Custom-Fitted In- and Around-the-Ear Sensors for Unobtrusive and On-the-Go EEG Acquisitions: Development and Validation
Abstract
1. Introduction
2. Materials
2.1. Prototyping of Conductive Silicone Electrodes
2.2. Intra-Aural Earpiece
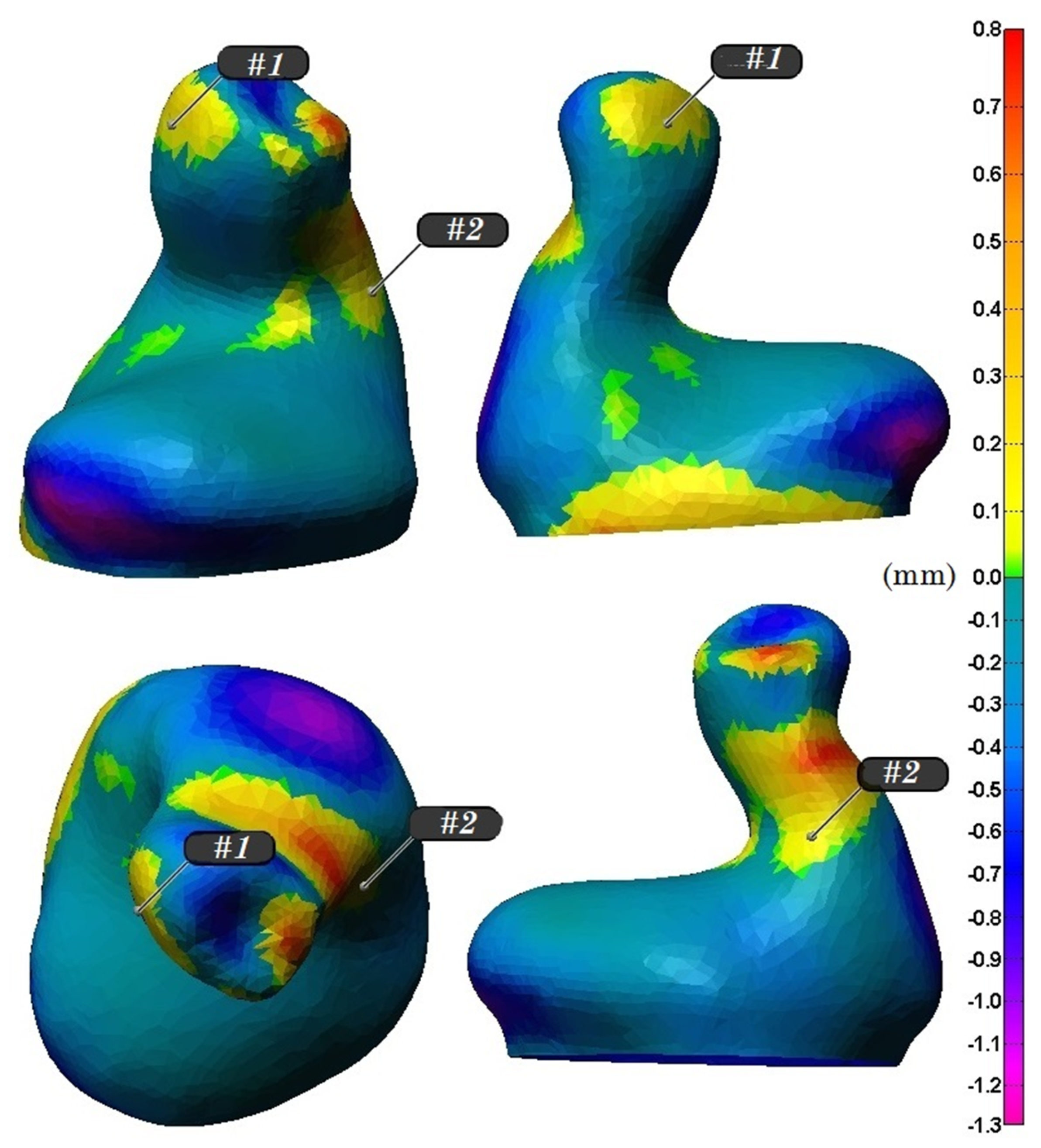
- The electrodes are close neither to the tip of the earplug nor to the concha of the ear since they would likely not be in contact with the ear canal at these two regions;
- The electrodes are preferably placed in nearly flat or large curvature regions of the ear canal;
- The choice of the relative positions of two electrodes in one ear mold should ensure the maximum possible distance between them;
- The electrodes are placed in an area with zero or most preferably positive deformations, so that, regardless of the jaw’s position, the electrode always stays in contact with the skin of the ear canal.
