Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes
Abstract
1. Introduction
2. Materials and Methods
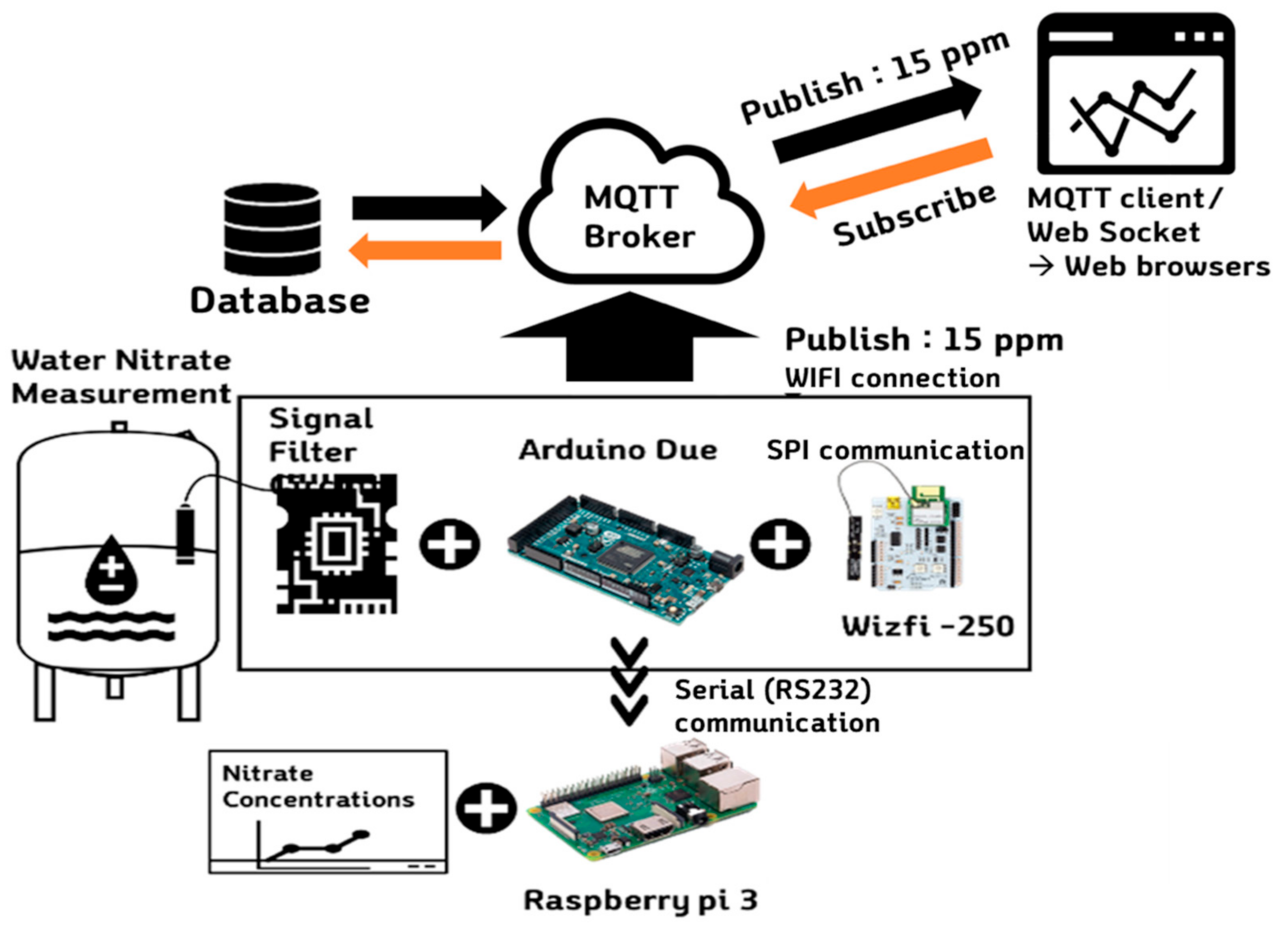
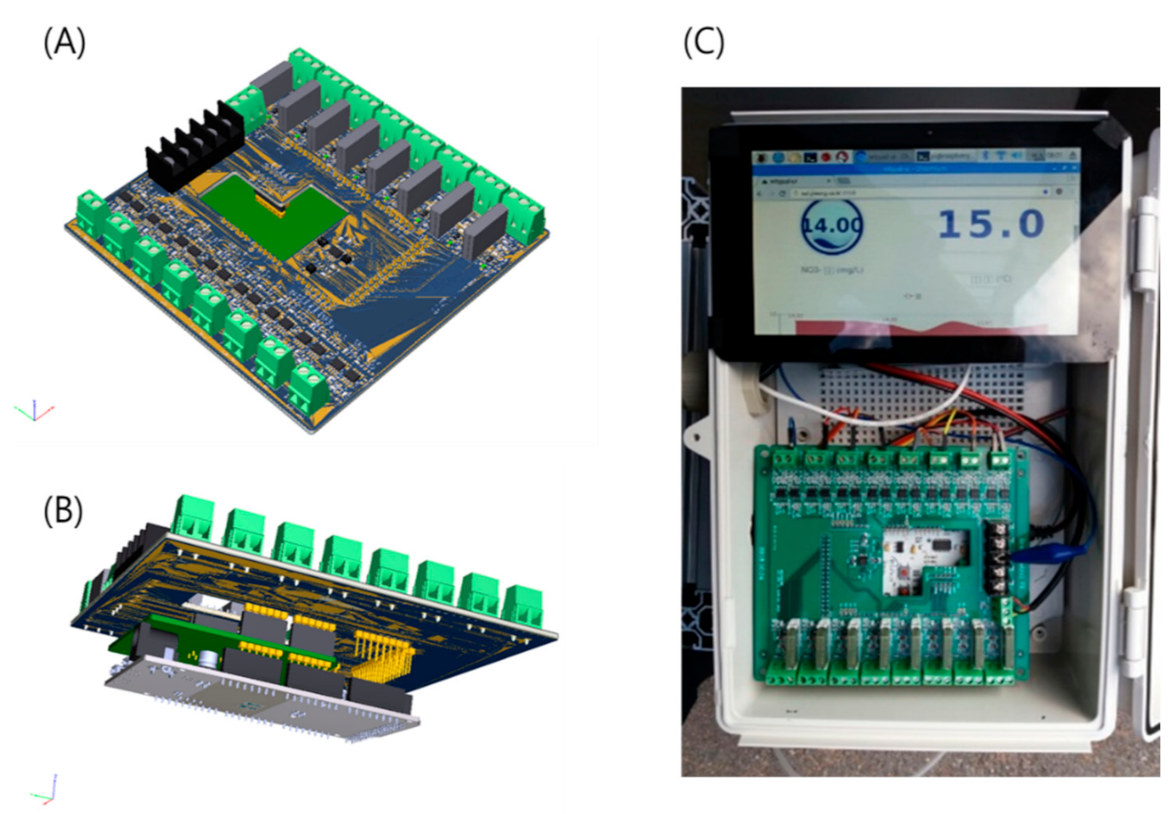
2.1. Configuration of IoT-Based Nitrate Measurement System
2.2. Self-Diagnostic Algorithm for ISE
Selection of SDI for Electrode
2.3. Experimental Procedure
2.3.1. Laboratory Test for Effectiveness of Self-Diagnostic Algorithm
2.3.2. Field Verification Experiment
2.3.3. Evaluation of Self-Diagnostic Methods
3. Results
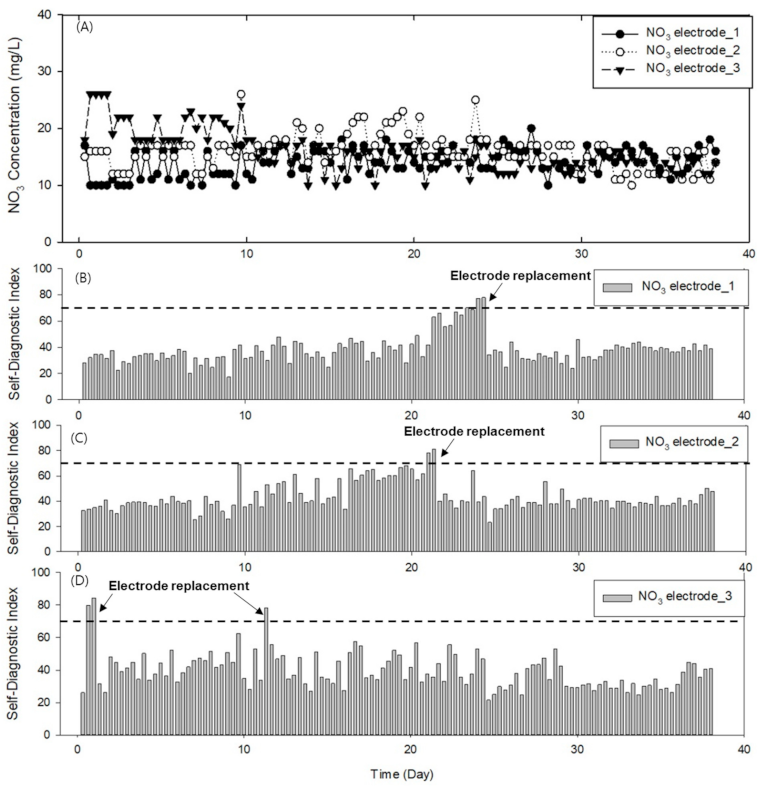
3.1. Lab Test Results Following Application of SDI
3.2. Learning Results of Deep Neural Network-Based Diagnostic Model for Electrode Status
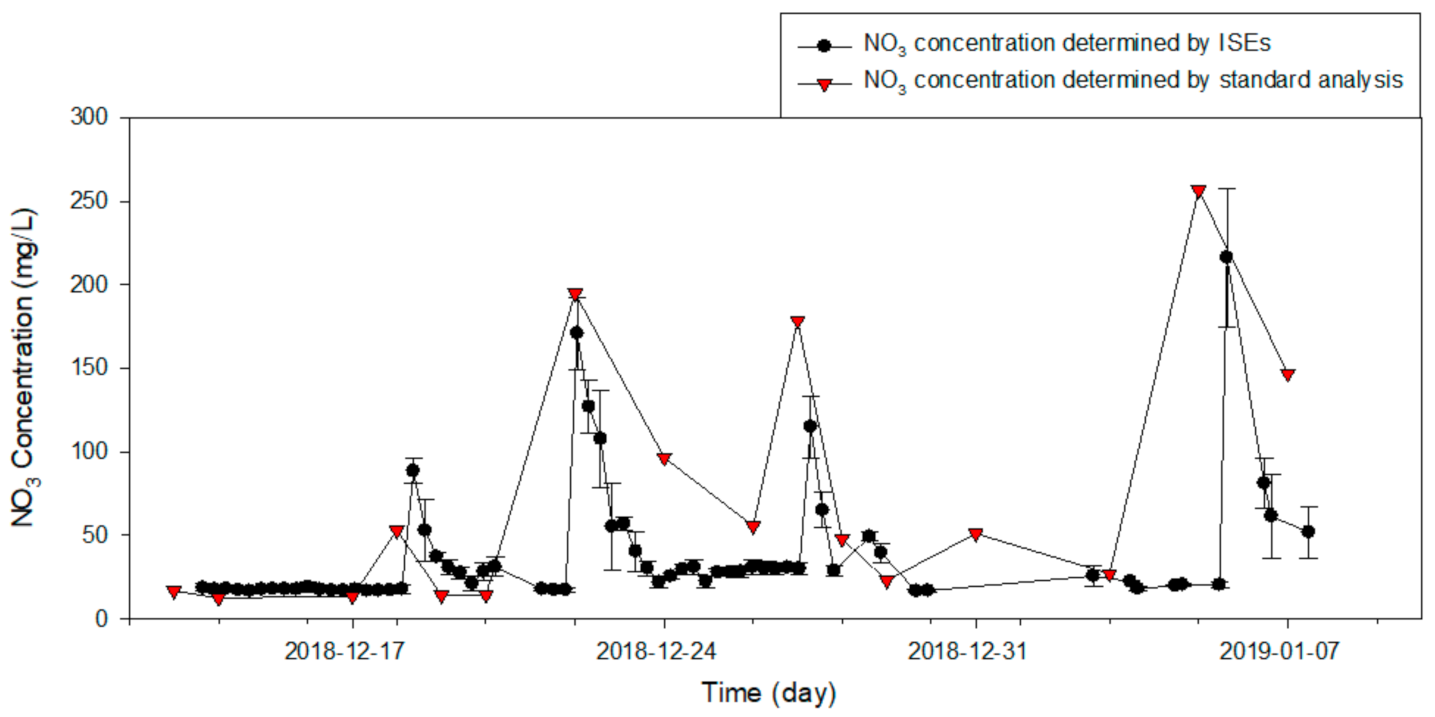
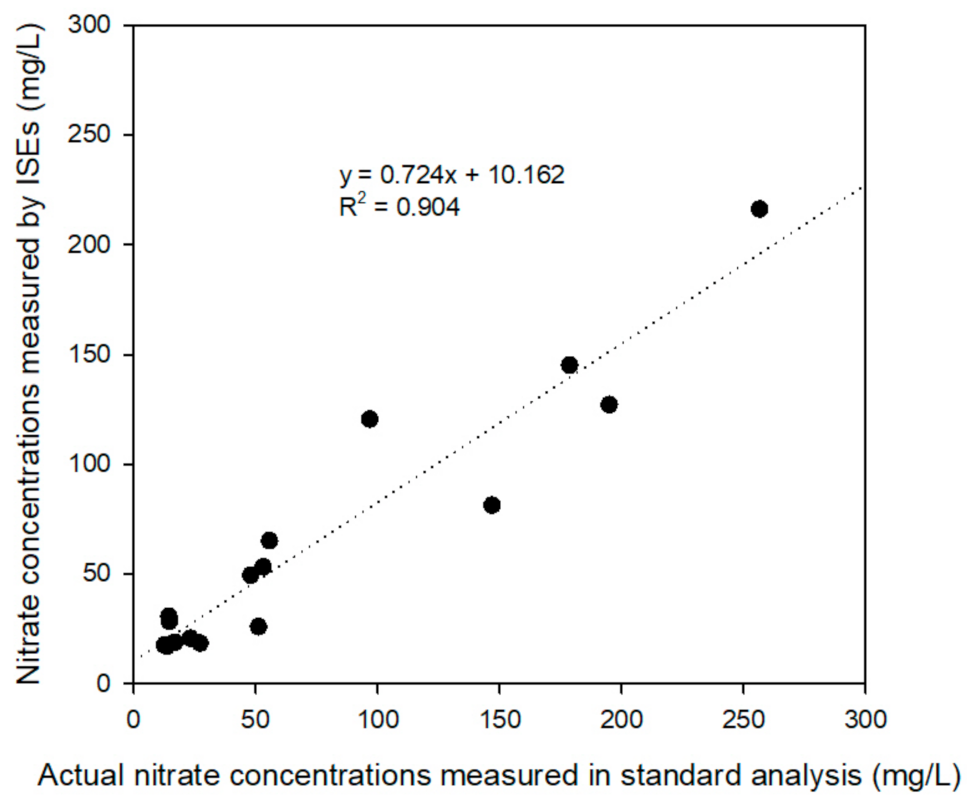
3.3. Field Verification Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, A.R. Nitrate removal in stream riparian zones. J. Environ. Qual. 1996, 25, 743–755. [Google Scholar] [CrossRef]
- Dymond, J.R.; Ausseil, A.-G.; Parfitt, R.L.; Herzig, A.; McDowell, R.W. Nitrate and phosphorus leaching in New Zealand: A national perspective. N. Z. J. Agric. Res. 2013, 56, 49–59. [Google Scholar] [CrossRef]
- Bourgeois, W.; Romain, A.-C.; Nicolas, J.; Stuetz, R.M. The use of sensor arrays for environmental monitoring: Interests and limitations. J. Environ. Monit. 2003, 5, 852. [Google Scholar] [CrossRef]
- Hierlemann, A.; Weimar, U.; Kraus, G.; Schweizer-Berberich, M.; Göpel, W. Polymer-based sensor arrays and multicomponent analysis for the detection of hazardous oragnic vapours in the environment. Sens. Actuators B Chem. 1995, 26, 126–134. [Google Scholar] [CrossRef]
