1. Introduction
Postural control is a complex motor function derived from several integrated neural components, including sensory and movement strategies, orientation in space, biomechanical constraints, and cognitive processing [
1]. It is also the ability to build up posture against gravity and ensure that balance is maintained. Force plates are frequently used to measure balance [
2,
3]. Force plate equipment and motion analysis machines allow therapists to accurately describe the center of gravity (COG) location, center of body mass (COM) position, center of pressure (COP) displacement, and kinematics of movement strategies for balance. COG is the average location of the weight of an object. COM is the average position of all the parts of the body, weighted according to mass. However, the movements of body parts make assessing postural control by measuring average location, position, or displacement (COG, COM, COP) challenging [
4]. Measuring postural control is difficult because postural changes may occur as a result of slight movements that are difficult to detect through simple observation by human eyes [
1]. Observational balance measures such as the Berg Balance Scale are used to evaluate balance. However, they evaluate performance and not balance movement strategies. The assessment scales used by therapists tend to be subjective, and their reliability and sensitivity can be limited [
5]. Measurements of postural control should identify how stably or quickly a subject performs or maintains an equilibrium position and the appropriateness and efficiency of movement strategies used to achieve or maintain the equilibrium position. Objective measures of postural control using computerized systems can allow more sensitive, specific, and responsive assessments in clinical practice.
Microsoft Kinect is a popular human motion capture tool. Kinect cameras are useful, as they provide joint center position data directly without additional processing of depth or image data [
6]. Recent evidence suggests that Kinect may enable low-cost balance assessments and gait analyses [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16]. The Kinect device has been reported to have validity for the evaluation of spatiotemporal gait parameters [
17]. Kinect’s kinematic information is generally accurate enough for ergonomic assessments [
18]. Postural control is the coordination of multiple joints to maintain postural stability, and the device can be used to collect large amounts of joints data to explore the coordinated relationships among the joints of the whole body during the maintenance of postural control [
19]. Kinect’s kinematic parameters follow joint trajectories and, thus, can be used as a tool for measuring spatiotemporal aspects of postural control. Recent studies have demonstrated that 3D motion analysis of data from the Kinect motion capture system can be used in clinical assessments of coordination and balance and could potentially be used to monitor gross motor performance and assess motor function [
20,
21,
22]. However, most studies have explored the displacement of COP, COM, or COG or the kinematics of body segments [
7,
11,
13,
23,
24,
25,
26], whereas few have endeavored to classify the quality of postural control or measure slight differences in similar situations of postural control.
Neural networks have advanced at a remarkable rate, and they have practical applications in various industries, including the medical and health care industry [
27,
28,
29,
30]. Deep learning has major applications in medical diagnosis, classification, and prediction, including but not limited to health informatics [
31] and biomedicine analysis [
32]. Other uses of deep learning in the medical field are in medical image segmentation, registration, and detection of various anatomical regions of interest, such as in magnetic resonance imaging [
33], ultrasound [
34], and radiography [
35]. The clinical use of images from digital cameras or depth sensors combined with deep and machine learning has promise for postural control assessment, body motion assessment, and fall detection. In one study, skeleton joints data from Kinect were used to determine human balance states, and a fall prediction algorithm based on recurrent neural networks and unbalanced posture features was proposed [
36]. One fall detection method based on 3D skeleton data obtained from Kinect employed long short-term memory networks [
37]. One study investigated the extent to which such deep learning–based systems provide satisfactory accuracy in exergame-relevant measures; a deep learning–based system was reported to perform as well as the gold standard system in the detection of temporal variations [
38]. In one study, a long short-term memory recurrent neural network was used in a supervised machine learning architecture and a novel deep learning–refined kinematic model with good kinematic accuracy for upper limb functional assessment was developed [
39]. Therefore, Kinect’s image information combined with machine and deep learning can be used to develop an effective limb functional assessment system for medical diagnosis or therapeutic evaluation.
Convolutional neural networks (CNNs) are the most widely represented class in deep learning and medical image analysis [
27,
28]. Deep learning methods are useful for extracting various image features, whereas machine learning approaches are efficient, rapid, and quantitative and can be used to build classification methods for numerous predictors. Hence, a combination of deep and machine learning methods was employed in this study.