2.3. Circum-Aural Earpiece
2.4. Custom EEG Research Platform
3. Methods
- The first study aimed to assess the comfort of a custom in-ear piece versus a generic in-ear piece with a less complex design.
- The second study aimed to validate the conductive silicone material by comparing the EEG data recorded with conventional gold-plated electrodes to those obtained with conductive silicone electrodes.
- The third study aimed to demonstrate the capability of CochlEEG in recording EEG data at 4 kHz, in addition to the 0.5 kHz, 1 kHz and 2 kHz sampling frequencies whose validation was presented in previous work [24].
- Finally, the fourth study aimed to demonstrate the device’s capability to reliably detect decision-making processes through an event-related potential (ERP) generated by an auditory oddball task with the behind-the-ear piece used concurrently with an in-ear piece.
3.1. Study #1: Comfort Evaluation
3.2. Study #2: Validation of the Conductive Silicone
3.2.1. ASSRs
3.2.2. Participants
3.2.3. Auditory Stimuli
3.2.4. Recordings
3.3. Study #3: Validation of CochlEEG’s 4 kHz Sampling Rate
3.4. Study #4: Event-Related Potentials (ERPs)
3.4.1. ERPs and the Oddball Paradigm
3.4.2. Participants
3.4.3. Auditory Stimuli
3.4.4. Recordings
4. Results
4.1. Comfort of the In-Ear Pieces
4.2. Validation of the Conductive Silicone Material
4.3. Recordings at 4 kHz
4.4. Event-Related Potentials (ERP)
5. Discussion
- The definition of the discomfort induced by in-ear devices has been the subject of debate for many years in the literature [41,42,43] and the comfort evaluations presented in this paper are not intended to tackle this question as several parameters (e.g., short wearing time and lack of activities performed by the participant while evaluating the comfort, among other considerations) narrow the conclusions that could be drawn from this limited study. However, even if a more extensive study is needed to conclude about the potential discomfort induced by wearing such in-ear devices, the comfort evaluations performed in this paper seem to indicate that there is no real difference between the custom and the generic in-ear piece, in terms of comfort. Nevertheless, several modifications should be done to improve the in-ear piece design. Indeed, according to the method proposed in this paper, there is more than one optimal position on the intra-aural earpiece where the electrodes could be placed; however, only one was tested in this study for each participant. Other positions should be tested individually or together in order to find the best possible electrode configuration. Furthermore, a handle to pull the earpiece out of the ear canal and a sound bore to let pass the auditory stimulation should be considered for mold design improvements.
- Even with the second behind-the-ear design, some difficulties remain the same for a few participants as to them not achieving acceptable electrical impedance at the point of skin–electrode contact. Therefore, the geometry and shape of the behind-the-ear piece should be further improved by increasing the flexibility of its structure in order to solve the problem of poor electrode-skin contact observed for a few electrodes.
- Results obtained with conductive silicone electrodes were compared with those obtained with gold-plated electrodes. Silver-plated (Ag/AgCl) electrodes are a good alternative to gold-plated electrodes. Such electrodes seem to be slightly more stable electrically, but need replacing more frequently because the plating layer (AgCl on top of Ag) is thin. Future work should investigate how silicone conductive electrodes perform compared to silver-plated electrodes.
- The impedance measurements were achieved using a medical grade impedance meter (Grass Technologies Electrode Impedance Meter), which does not provide measurements over a wider frequency range. Consequently, the electrode characterization presented in this paper remains limited. A thorough investigation over a wider frequency range should be conducted to complete the electrode characterization and to find out how the electrode-skin impedance might vary with time.
- While the EARtrodes have been validated with wet electrodes made of conductive silicone, the use of dry conductive silicon electrodes should also be investigated. Indeed, such dry electrodes would not require the abrasion of the Stratum Corneum and the use of electrolytic gel to reduce the skin’s impedance, which would make them easier to use and more acceptable in social settings [44,45,46]. Dry EARtrodes would also be great alternatives to semidry electrodes, which are not really user-friendly due to their cumbersome design [47,48,49].
