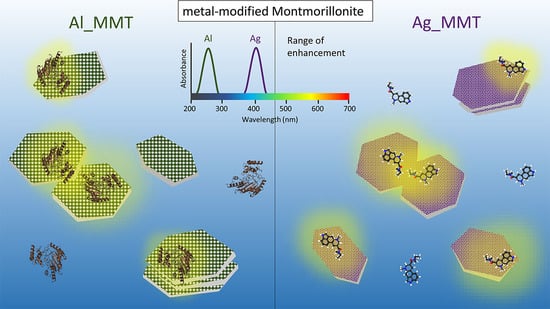
Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- Synthesis of metal-modified MMT:
- Transmission electron microscopy (TEM):
- Scanning electron microscopy (SEM):
- Energy-dispersive X-ray (EDX) analysis:
- Surface Enhanced Raman Spectroscopy (SERS):
- X-ray photoelectron spectroscopy (XPS):
- X-ray diffraction (XRD):
- UV-Vis measurements:
- Fluorescent measurements:
3. Results and Discussion
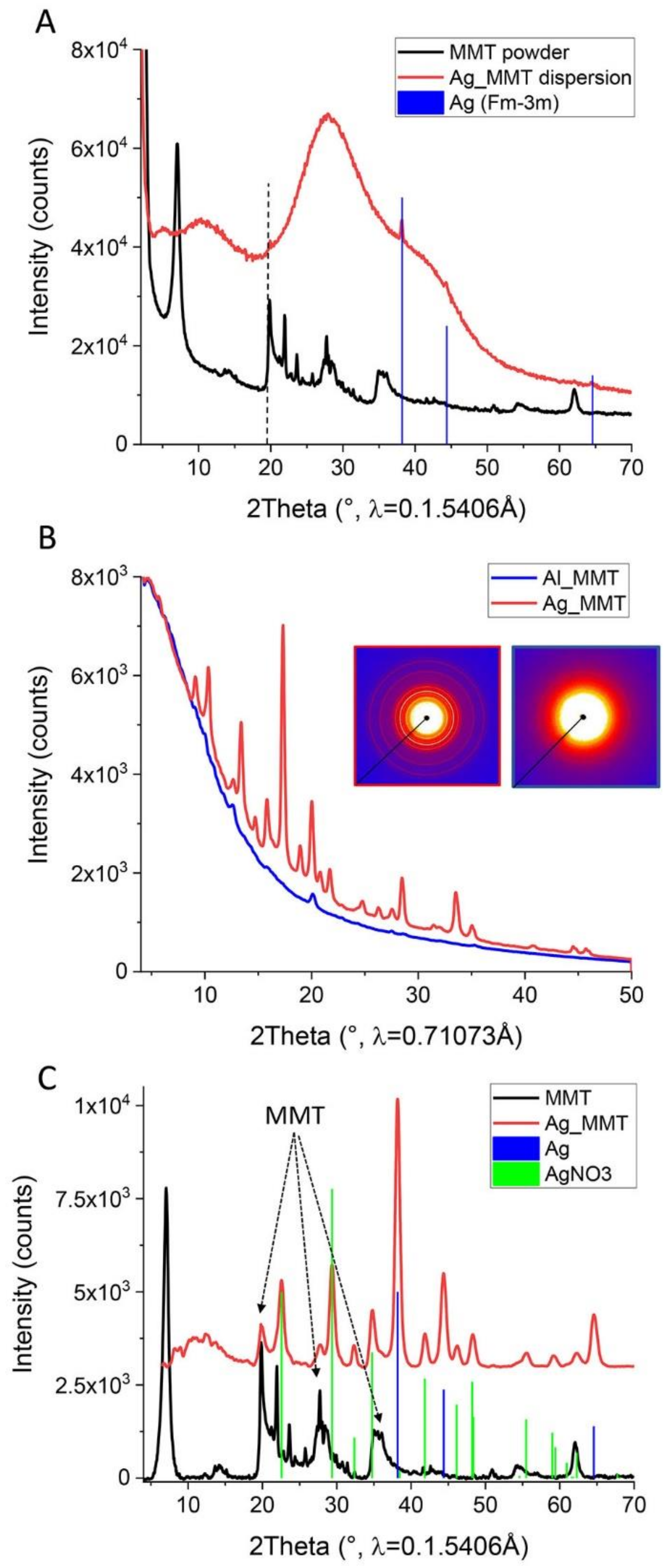
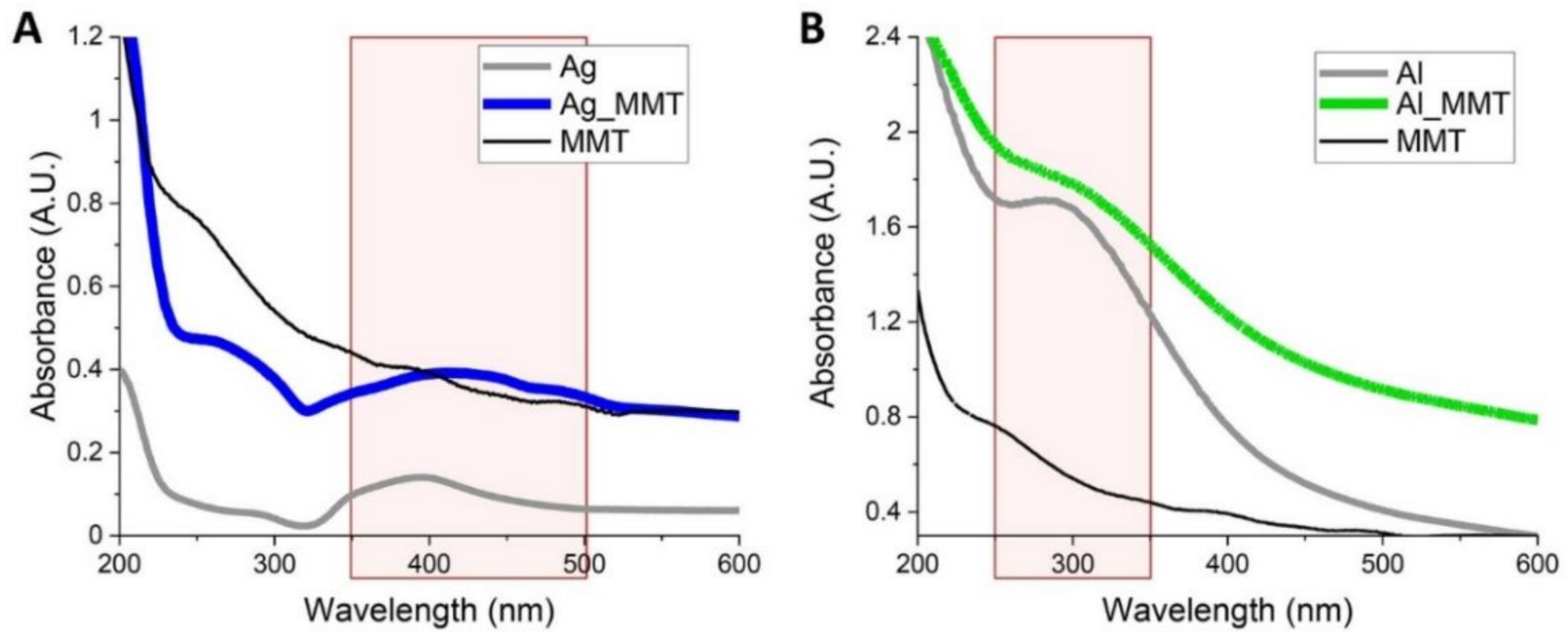
3.1. Synthesis and Characterization of Metal-Modified MMT
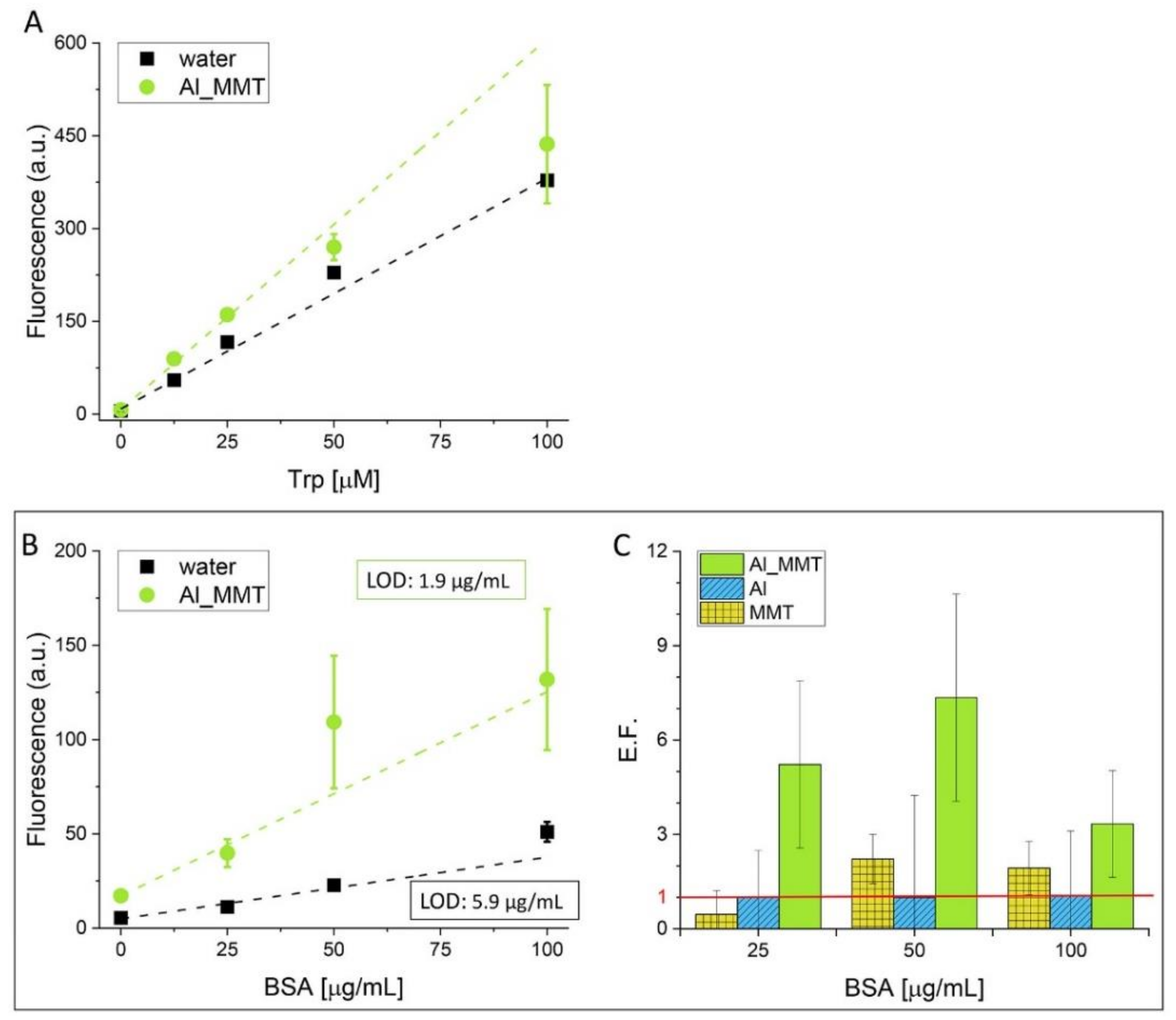
3.2. Fluorescence-Based Detection of Biomolecules with Al_MMT
3.3. Fluorescence-Based Detection of FITC with Ag_MMT
3.4. Label-Free Detection: SERS Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaharwar, A.K.; Cross, L.M.; Peak, C.W.; Gold, K.; Carrow, J.K.; Brokesh, A.; Singh, K.A. 2D Nanoclay for Biomedical Applications: Regenerative Medicine, Therapeutic Delivery, and Additive Manufacturing. Adv. Mater. 2019, 31, 1900332. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Otoni, C.G.; Corrêa, D.S.; Assis, O.B.G.; de Moura, M.R.; Mattoso, L.H.C. Nanostructured Antimicrobials in Food Packaging—Recent Advances. Biotechnol. J. 2019, 14, 1900068. [Google Scholar] [CrossRef]