Objective measurements of postural control made with a computerized system using Kinect combined with machine and deep learning can enable sensitive postural control assessment in clinical practice. Such a system might effectively classify the quality of postural control or identify minute differences between cases of similar postural control. This study is the first to combine joint node motion information with machine learning to extract joint node trajectory features and to use deep learning to classify postural control stability according to joint node trajectory patterns. This work had a twofold aim: to extract joint node trajectory plot features in order to explore the relative motion and to classify the stability of postural control according to joint node trajectory patterns.
The remainder of the paper is organized as follows. The research methodology is described in
Section 2. The experimental results are presented in
Section 3. The proposed features for assessing postural control performance and the joint–node plot (JNP) are discussed in
Section 4.
Section 5 presents the conclusion and proposes future research directions.
2. Materials and Methods
2.1. Experimental Design in Young and Elderly Adults
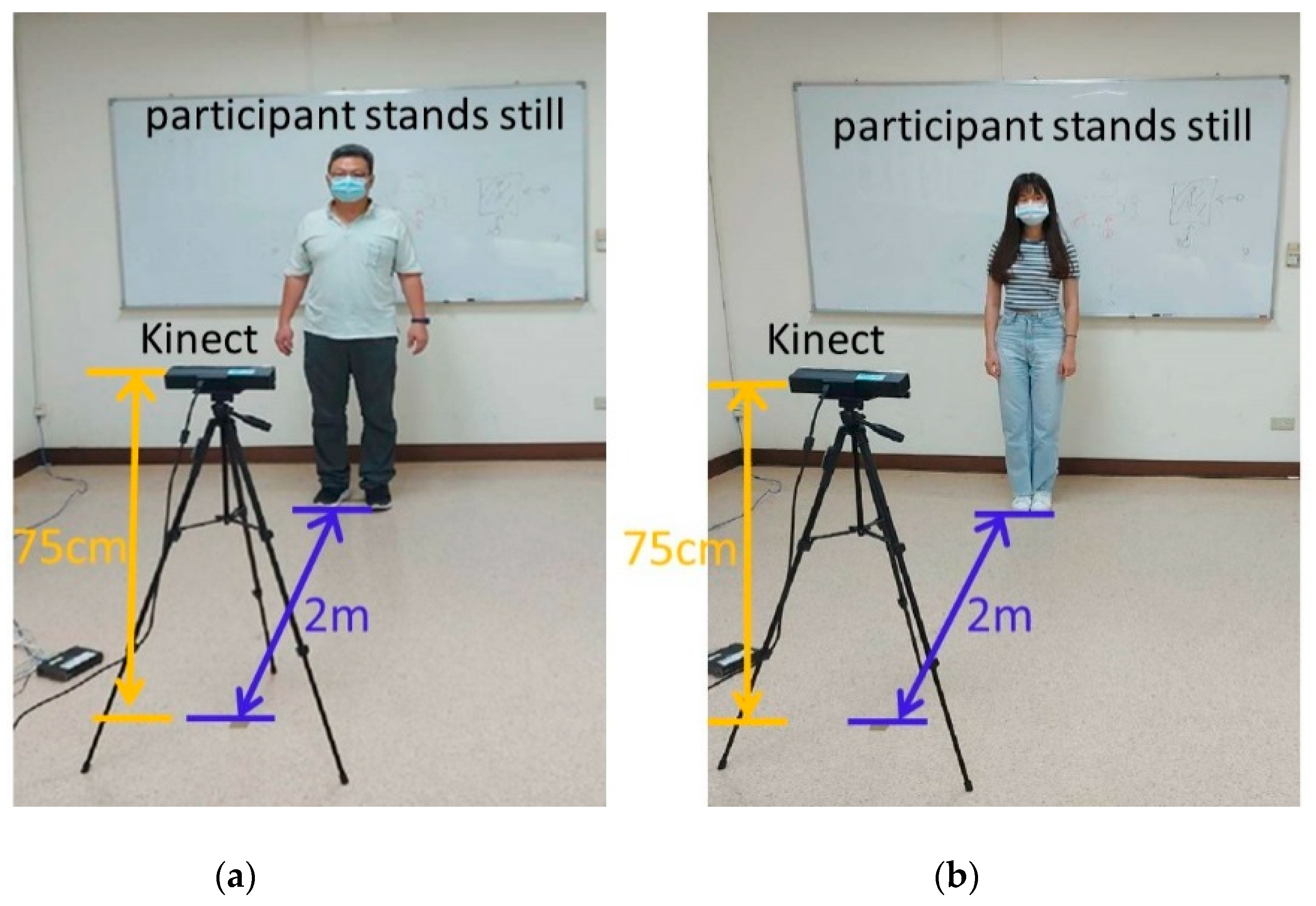
The experimental group was composed of elderly people who had a medical history and disabilities in daily life. They resided in a nursing home. In general, they might or could be regarded as a poor postural control group. In addition, the young adults had no medical history or any tremor problems. Therefore, the young group might or could be regarded as the control group. The study was conducted at a nursing home and on a college campus. Participants were recruited by a clinic nurse and study staff. To be included, participants had to meet the following criteria: be adults (>20 years old) to rule out developmental problems; have no restriction on