- Kim, D.; Jung, D.; Cho, W.; Sim, K.; Kim, H. On-site Water Nitrate Monitoring System based on Automatic Sampling and Direct Measurement with Ion-Selective Electrodes. J. Biosyst. Eng. 2017, 42, 350–357. [Google Scholar]
- Jung, D.H.; Kim, H.-J.; Choi, G.L.; Ahn, T.-I.; Son, J.-E.; Sudduth, K.A. Automated lettuce nutrient solution management using an array of ion-selective electrodes. Trans. ASABE 2015, 58, 1309–1319. [Google Scholar]
- Cho, W.-J.; Kim, H.-J.; Jung, D.-H.; Kim, D.-W.; Ahn, T.I.; Son, J.-E. On-site ion monitoring system for precision hydroponic nutrient management. Comput. Electron. Agric. 2018, 146, 51–58. [Google Scholar] [CrossRef]
- Carey, C.M.; Riggan, W.B. Cyclic polyamine ionophore for use in a dibasic phosphate-selective electrode. Anal. Chem. 1994, 66, 3587–3591. [Google Scholar] [CrossRef]
- Winkler, S.; Rieger, L.; Saracevic, E.; Pressl, A.; Gruber, G. Application of ion-sensitive sensors in water quality monitoring. Water Sci. Technol. 2004, 50, 105–114. [Google Scholar] [CrossRef]
- Sharif-Khodaei, Z.; Ghajari, M.; Aliabadi, M.H. Impact damage detection in composite plates using a self-diagnostic electro-mechanical impedance-based structural health monitoring system. J. Multiscale Model. 2015, 6, 1550013. [Google Scholar] [CrossRef]
- Pinto, R.; Reis, J.; Sousa, V.; Silva, R.; Gonçalves, G.M. Self-Diagnosis and Automatic Configuration of Smart Components in Advanced Manufacturing Systems. In Proceedings of the INTELLI 2015, The Fourth International Conference on Intelligent Systems and Applications, Julians, Malta, 11–16 October 2015. [Google Scholar]