- Zhang, J.R.; Xu, M.D.; Christidis, G.E.; Zhou, C.H. Clay minerals in drilling fluids: Functions and challenges. Clay Miner. 2020, 55, 1–11. [Google Scholar] [CrossRef]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K.; Nyamori, V.O. Transforming inorganic layered montmorillonite into inorganic–organic hybrid materials for various applications: A brief overview. Inorg. Chem. Front. 2016, 3, 1100–1111. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Parida, K.M. Montmorillonite supported metal nanoparticles: An update on syntheses and applications. RSC Adv. 2013, 3, 13583–13593. [Google Scholar] [CrossRef]
- De Almeida, J.C.; De Barros, A.; Odone Mazali, I.; Ferreira, M. Influence of gold nanostructures incorporated into sodium montmorillonite clay based on LbL films for detection of metal traces ions. Appl. Surf. Sci. 2020, 507, 144972. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Brazovskaya, E.Y.; Shamova, O.V. Biological activity and sorption ability of synthetic montmorillonite modified by silver/lysozyme nanoparticles. Appl. Clay Sci. 2018, 163, 56–62. [Google Scholar] [CrossRef]
- Giovannini, G.; Rossi, M.R.; Boesel, F.L. Changes in optical properties upon dye–clay interaction: Experimental evaluation and applications. Nanomaterials 2021, 11, 197. [Google Scholar] [CrossRef]
- Park, K.M.; Chung, D.J.; Choi, M.; Kang, T.; Jeong, J. Fluorescent fullerene nanoparticle-based lateral flow immunochromatographic assay for rapid quantitative detection of C-reactive protein. Nano Converg. 2019, 6, 35. [Google Scholar] [CrossRef]
