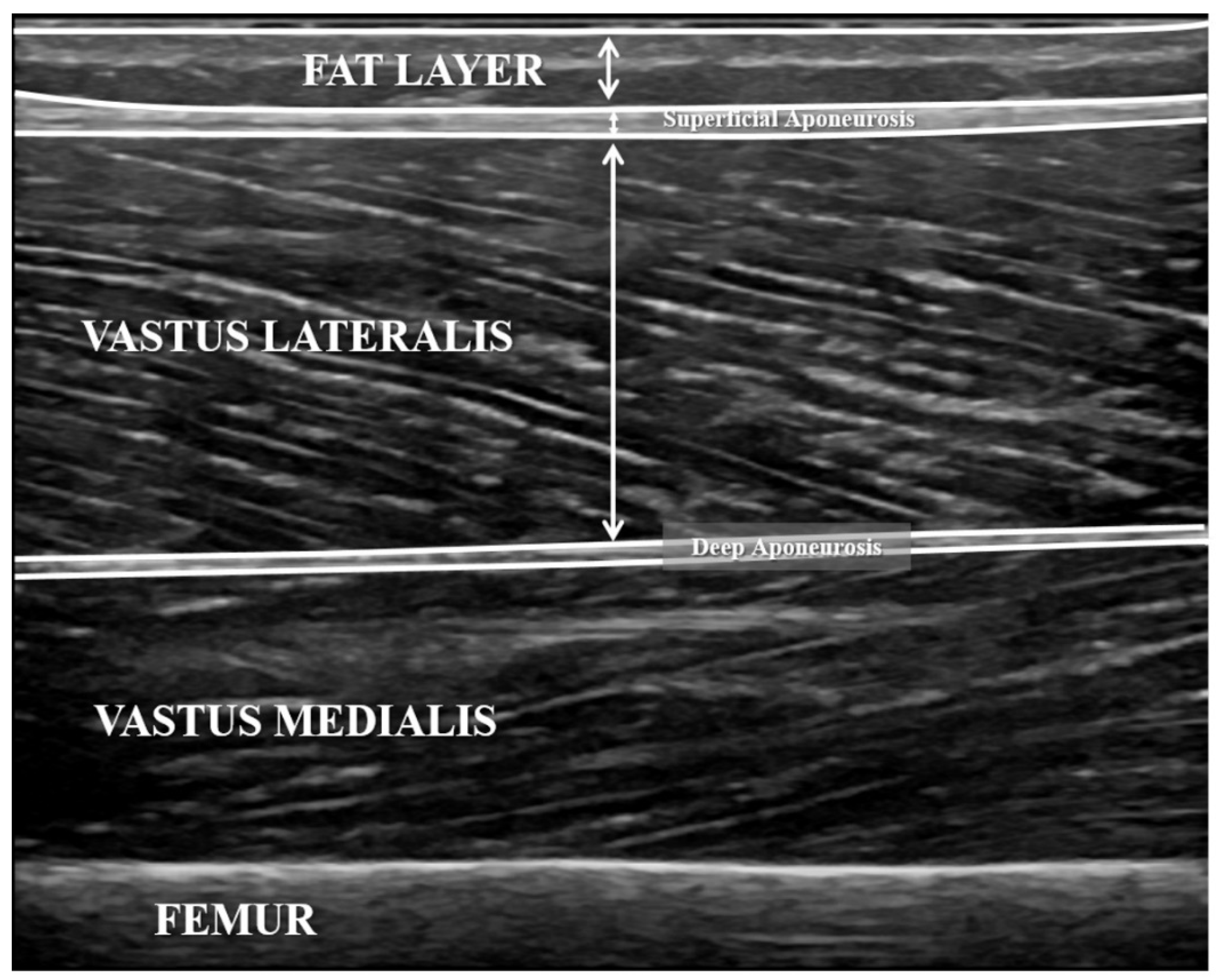
2.3. Ultrasound Measurements
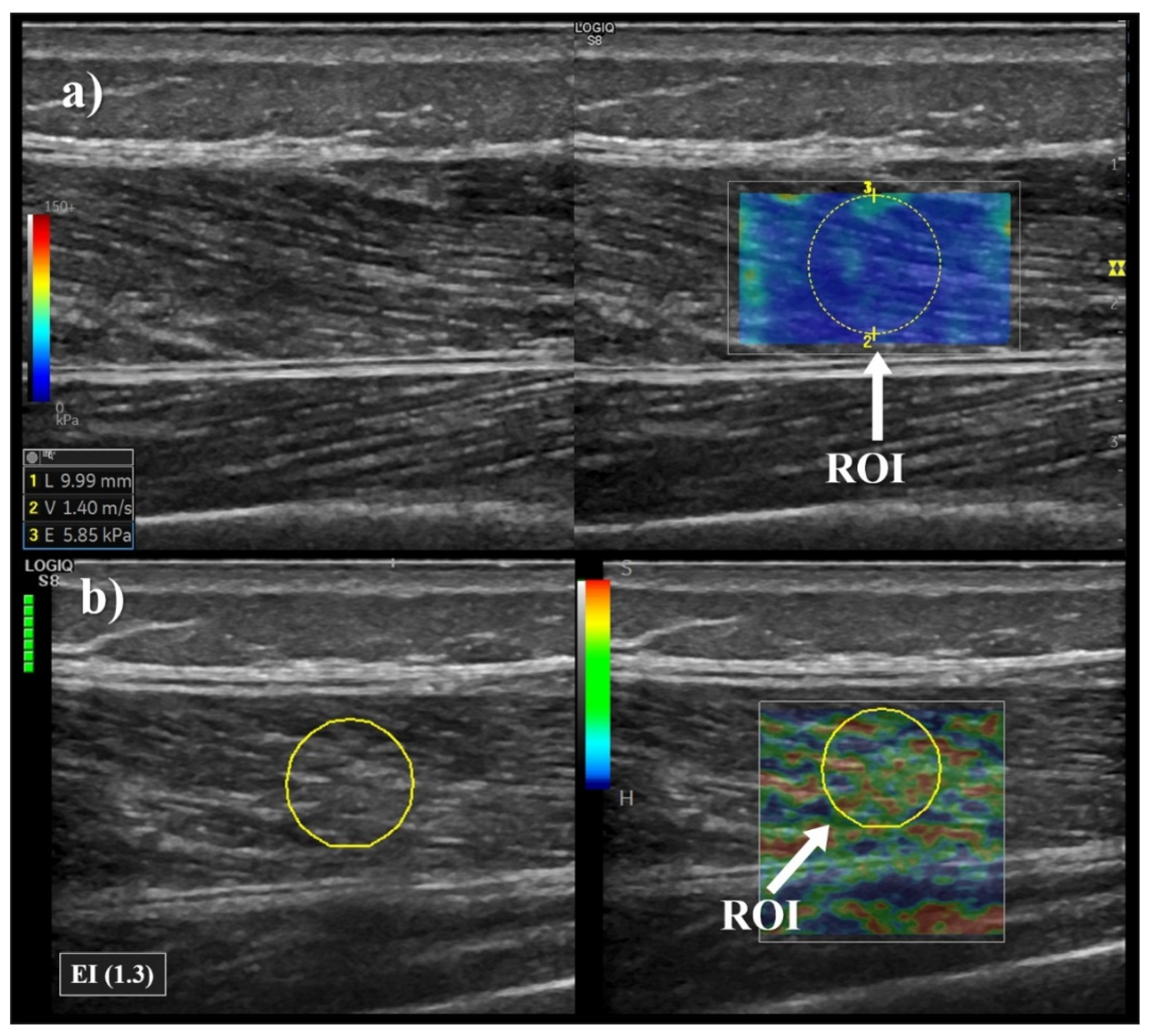
2.4. Elastography Measurements
2.5. MyotonPRO Measurements
2.6. Tensiomyography Measurements
2.7. Statistical Analysis
3. Results
3.1. Test-Retest Reliability
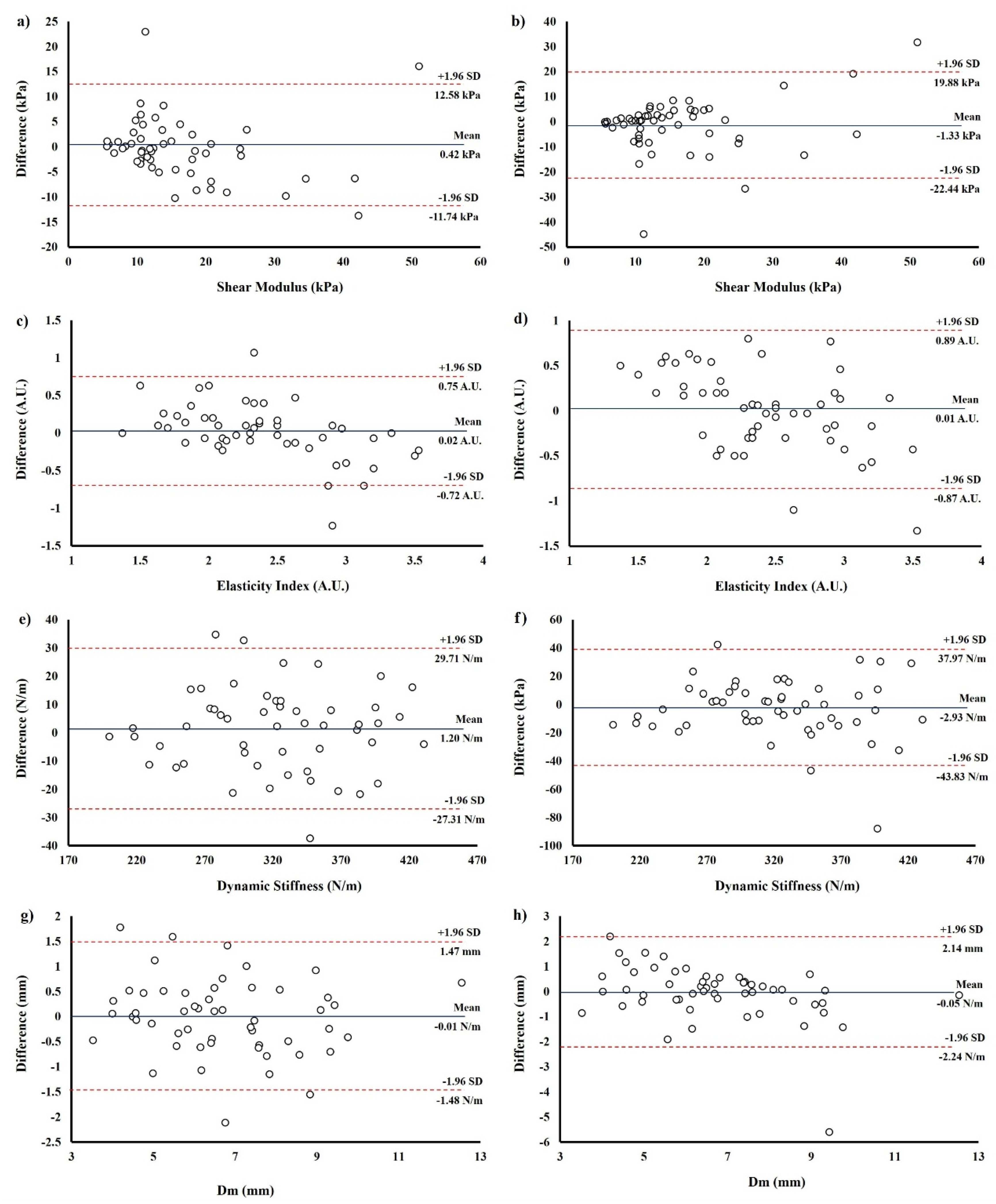
3.2. Bland-Altman Results
3.3. Correlation Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brughelli, M.; Cronin, J. A review of research on the mechanical stiffness in running and jumping: Methodology and implications. Scand J. Med. Sci. Sports 2008, 18, 417–426. [Google Scholar] [CrossRef]
- Bravo-Sanchez, A.; Abian, P.; Sousa, F.; Jimenez, F.; Abian-Vicen, J. Influence of Badminton Practice on Age-Related Changes in Patellar and Achilles Tendons. J. Aging Phys. Act. 2020. [Google Scholar] [CrossRef]
- Aird, L.; Samuel, D.; Stokes, M. Quadriceps muscle tone, elasticity and stiffness in older males: Reliability and symmetry using the MyotonPRO. Arch. Gerontol. Geriatr. 2012, 55, e31–e39. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Chen, G.; Lu, Y.; Ren, W.; Xu, W.; Xu, X.; Wu, Z.; Guan, Y.; Zheng, Y.; et al. Reliability of a portable device for quantifying tone and stiffness of quadriceps femoris and patellar tendon at different knee flexion angles. PLoS ONE 2019, 14, e0220521. [Google Scholar] [CrossRef]
- Dickson, D.M.; Fawole, H.O.; Newcombe, L.; Smith, S.L.; Hendry, G.J. Reliability of ultrasound strain elastography in the assessment of the quadriceps and patellar tendon in healthy adults. Ultrasound 2019, 27, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Simunic, B. Between-day reliability of a method for non-invasive estimation of muscle composition. J. Electromyogr. Kinesiol. 2012, 22, 527–530. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef] [PubMed]