- Li, H.; Price, M.C.; Stott, J.; Marshall, I.W. The Development of a Wireless Sensor Network Sensing Node Utilising Adaptive Self-Diagnostics. In International Workshop on Self-Organizing Systems; Springer: Berlin/Heidelberg, Germany, 2007; pp. 30–43. [Google Scholar]
- Bertrand-Krajewski, J.-L.; Bardin, J.-P.; Mourad, M.; Beranger, Y. Accounting for sensor calibration, concentration heterogeneity, measurement and sampling uncertainties in monitoring urban drainage systems. Water Sci. Technol. 2003, 47, 95–102. [Google Scholar] [CrossRef]
- Ramanathan, N.; Balzano, L.; Burt, M.; Estrin, D.; Harmon, T.; Harvey, C.; Jay, J.; Kohler, E.; Rothenberg, S.; Srivastava, M. Rapid Deployment with Confidence: Calibration and Fault Detection in Environmental Sensor Networks; UCLA Technical Reports: Los Angeles, SC, USA, 2006. [Google Scholar]
- Cho, W.J.; Kim, D.-W.; Jung, D.H.; Cho, S.S.; Kim, H.-J. An Automated Water Nitrate Monitoring System based on Ion-Selective Electrodes. J. Biosyst. Eng. 2016, 41, 75–84. [Google Scholar] [CrossRef][Green Version]
- Khomfoi, S.; Tolbert, L.M. Fault Diagnosis and Reconfiguration for Multilevel Inverter Drive Using AI-Based Techniques. IEEE Trans. Ind. Electron. 2007, 54, 2954–2968. [Google Scholar] [CrossRef]
- Vas, P. Artificial-Intelligence-Based Electrical Machines and Drives: Application of Fuzzy, Neural, Fuzzy-Neural, and Genetic-Algorithm-Based Techniques; Oxford university press: Oxford, UK, 1999; Volume 45, ISBN 019859397X. [Google Scholar]