physical activity; have no lower-limb discomfort and be able to maintain a double-leg stance with both eyes open for at least 40 s; and be willing to provide consent to participate in the study. The selected participants underwent the Mini-Mental State Examination (MMSE), Barthel Index (BI), and Berg Balance Scale (BBS) examinations in both young and elderly groups (
Table 1). The young participants got full marks in the MMSE, BI, BBS examinations, and without any medical history. The elderly participants must be 65 years of age or older, able to cooperate balance test, communicate with each other, and read words well. Exclusion criteria were severe somatic illness or neurological or musculoskeletal impairment including cognitive impairment, chest pain, angina pectoris, joint pain during recent exercise, congestive heart failure, and advised by doctors not to exercise. In all, 35 elderly adults (aged 82.06 ± 8.74 years) and 20 healthy young adults (aged 21.60 ± 0.60 years) participated. Postural control was measured according to the records of 15 joint coordinates. All participants were required to statically stand for 40 s while measurements were captured by a Kinect device. The recording procedure was performed daily for 6 days. The participants were instructed to stand and look straight at a visual reference and stand still with their shoulders relaxed, arms at the side of the trunk, feet slightly spread apart, and knee and hip joints in the upright position for 40 s. The participants were defined as young (control group) or elderly (experimental group) adults. The target class was the elderly group (experimental group) due to the lack of postural control. The experimental setup is depicted in
Figure 1. All experimental procedures were approved by the Institutional Review Board of E-DA Hospital [with approval number EMRP-107-103 (2019/01/28)].
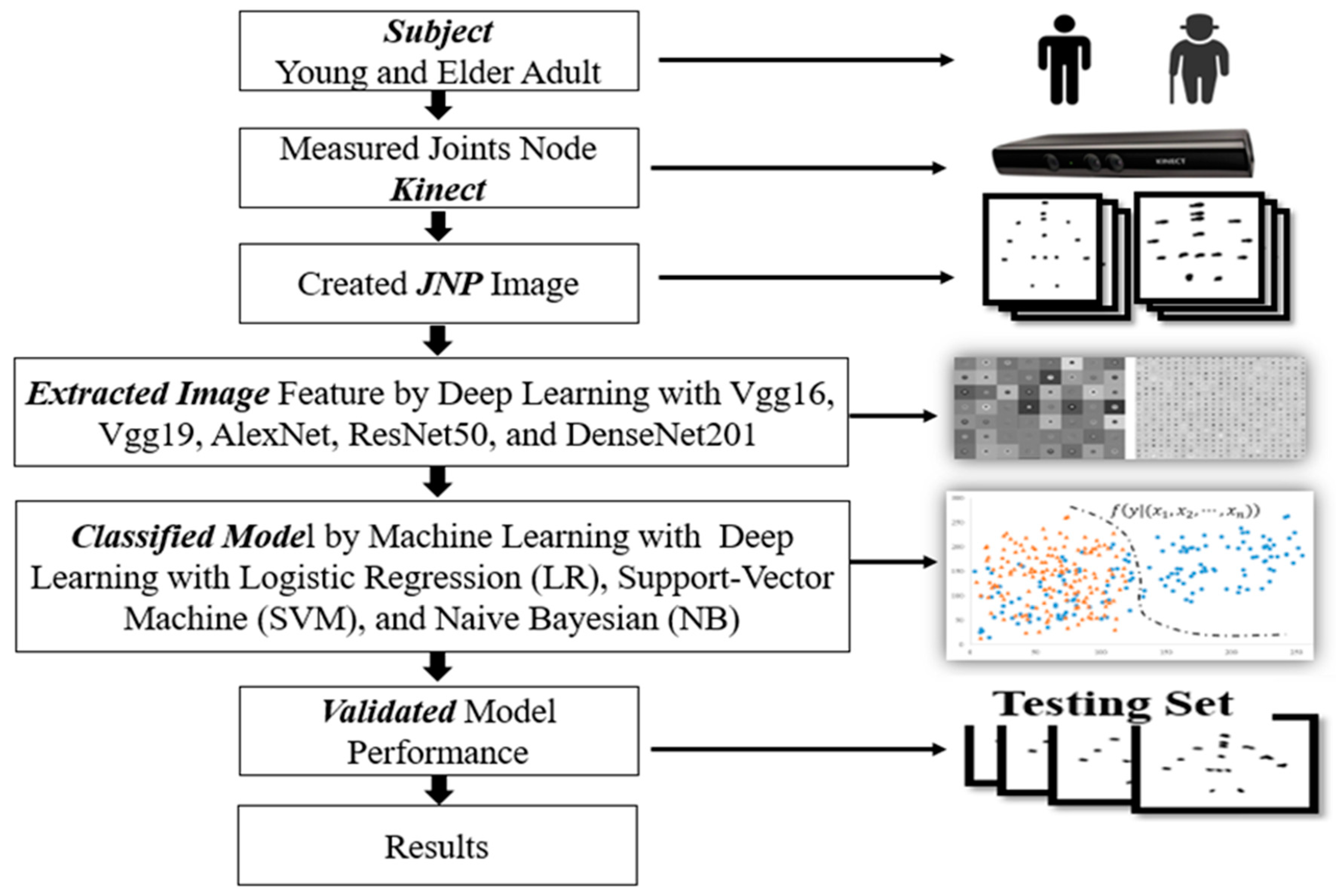