- Rus, G.; Melchor, J.M.; Faris, I.; Callejas, A.; Riveiro, M.; Molina, F.; Torres, J. Mechanical biomarkers by torsional shear ultrasound for medical diagnosis. J. Acoust. Soc. Am. 2018, 144, 1747. [Google Scholar] [CrossRef]
- Rus, G.; Faris, I.H.; Torres, J.; Callejas, A.; Melchor, J. Why Are Viscosity and Nonlinearity Bound to Make an Impact in Clinical Elastographic Diagnosis? Sensors 2020, 20, 2379. [Google Scholar] [CrossRef]
- Brandenburg, J.E.; Eby, S.F.; Song, P.; Zhao, H.; Brault, J.S.; Chen, S.; An, K.-N. Ultrasound Elastography: The New Frontier in Direct Measurement of Muscle Stiffness. Arch. Phys. Med. Rehab. 2014, 95, 2207–2219. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; O’Connor, P.; Hensor, E.; Tan, A.L.; Emery, P.; Wakefield, R.J. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J. Clin. Ultrasound 2018, 46, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Klauser, A.S.; Miyamoto, H.; Tamegger, M.; Faschingbauer, R.; Moriggl, B.; Klima, G.; Feuchtner, G.M.; Kastlunger, M.; Jaschke, W.R. Achilles tendon assessed with sonoelastography: Histologic agreement. Radiology 2013, 267, 837–842. [Google Scholar] [CrossRef]
- Lacourpaille, L.; Hug, F.; Bouillard, K.; Hogrel, J.Y.; Nordez, A. Supersonic shear imaging provides a reliable measurement of resting muscle shear elastic modulus. Physiol. Meas. 2012, 33, N19–N28. [Google Scholar] [CrossRef] [PubMed]
- Andonian, P.; Viallon, M.; Le Goff, C.; de Bourguignon, C.; Tourel, C.; Morel, J.; Giardini, G.; Gergele, L.; Millet, G.P.; Croisille, P. Shear-Wave Elastography Assessments of Quadriceps Stiffness Changes prior to, during and after Prolonged Exercise: A Longitudinal Study during an Extreme Mountain Ultra-Marathon. PLoS ONE 2016, 11, e0161855. [Google Scholar] [CrossRef]
- Porta, F.; Damjanov, N.; Galluccio, F.; Iagnocco, A.; Matucci-Cerinic, M. Ultrasound elastography is a reproducible and feasible tool for the evaluation of the patellar tendon in healthy subjects. Int. J. Rheum. Dis. 2014, 17, 762–766. [Google Scholar] [CrossRef]