- Robidillo, C.J.T.; Wandelt, S.; Dalangin, R.; Zhang, L.; Yu, H.; Meldrum, A.; Campbell, R.E.; Veinot, J.G.C. Ratiometric Detection of Nerve Agents by Coupling Complementary Properties of Silicon-Based Quantum Dots and Green Fluorescent Protein. ACS Appl. Mater. Interfaces 2019, 11, 33478–33488. [Google Scholar] [CrossRef] [PubMed]
- Faccio, G.; Bannwarth, M.B.; Schulenburg, C.; Steffen, V.; Jankowska, D.; Pohl, M.; Rossi, R.M.; Maniura-Weber, K.; Boesel, L.F.; Richter, M. Encapsulation of FRET-based glucose and maltose biosensors to develop functionalized silica nanoparticles. Analyst 2016, 141, 3982–3984. [Google Scholar] [CrossRef]
- Giovannini, G.; De Angelis, F. Novel electro-magnetophoretic separation method for the highly sensitive detection of analytes. Nanoscale Horiz. 2020, 5, 95–101. [Google Scholar] [CrossRef]
- Tam, F.; Goodrich, G.P.; Johnson, B.R.; Halas, N.J. Plasmonic enhancement of molecular fluorescence. Nano Lett. 2007, 7, 496–501. [Google Scholar] [CrossRef]
- Chekini, M.; Filter, R.; Bierwagen, J.; Cunningham, A.; Rockstuhl, C.; Bürgi, T. Fluorescence enhancement in large-scale self-assembled gold nanoparticle double arrays. J. Appl. Phys. 2015, 118. [Google Scholar] [CrossRef]
- Huang, J.-A.; Mousavi, M.Z.; Zhao, Y.; Hubarevich, A.; Omeis, F.; Giovannini, G.; Schütte, M.; Garoli, D.; De Angelis, F. SERS discrimination of single DNA bases in single oligonucleotides by electro-plasmonic trapping. Nat. Commun. 2019, 10, 5321. [Google Scholar] [CrossRef]