The study flowchart includes the participants coordinates of joint nodes measured by Kinect, creation of joint node images with the coordinates, features extracted from images, and training of the classification models. The models were validated with a testing set, and the final results were recorded (
Figure 2).
2.2. Measurement of Joint Coordinates
The Kinect device was made by Microsoft (Microsoft Inc., Redmond, WA, USA). It recorded joint node locations and was connected to a personal computer–based signal processing system. A data point of a joint node signal includes X, Y, and Z coordinates. Only X and Y coordinates were considered in this study because when standing still, vertical movement is negligible. The signals of the joint nodes were recorded at a frequency of 30 Hz.
2.3. Creating the JNP
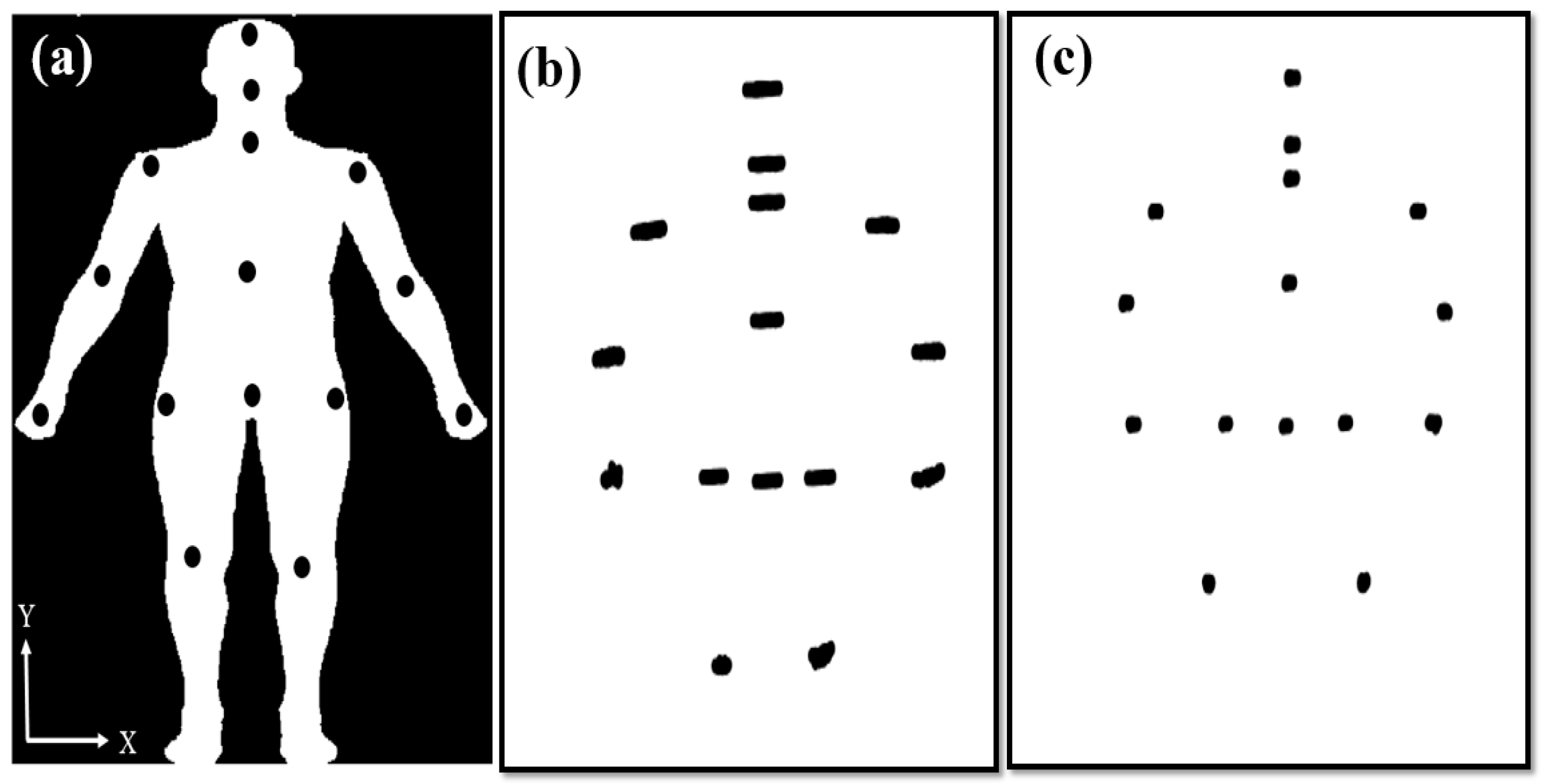
The 15 joints were recorded by Kinect for 40 s (
Figure 3a). However, the 1200 coordinates (
X,
Y) of the joints were recorded over 40 s. Hence, the JNP was created to observe postural control and examine stability over a period of 40 s (
Figure 3b,c). The JNPs clearly visualized good or poor postural control and provided positioning information for the deep and machine learning approaches.
2.4. Deep and Machine Learning Methods
Combinations of deep and machine learning methods were used to classify and predict postural control in young and elderly adults. The 90 model combinations involved five CNNs, three classifiers, three epochs (10, 15, and 20), and two random splitting ratios for the training set (60% and 70%) (i.e., 5 × 3 × 3 × 2 combinations).
2.4.1. Deep Learning Methods
The pre-trained CNNs applied to extract features of the JNPs were Vgg16, Vgg19, AlexNet, ResNet50, and DenseNet201. Deep CNN network technology has five primary layers: a convolutional layer, a pooling layer, a rectified linear unit layer, fully connected layers, and a softmax layer. The layers are listed in