- Corrigan, P.; Zellers, J.A.; Balascio, P.; Silbernagel, K.G.; Cortes, D.H. Quantification of Mechanical Properties in Healthy Achilles Tendon Using Continuous Shear Wave Elastography: A Reliability and Validation Study. Ultrasound Med. Biol. 2019, 45, 1574–1585. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hara, K.; Mizuno, N.; Haba, S.; Okuno, N. Present status of ultrasound elastography for the diagnosis of pancreatic tumors: Review of the literature. J. Med. Ultrason. (2001) 2020, 47, 413–420. [Google Scholar] [CrossRef]
- Gavronski, G.; Veraksits, A.; Vasar, E.; Maaroos, J. Evaluation of viscoelastic parameters of the skeletal muscles in junior triathletes. Physiol. Meas. 2007, 28, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bellmunt, A.; Llurda-Almuzara, L.; Simon, M.; Navarro, R.; Casasayas, O.; López-De-Celis, C. Neuromuscular Response What is it and How to Measure it. Phys. Med. Rehabil. J. 2019, 2, 118. [Google Scholar]
- Simunic, B. Two-dimensional spatial error distribution of key tensiomyographic parameters. J. Biomech. 2019, 92, 92–97. [Google Scholar] [CrossRef]
- Tous-Fajardo, J.; Moras, G.; Rodríguez-Jiménez, S.; Usach, R.; Doutres, D.M.; Maffiuletti, N.A. Inter-rater reliability of muscle contractile property measurements using non-invasive tensiomyography. J. Electromyogr. Kinesiol. 2010, 20, 761–766. [Google Scholar] [CrossRef]
- Lohr, C.; Braumann, K.M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of tensiomyography and myotonometry in detecting mechanical and contractile characteristics of the lumbar erector spinae in healthy volunteers. Eur. J. Appl. Physiol. 2018, 118, 1349–1359. [Google Scholar] [CrossRef]
- Rouissi, M.; Chtara, M.; Owen, A.; Chaalali, A.; Chaouachi, A.; Gabbett, T.; Chamari, K. Effect of leg dominance on change of direction ability amongst young elite soccer players. J. Sports Sci. 2016, 34, 542–548. [Google Scholar] [CrossRef]