- Wang, J.; Koo, K.M.; Wang, Y.; Trau, M. Engineering State-of-the-Art Plasmonic Nanomaterials for SERS-Based Clinical Liquid Biopsy Applications. Adv. Sci. 2019, 6, 1900730. [Google Scholar] [CrossRef]
- Liu, J.; Jalali, M.; Mahshid, S.; Wachsmann-Hogiu, S. Are plasmonic optical biosensors ready for use in point-of-need applications? Analyst 2020, 145, 364–384. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1995. [Google Scholar]
- HighScorePlus Software; Version V.4.9; Malvern Panalytical: Malvern, UK, 2020.
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V.; Zhukhlistov, A.P.; Belousov, P.E.; Timofeeva, M.N. Experimental study of montmorillonite structure and transformation of its properties under treatment with inorganic acid solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Iannuccelli, V.; Maretti, E.; Sacchetti, F.; Romagnoli, M.; Bellini, A.; Truzzi, E.; Miselli, P.; Leo, E. Characterization of natural clays from italian deposits with focus on elemental composition and exchange estimated by EDX analysis: Potential pharmaceutical and cosmetic uses. Clays Clay Miner. 2016, 64, 719–731. [Google Scholar] [CrossRef]
- McGuire, G.E.; Schweitzer, G.K.; Carlson, T.A. Core electron binding energies in some Group IIIA, VB, and VIB compounds. Inorg. Chem. 1973, 12, 2450. [Google Scholar] [CrossRef]