- Lin, W.-C.; Brondum, K.; Monroe, C.W.; Burns, M.A. Multifunctional Water Sensors for pH, ORP, and Conductivity Using Only Microfabricated Platinum Electrodes. Sensors 2017, 17, 1655. [Google Scholar] [CrossRef] [PubMed]
- Adu-Manu, K.S.; Tapparello, C.; Heinzelman, W.; Katsriku, F.A.; Abdulai, J.-D. Water quality monitoring using wireless sensor networks: Current trends and future research directions. ACM Trans. Sens. Netw. 2017, 13, 4. [Google Scholar] [CrossRef]
- Ayaz, M.; Ammad-uddin, M.; Baig, I. Wireless Sensor’s Civil Applications, Prototypes, and Future Integration Possibilities: A Review. IEEE Sens. J. 2017, 18, 4–30. [Google Scholar] [CrossRef]
- Taher, T.; Weymouth, G.D.; Varghese, T. Novel Platform for Ocean Survey and Autonomous Sampling Using Multi-Agent System. In Proceedings of the OCEANS-Bergen, 2013 MTS/IEEE, Bergen, Norway, 26 September 2013; pp. 1–5. [Google Scholar]
- Dolan, J.M.; Podnar, G.W.; Stancliff, S.; Low, K.H.; Elfes, A.; Higinbotham, J.; Hosler, J.; Moisan, T.; Moisan, J. Cooperative Aquatic Sensing Using the Telesupervised Adaptive Ocean Sensor Fleet. In Proceedings of the Remote Sensing of the Ocean, Sea Ice, and Large Water Regions 2009, International Society for Optics and Photonics, Berlin, Germany, 31 August–3 September 2009; Volume 7473, p. 747307. [Google Scholar]
- Sukhatme, G.S.; Dhariwal, A.; Zhang, B.; Oberg, C.; Stauffer, B.; Caron, D.A. Design and development of a wireless robotic networked aquatic microbial observing system. Environ. Eng. Sci. 2007, 24, 205–215. [Google Scholar] [CrossRef]
- Yu, L.; Nazir, B.; Wang, Y. Intelligent power monitoring of building equipment based on Internet of Things technology. Comput. Commun. 2020, 157, 76–84. [Google Scholar] [CrossRef]