- Lopez, P.; Pinto, M.D.; Pinto, R.S. Does Rest Time before Ultrasonography Imaging Affect Quadriceps Femoris Muscle Thickness, Cross-Sectional Area and Echo Intensity Measurements? Ultrasound Med. Biol. 2019, 45, 612–616. [Google Scholar] [CrossRef]
- Mannarino, P.; Matta, T.T.d.; Oliveira, L.F.D. An 8-week resistance training protocol is effective in adapting quadriceps but not patellar tendon shear modulus measured by Shear Wave Elastography. PLoS ONE 2019, 14, e0205782. [Google Scholar] [CrossRef]
- Ling, S.; Zhou, Y.; Chen, Y.; Zhao, Y.Q.; Wang, L.; Zheng, Y.P. Automatic tracking of aponeuroses and estimation of muscle thickness in ultrasonography: A feasibility study. IEEE J. Biomed. Health Inform. 2013, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Bolsterlee, B.; Gandevia, S.C.; Herbert, R.D. Effect of Transducer Orientation on Errors in Ultrasound Image-Based Measurements of Human Medial Gastrocnemius Muscle Fascicle Length and Pennation. PLoS ONE 2016, 11, e0157273. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Wilson, H.V.; Jones, A.; Johnson, M.I.; Francis, P. The effect of inter-electrode distance on radial muscle displacement and contraction time of the biceps femoris, gastrocnemius medialis and biceps brachii, using tensiomyography in healthy participants. Physiol. Meas. 2019, 40, 075007. [Google Scholar] [CrossRef]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, M.J.; Kim, H.Y.; Roh, Y.H.; Lee, M.J. Comparison of shear wave velocities on ultrasound elastography between different machines, transducers, and acquisition depths: A phantom study. Eur. Radiol. 2016, 26, 3361–3367. [Google Scholar] [CrossRef]
- Ewertsen, C.; Carlsen, J.F.; Christiansen, I.R.; Jensen, J.A.; Nielsen, M.B. Evaluation of healthy muscle tissue by strain and shear wave elastography—Dependency on depth and ROI position in relation to underlying bone. Ultrasonics 2016, 71, 127–133. [Google Scholar] [CrossRef]