Table 2. The fully connected CNN layers extracted and stored the features of the input image. The used CNNs were described by Hsu et al. [
40] (
Table 2). The CNN has been confirmed to be efficient and useful for image feature extraction in the fields of biomedicine and biology [
41,
42,
43]. Again, in the current study, the size of the epoch was set as 10, 15, or 20, and the training set percentage was 60% or 70% of data, randomly selected from the groups.
2.4.2. Machine Learning Methods
Logistic regression (LR) is often applied to analyze associations between two or more predictors or variables. Regression analysis is commonly adopted to describe relations between predictors or variables to build a linear functional model, whereas regression modeling is usually used to predict an outcome with a new predictor. LR is a binary regression model. The LR method is used in the field of machine learning and is applied for the development of classification models because of its capacity to provide tree-like or hierarchical structures. Many fields have adopted LR for prediction and classification.
A support vector machine (SVM) is a supervised learning method with the ability to powerfully generate a Hyper Plan for classifying categorical data. The SVM is generally utilized in high-dimensional or nonlinear categorization. Many useful kernels are available to improve classification performance and reduce false rates.
Naive Bayes (NB) classifiers are based on the Bayesian theorem with a naïve independence hypothesis between the adopted predictors or features. NB classifiers provide higher accuracy under bundle with kernel density estimation [
44]. They also offer high flexibility for linear or nonlinear relations among variables (features/predictors) in classification problems. The computing cost takes linear time by compared those of expensive iterative approximations of classifiers.