- Liang, P.; Deng, C.; Wu, J.; Yang, Z. Intelligent fault diagnosis of rotating machinery via wavelet transform, generative adversarial nets and convolutional neural network. Measurement 2020, 159, 107768. [Google Scholar] [CrossRef]
- Thangavel, D.; Ma, X.; Valera, A.; Tan, H.-X.; Tan, C.K.-Y. Performance Evaluation of MQTT and CoAP via a Common Middleware. In Proceedings of the 2014 IEEE Ninth International Conference on Intelligent Sensors, Sensor Networks and Information Processing (ISSNIP), Singapore, 9 June 2014; pp. 1–6. [Google Scholar]
- Van Stralen, K.J.; Stel, V.S.; Reitsma, J.B.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Diagnostic methods I: Sensitivity, specificity, and other measures of accuracy. Kidney Int. 2009, 75, 1257–1263. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, H.J.; Cho, W.J.; Park, S.H.; Yang, S.H. Validation testing of an ion-specific sensing and control system for precision hydroponic macronutrient management. Comput. Electron. Agric. 2019, 156, 660–668. [Google Scholar] [CrossRef]
- Gieling, T.H.; van Straten, G.; Janssen, H.J.J.; Wouters, H. ISE and Chemfet sensors in greenhouse cultivation. Sens. Actuators B Chem. 2005, 105, 74–80. [Google Scholar] [CrossRef]
- Mimendia, A.; Gutiérrez, J.M.; Leija, L.; Hernández, P.R.; Favari, L.; Muñoz, R.; del Valle, M. A review of the use of the potentiometric electronic tongue in the monitoring of environmental systems. Environ. Model. Softw. 2010, 25, 1023–1030. [Google Scholar] [CrossRef]