- Taş, S.; Onur, M.R.; Yılmaz, S.; Soylu, A.R.; Korkusuz, F. Shear Wave Elastography Is a Reliable and Repeatable Method for Measuring the Elastic Modulus of the Rectus Femoris Muscle and Patellar Tendon. J. Ultrasound Med. 2017, 36, 565–570. [Google Scholar] [CrossRef]
- Yoshida, K.; Itoigawa, Y.; Maruyama, Y.; Saita, Y.; Takazawa, Y.; Ikeda, H.; Kaneko, K.; Sakai, T.; Okuwaki, T. Application of shear wave elastography for the gastrocnemius medial head to tennis leg. Clin. Anat. 2017, 30, 114–119. [Google Scholar] [CrossRef]
- Bortolotto, C.; Turpini, E.; Felisaz, P.; Fresilli, D.; Fiorina, I.; Raciti, M.V.; Belloni, E.; Bottinelli, O.; Cantisani, V.; Calliada, F. Median nerve evaluation by shear wave elastosonography: Impact of "bone-proximity" hardening artifacts and inter-observer agreement. J. Ultrasound 2017, 20, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.; Costa Junior, J.F.S.; Pereira, W.C.A.; Oliveira, L.F. Assessment of the mechanical properties of the muscle-tendon unit by supersonic shear wave imaging elastography: A review. Ultrasonography 2018, 37, 3–15. [Google Scholar] [CrossRef]
- Piqueras-Sanchiz, F.; Martín-Rodríguez, S.; González-Hernández, J.M.; García, Ó.G. In-season analysis of the muscle response speed of knee extensors and flexors in elite futsal players. Adv. Skelet. Muscle Funct. Assess. 2017, 1, 17. [Google Scholar]
- Mustalampi, S.; Ylinen, J.; Kautiainen, H.; Weir, A.; Hakkinen, A. Acute effects of cold pack on mechanical properties of the quadriceps muscle in healthy subjects. Phys. Ther. Sport 2012, 13, 265–269. [Google Scholar] [CrossRef] [PubMed]
| Set 1 | Set 2 | Set 3 | P2-1 | P3-1 | ICC2-1 (95%CI) | ICC3-1 (95%CI) | MDC2-1 | MDC3-1 | |
|---|---|---|---|---|---|---|---|---|---|
| Shear Wave Elastography | |||||||||