- The major concerns for most composite materials tend to relate to the loss of durability and reliability over time. Therefore, a more comprehensive study should be conducted to evaluate the conductive silicone material’s aging profile, including physical and thermal performance over time.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASSR | Auditory steady-state response |
| BCI | Brain–computer interface |
| dB | Decibel |
| EEG | Electroencephalography |
| ERP | Event-related potentials |
| FFT | Fast Fourier transform |
| HL | Hearing level |
| HRA | Rockwell A-scale hardness |
| ICA | Independent component analysis |
| MMN | Mismatch negativity |
| RMS | Root mean square |
| TMJ | Temporomandibular joint |
References
- Machado, S.; Arauro, F.; Paes, F.; Velasques, B.; Cunha, M.; Budde, H.; Basile, L.; Anghinah, R.; Arias-Carrión, O.; Cagy, M.; et al. EEG-based Brain-Computer Interfaces: An Overview of Basic Concepts and Clinical Applications in Neurorehabilitation. Rev. Neurosci. 2010, 21, 451–468. [Google Scholar] [CrossRef]
- Kappel, S.L.; Looney, D.; Mandic, D.P.; Kidmose, P. Physiological artifacts in scalp EEG and ear-EEG. Biomed. Eng. Online 2017, 16, 103. [Google Scholar] [CrossRef]
- Kidmose, P.; Looney, D.; Mandic, D.P. Auditory evoked responses from Ear-EEG recordings. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, p. 586. [Google Scholar]
- Kidmose, P.; Looney, D.; Ungstrup, M.; Rank, M.; Mandic, D. A Study of Evoked Potentials from Ear-EEG. IEEE Trans. Biomed. Eng. 2013, 60, 2824–2830. [Google Scholar] [CrossRef]
- Mikkelsen, K.B.; Kappel, S.L.; Mandic, D.P.; Kidmose, P. EEG Recorded from the Ear: Characterizing the Ear-EEG Method. Front. Neurosci. 2015, 9, 438. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Looney, D.; Kidmose, P.; Mandic, D.P. In-Ear EEG From Viscoelastic Generic Earpieces: Robust and Unobtrusive 24/7 Monitoring. IEEE Sens. J. 2016, 16, 271–277. [Google Scholar] [CrossRef]
- Goverdovsky, V.; Rosenberg, W.V.; Nakamura, T.; Looney, D.; Sharp, D.; Papavassiliou, C.; Morell, M.; Mandic, D. Hearables: Multimodal physiological in-ear sensing. Sci. Rep. 2017, 7, 6948. [Google Scholar] [CrossRef]
- Simon, P.K.; Kappel, L.; Rank, M.L.; Toft, H.O.; Andersen, M. Dry-Contact Electrode Ear-EEG. IEEE Trans. Biomed. Eng. 2018, 66, 150–158. [Google Scholar]
- Nguyen, A.; Alqurashi, R.; Raghebi, Z.; Banaei-Kashani, F.; Halbower, A.C.; Vu, T. LIBS: A Bioelectrical Sensing System from Human Ears for Staging Whole-Night Sleep Study. Commun. ACM 2018, 61, 157–165. [Google Scholar] [CrossRef]
- Valle, B.G.D.; Cash, S.S.; Sodini, C.G. Wireless behind-the-ear EEG recording device with wireless interface to a mobile device (iPhone/iPod touch). In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 5952–5955. [Google Scholar]
- Norton, J.S.; Sup, L.D.; Woo, L.J.; Woosik, L.; Ohjin, K.; Won, P.; Jung, S.Y. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Debener, S.; Emkes, R.; Vos, M.D.; Bleichner, M. Unobtrusive ambulatory EEG using a smartphone and flexible printed electrodes around the ear. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bleichner, M.G.; Debener, S. Concealed, Unobtrusive Ear-Centered EEG Acquisition: cEEGrids for Transparent EEG. Front. Hum. Neurosci. 2017, 11, 163. [Google Scholar] [CrossRef]
- Mirkovic, B.; Bleichner, M.G.; Vos, M.D.; Debener, S. Target Speaker Detection with Concealed EEG Around the Ear. Front. Neurosci. 2016, 10, 349. [Google Scholar] [CrossRef]
- Sterr, A.; Ebajemito, J.K.; Mikkelsen, K.B.; Bonmati-Carrion, M.A.; Santhi, N.; della, M.C.; Grainger, L. Sleep EEG Derived From Behind-the-Ear Electrodes (cEEGrid) Compared to Standard Polysomnography: A Proof of Concept Study. Front. Hum. Neurosci. 2018, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Cleeren, E.; Dan, J.; Claes, K.; Paesschen, W.V.; Huffel, S.V.; Hunyadi, B. Comparison between Scalp EEG and Behind-the-Ear EEG for Development of a Wearable Seizure Detection System for Patients with Focal Epilepsy. Sensors 2017, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Bleichner, M.G.; Lundbeck, M.; Selisky, M.; Minow, F.; Jager, M.; Emkes, R.; Debener, S.; Vos, M.D. Exploring miniaturized EEG electrodes for brain-computer interfaces. An EEG you do not see? Physiol. Rep. 2015, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.W.; Ku, Y.; Kim, D.Y.; Sohn, J.; Kim, J.-H.; Kim, H.C. Wearable in-the-ear EEG system for SSVEP-based brain–computer interface. Electron. Lett. 2018, 54, 413–414. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.M.; Byeon, H.J.; Hong, J.S.; Park, K.S.; Lee, S.-H. CNT/PDMS-based canal-typed ear electrodes for inconspicuous EEG recording. J. Neural Eng. 2014, 11, 046014. [Google Scholar]