- Goodchild, R.G. EU policies for the reduction of nitrogen in water: The example of the Nitrates Directive. In Nitrogen, the Confer-Ns; Elsevier: Amsterdam, The Netherlands, 1998; pp. 737–740. [Google Scholar]
- Massa, D.; Incrocci, L.; Maggini, R.; Carmassi, G.; Campiotti, C.A.; Pardossi, A. Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato. Agric. Water Manag. 2010, 97, 971–980. [Google Scholar] [CrossRef]






| Component | Reagent | Composition |
|---|---|---|
| Ionophore | TDDA | 4.0% (8 mg) |
| Plasticizer | NPOE | 67.75% (135.5 mg) |
| Matrix | PVC | 28.25% (56.5 mg) |
| Inner solution | 0.01 M NaNO3 + 0.01 M NaCl | |
| Actual Results | |||
|---|---|---|---|
| True | False | ||
| Model classification result | True | True Positive (TP) | False Positive (FP) |
| False | False Negative (FN) | True Negative (TN) | |
| SDI | |
|---|---|
| Precision () | 0.65 |
| Recall () | 0.87 |
| Accuracy () | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, D.-H.; Kim, H.-J.; Kim, J.Y.; Park, S.H.; Cho, W.J. Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes. Sensors 2021, 21, 2703. https://doi.org/10.3390/s21082703
Jung D-H, Kim H-J, Kim JY, Park SH, Cho WJ. Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes. Sensors. 2021; 21(8):2703. https://doi.org/10.3390/s21082703
Chicago/Turabian StyleJung, Dae-Hyun, Hak-Jin Kim, Joon Yong Kim, Soo Hyun Park, and Woo Jae Cho. 2021. "Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes" Sensors 21, no. 8: 2703. https://doi.org/10.3390/s21082703
APA StyleJung, D.-H., Kim, H.-J., Kim, J. Y., Park, S. H., & Cho, W. J. (2021). Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes. Sensors, 21(8), 2703. https://doi.org/10.3390/s21082703