| Shear Modulus (kPa) | 16.16 ± 9.56 | 15.74 ± 10.33 | 17.49 ± 12.29 | 0.623 | 0.375 | 0.80 (0.68, 0.88) | 0.62 (0.41, 0.77) | 8.80 | 15.52 |
| Strain Elastography | |||||||||
| Elastography Index (A.U.) | 2.39 ± 0.51 | 2.42 ± 0.47 | 2.42 ± 0.49 | 0.762 | 0.922 | 0.71 (0.57, 0.81) | 0.71 (0.57, 0.81) | 0.73 | 0.75 |
| MyotonPRO | |||||||||
| Dynamic Stiffness (N/m) | 321.45 ± 56.89 | 322.65 ± 58.15 | 318.19 ± 58.24 | 0.555 | 0.311 | 0.97 (0.95, 0.98) | 0.93 (0.89, 0.96) | 28.37 | 40.38 |
| Tensiomyography | |||||||||
| Radial Displacement (mm) | 6.71 ± 1.83 | 6.70 ± 1.88 | 6.66 ± 1.74 | 0.946 | 0.744 | 0.91 (0.85, 0.95) | 0.89 (0.82, 0.94) | 1.48 | 1.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Sánchez, A.; Abián, P.; Sánchez-Infante, J.; Esteban-Gacía, P.; Jiménez, F.; Abián-Vicén, J. Objective Assessment of Regional Stiffness in Vastus Lateralis with Different Measurement Methods: A Reliability Study. Sensors 2021, 21, 3213. https://doi.org/10.3390/s21093213
Bravo-Sánchez A, Abián P, Sánchez-Infante J, Esteban-Gacía P, Jiménez F, Abián-Vicén J. Objective Assessment of Regional Stiffness in Vastus Lateralis with Different Measurement Methods: A Reliability Study. Sensors. 2021; 21(9):3213. https://doi.org/10.3390/s21093213
Chicago/Turabian StyleBravo-Sánchez, Alfredo, Pablo Abián, Jorge Sánchez-Infante, Paula Esteban-Gacía, Fernando Jiménez, and Javier Abián-Vicén. 2021. "Objective Assessment of Regional Stiffness in Vastus Lateralis with Different Measurement Methods: A Reliability Study" Sensors 21, no. 9: 3213. https://doi.org/10.3390/s21093213
APA StyleBravo-Sánchez, A., Abián, P., Sánchez-Infante, J., Esteban-Gacía, P., Jiménez, F., & Abián-Vicén, J. (2021). Objective Assessment of Regional Stiffness in Vastus Lateralis with Different Measurement Methods: A Reliability Study. Sensors, 21(9), 3213. https://doi.org/10.3390/s21093213