- He, W.; Sun, Y.; Xi, J.; Abdurhman, A.A.M.; Ren, J.; Duan, H. Printing graphene-carbon nanotube-ionic liquid gel on graphene paper: Towards flexible electrodes with efficient loading of PtAu alloy nanoparticles for electrochemical sensing of blood glucose. Anal. Chim. Acta 2016, 903, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Manabe, H.; Fukumoto, M.; Yagi, T. Conductive rubber electrodes for earphone-based eye gesture input interface. Pers. Ubiquit. Comput. 2014, 19, 143–154. [Google Scholar] [CrossRef]
- Delnavaz, A.; Voix, J. Ear Canal Dynamic Motion as a Source of Power for In-Ear Devices. J. Appl. Phys. 2013, 113, 1–9. [Google Scholar] [CrossRef]
- Kothe, C. Lab Streaming Layer (LSL). Available online: https://github.com/sccn/labstreaminglayer/ (accessed on 17 June 2019).
- Valentin, O.; Ducharme, M.; Cretot-Richert, G.; Monsarrat-Chanon, H.; Viallet, G.; Delnavaz, A.; Voix, J. Validation and Benchmarking of a Wearable EEG Acquisition Platform for Real-World Applications. IEEE Trans. Biomed. Circuits Syst. 2018, 13, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Casali, J.G. An empirical study if comfort afforded by various hearing protection devices: Laboratory versus field results. Appl. Acoust. 1991, 34, 151–179. [Google Scholar] [CrossRef]
- Cone-Wesson, B.; Dowell, R.C.; Tomlin, D.; Rance, G.; Ming, W.J. The auditory steady-state response: Comparisons with the auditory brainstem response. J. Am. Acad. Audiol. 2002, 13, 173–183. [Google Scholar]
- Picton, T.W.; John, M.S.; Dimitrijevic, A.; Purcell, D. Human auditory steady state responses. Int. J. Audiol. 2003, 42, 177–219. [Google Scholar] [CrossRef]
- John, M.S.; Lins, O.G.; Boucher, B.L.; Picton, T.W. Multiple auditory steady-state responses (MASTER): Stimulus and recording parameters. Audiology 1998, 37, 59–82. [Google Scholar] [CrossRef]
- Galambos, R.; Makeig, A.; Talmachoff, P.J. A 40-Hz auditory potential recorded from the human scalp. Proc. Natl. Acad. Sci. USA 1981, 78, 2643–2647. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; John, M.S.; Roon, P.V.; Picton, T.W. Human auditory steady-state responses to tones independently modulated in both frequency and amplitude. Ear Hear 2001, 22, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Maanen, A.V.; Stapells, D.R. Comparison of multiple auditory steady state responses (80 versus 40Hz) and slow cortical potentials for threshold estimation in hearing-impaired adults. Int. J. Audiol. 2005, 44, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 15, 9–21. [Google Scholar] [CrossRef]
- Luck, S.J.; Kappenman, E.S. The Oxford Handbook of Event-Related Potential Components; Oxford University Press: Hong Kong, China, 2012. [Google Scholar]
- Graimann, B.; Allison, B.; Pfurtscheller, G. Brain-Computer Interfaces; Springer: Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Donchin, E.; Coles, M.G. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988, 11, 357–427. [Google Scholar] [CrossRef]
- Bennington, J.Y.; Polich, J. Comparison of P300 from passive and active tasks for auditory and visual stimuli. Int. J. Psychophysiol. 1999, 34, 171–177. [Google Scholar] [CrossRef]
- Hoffmann, S.; Falkenstein, M. The correction of eye blink artefacts in the EEG: A comparison of two prominent methods. PLoS ONE 2008, 3, e3004. [Google Scholar] [CrossRef]
- Wellek, S. Testing Statistical Hypotheses of Equivalence and Noniferiority, 2nd ed.; Chapman & Hall: Boca Raton, FL, USA, 2010. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 17 June 2019).