To classify the postural control of the young and elderly groups, these algorithms were applied to the extracted features as deep and machine learning methods with JNP.
2.5. Evaluating Model Performance
The coordinates of 15 joints continually measured for 40 s were plotted in one figure for each candidate. Each participant had six figures as a result of the replicated runs. Hence, a total of 120 and 150 JNPs were created for the young and elderly groups, respectively. The size of a JNP was 875 × 656 pixels with 24 bits per pixel. The testing sets were 48 and 60 JNPs (40%) or 36 and 45 JNPs (30%), randomly selected from the young and elderly groups, respectively. The original data were partitioned into training and testing sets randomly without overlapping samples in the sets.
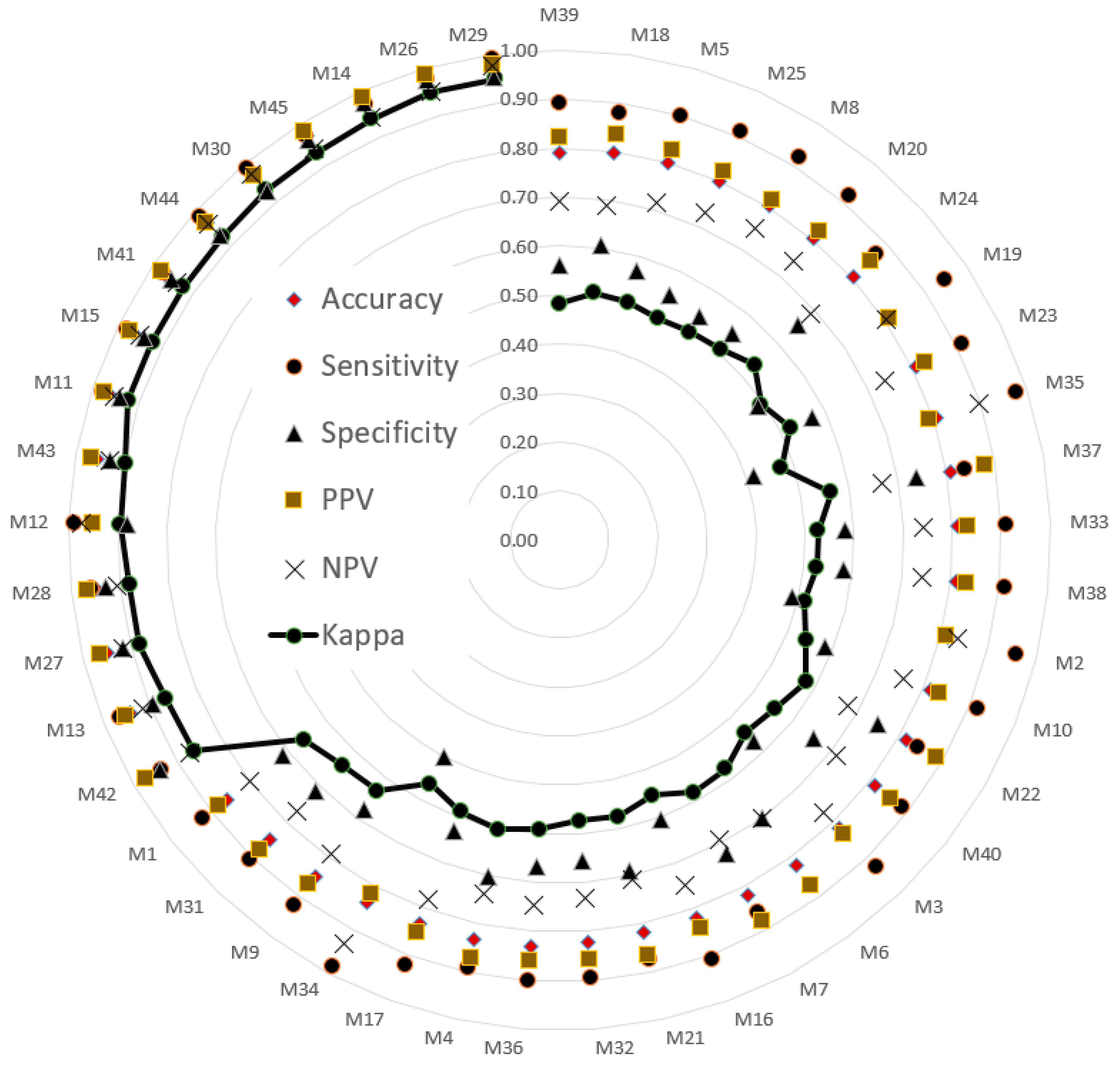
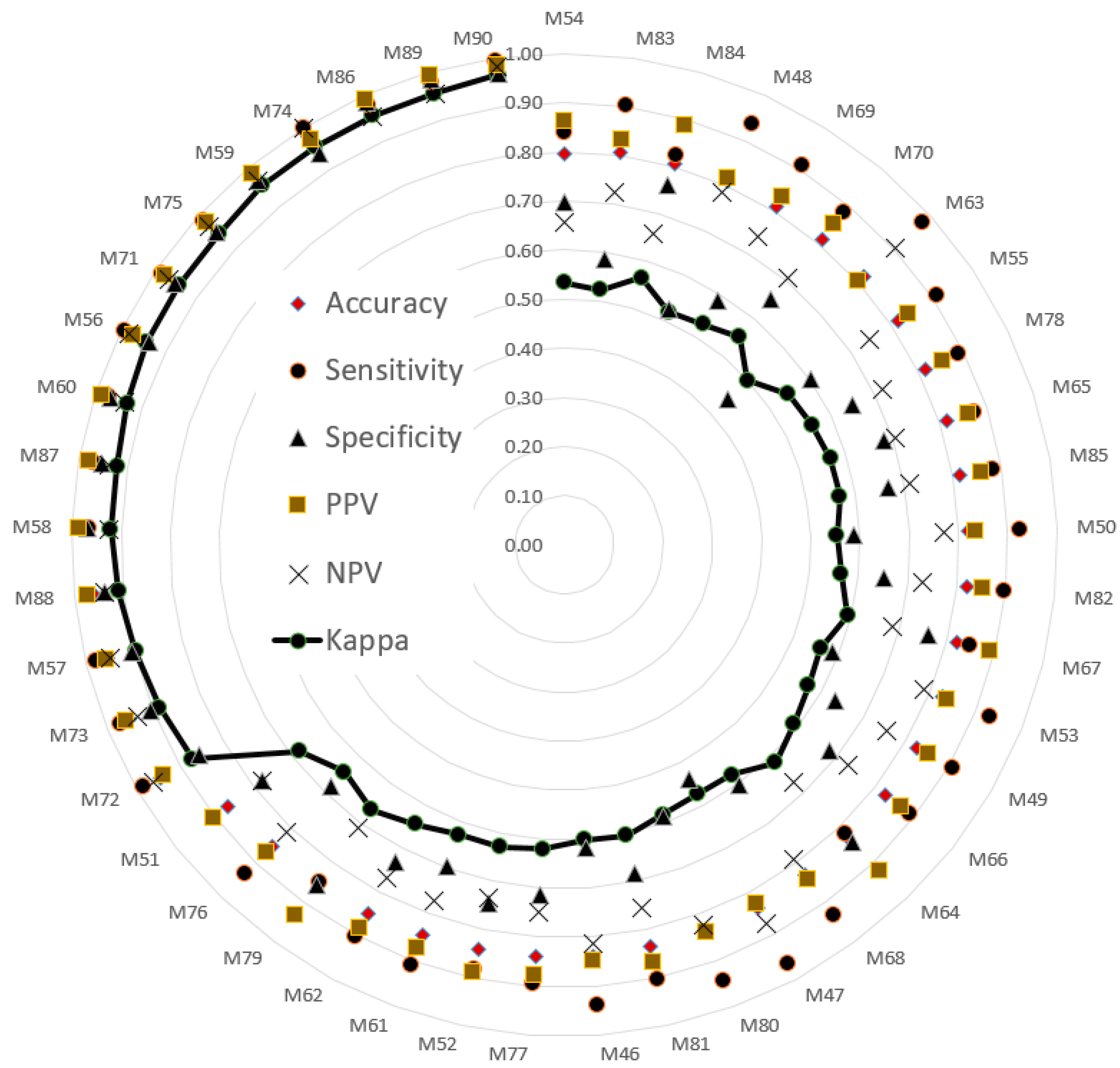
The testing sets were used to evaluate model performance. The validated performance of the presented methods is typically used to popular index. A confusion matrix is often used to assess model suitability, including its accuracy, sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and kappa value. The indices (i.e., six evaluated values) were sorted in ascending order according to the kappa value. Then, a radar plot was developed to display the indexes for the models. A radar plot was developed to display those indexes for the presented models.