- Wayne, W.D. Friedman Two-Way Analysis of Variance by Ranks, 2nd ed.; Applied Nonparametric Statistics; PWS-Kent: Boston, MA, USA, 1990; pp. 262–274. [Google Scholar]
- Davis, R.R. What do we know about hearing protector comfort? Noise Health 2008, 10, 83–89. [Google Scholar] [CrossRef]
- Kuijt-Evers, L.F.M.; Groenesteijn, L.; de Looze, M.P.; Vink, P. Identifying factors of comfort in using hand tools. Appl. Ergon. 2004, 35, 453–458. [Google Scholar] [CrossRef]
- Pearson, E.J.M. Comfort and its measurement—A literature review. Disabil. Rehabil. Assist. Technol. 2009, 4, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Liao, L.D.; Liu, Y.H.; Wang, I.J.; Lin, B.S.; Chang, J.Y. Novel dry polymer foam electrodes for long-term EEG measurement. IEEE Trans. Biomed. Eng. 2011, 58, 1200–1207. [Google Scholar] [PubMed]
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG Electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, J.; Xia, Y.; Wu, Y.; Tian, Y.; Liu, J.; Chen, D.; He, Q. Towards emerging EEG applications: A novel printable flexible Ag/AgCl dry electrode array for robust recording of EEG signals at forehead sites. J. Neural Eng. 2020, 17, 026001. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, D.; Wang, S.; Duan, Y.Y. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sens. Actuators Chem. 2016, 237, 167–178. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 2020, 17, 051004. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Li, M.; Duan, Y.Y. Towards Real-life EEG applications: Novel Superporous Hydrogel-Based Semi-dry EEG Electrodes Enabling Automatically “Charge-discharge” Electrolyte. J. Neural Eng. 2021, 8, 046016. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, B.; Dajani, H.R.; Giguère, C. Towards Developing a Brain-computer Interface for Automatic Hearing Aid Fitting based on the Speech-evoked Frequency Following Response. In NEUROTECHNIX 2017-Extended Abstracts; SciTePress—Science and Technology Publications: Funchal, Portugal, 2017; pp. 3–4. [Google Scholar]
- Kraus, N.; Thompson, E.; Krizman, J.; Cook, K.; White-Schwoch, T.; LaBella, C.-R. Auditory biological marker of concussion in children. Sci. Rep. 2016, 6, 39009. [Google Scholar] [CrossRef] [PubMed]










| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|
| Number of exploring electrodes | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentin, O.; Viallet, G.; Delnavaz, A.; Cretot-Richert, G.; Ducharme, M.; Monsarat-Chanon, H.; Voix, J. Custom-Fitted In- and Around-the-Ear Sensors for Unobtrusive and On-the-Go EEG Acquisitions: Development and Validation. Sensors 2021, 21, 2953. https://doi.org/10.3390/s21092953
Valentin O, Viallet G, Delnavaz A, Cretot-Richert G, Ducharme M, Monsarat-Chanon H, Voix J. Custom-Fitted In- and Around-the-Ear Sensors for Unobtrusive and On-the-Go EEG Acquisitions: Development and Validation. Sensors. 2021; 21(9):2953. https://doi.org/10.3390/s21092953
Chicago/Turabian StyleValentin, Olivier, Guilhem Viallet, Aidin Delnavaz, Gabrielle Cretot-Richert, Mikaël Ducharme, Hami Monsarat-Chanon, and Jérémie Voix. 2021. "Custom-Fitted In- and Around-the-Ear Sensors for Unobtrusive and On-the-Go EEG Acquisitions: Development and Validation" Sensors 21, no. 9: 2953. https://doi.org/10.3390/s21092953
APA StyleValentin, O., Viallet, G., Delnavaz, A., Cretot-Richert, G., Ducharme, M., Monsarat-Chanon, H., & Voix, J. (2021). Custom-Fitted In- and Around-the-Ear Sensors for Unobtrusive and On-the-Go EEG Acquisitions: Development and Validation. Sensors, 21(9), 2953. https://doi.org/10.3390/s21092953