4. Discussion
4.1. The Informative JNP
Using joint motion trajectories instead of COP or COM displacement for analysis enables the evaluation of posture control ability as well as the posture control strategies used to achieve balance [
45,
46]. In the current study, the JNPs of the elderly group indicated that they tended to use an extreme joint coordination mode, an inter-joint coordination strategy characterized by total joint dependence, to maintain balance when standing still [
19].
The JNP provided information on postural control, but also on tremors. No screening test or tool is available for the early detection of Parkinson’s disease. The JNP map can help in evaluating coordinated interactions among joints and discovering involuntary tremors of each segment when an individual is standing still [
47]. The stability of the torso and proximal joints in the elderly adult group was similar to that in the young adult group, but the forearm and knee joints exhibited slight tremors (
Figure 6a), which may have been psychogenic or physiological tremors.
Figure 6b displays the postural stability of joints in various parts of the body, which was better than most of elderly people in the study but the forearm and hand joints exhibited obviously psychogenic or physiological tremors. The postural stability of the joints of various parts of the body in
Figure 6d is similar to that in
Figure 6c and may indicate a postural tremor. In some cases, the left forearm shook more, but the whole body shook horizontally (
Figure 6c).
Figure 6c displayed a typical pattern of postural stability of the joints in the elderly adult group and possibly indicating a postural tremor and or psychogenic tremor. When a postural tremor occurs, further testing is required to confirm its cause, which may be, for example, primary cerebellar disease, brain injury, dystonia, alcohol, or drugs. In
Figure 6e, symmetrical shaking of the wrists and lower limbs occurs on both sides; this is suspected to be an essential tremor or Parkinsonian tremor. In
Figure 6f, whole-body shaking, including shaking of the feet, is intense and asymmetrical and leads to instability when the individual is standing; in such cases, a Parkinsonian tremor is suspected. When a postural tremor occurs in a case, further testing is required to confirm the cause of the jitter, which may be caused by other diseases, such as primary cerebellar disease, dystonia, Parkinson’s disease, drugs, etc. Hence, the JNP may be used to visualize shaking and relations between tremors and diseases.
4.2. Combined Deep and Machine Learning
In this study, the VGG16, VGG19, AlexNet, ResNet50, and DenseNet201 were used to extract image features for the development of SVM classification models. Although several fully connected layers (FCLs) were present in the CNN, we did not survey and compare all of them. Only the last FCL of the CNN was applied to extract features of images for the SVM, LR, and NB classifiers. The SVM was regarded as an efficient classifier for detecting and classifying postural control in young and elderly adults on the basis of their JNPs.
M29 combined VGG16 and the SVM (training set, 70%; validation set, 30%), and M90 combined VGG19 and SVM to classify balance function (training set, 80%; validation set, 20%). The accuracy and kappa values of M29 and M90 were (98%, 95%) and (99%, 97%), respectively. The validation results indicated that both M29 and M90 could classify balance function in the elderly group with high agreement and consistency. Additionally, the deep learning component of the VGG architecture provided useful features of images for the SVM. Therefore, the SVM archived to classify the task of detected balance function between the young and elderly adults.
Table 5 summarizes the results in
Table 3 and
Table 4. All 27 methods selected achieved kappa values of 0.88 or higher. AlexNet, VGG16, and VGG19 each appeared six times. DenseNet201 and ResNet50 appeared four and five times, respectively. The minimum accuracy generated by AlexNet, VGG16, and VGG19 was 0.97. VGG19 combined with the SVM had the highest maximum accuracy among the five deep learning methods with the SVM classifier.
4.3. Comparison with Reported Results
The proposed methods were compared with previously developed methods with respect to the results listed in
Table 5. SVMs, random forest models, and cohorts have been applied to detect motor [
48,
49], balance, or gait function [
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60]. The highest accuracy in classifying motor function was 97%, achieved by an SVM. The highest accuracy for classifying gait or balance function was 96.7%, also achieved by an SVM. Thus, SVMs were proven successful in classification tasks. However, the proposed methods achieved higher accuracies in terms of reasonability and feasibility than did the other methods listed in
Table 6.
To further test the reliability of the proposed methods in classifying postural control, a future study might compare the results a gold standard detection method, such as functional assessment or balance assessment.