- Alexander, M.R.; Thompson, G.E.; Beamson, G. Characterization of the oxide/hydroxide surface of aluminium using X-ray photoelectron spectroscopy: A procedure for curve fitting the O 1s core level. Surf. Interface Anal. 2000, 29, 468–477. [Google Scholar] [CrossRef]
- Barr, T.L. An XPS study of Si as it occurs in adsorbents, catalysts, and thin films. Appl. Surf. Sci. 1983, 15, 1–35. [Google Scholar] [CrossRef]
- Rupper, P.; Amberg, M.; Hegemann, D.; Heuberger, M. Optimization of mica surface hydroxylation in water vapor plasma monitored by optical emission spectroscopy. Appl. Surf. Sci. 2020, 509, 145362. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Jani, P.; Wilson, L.D. Redox-Responsive Polymer Template as an Advanced Multifunctional Catalyst Support for Silver Nanoparticles. Langmuir 2018, 34, 10560–10568. [Google Scholar] [CrossRef]
- Hoflund, G.B.; Hazos, Z.F.; Salaita, G.N. Surface characterization study of Ag, AgO, and Ag2O using X-ray photoelectron spectroscopy and electron energy-loss spectroscopy. Phys. Rev. B 2000, 62, 11126–11133. [Google Scholar] [CrossRef]
- Kaspar, T.C.; Droubay, T.; Chambers, S.A.; Bagus, P.S. Spectroscopic Evidence for Ag(III) in Highly Oxidized Silver Films by X-ray Photoelectron Spectroscopy. J. Phys. Chem. C 2010, 114, 21562–21571. [Google Scholar] [CrossRef]
- Kaushik, V.K. XPS core level spectra and Auger parameters for some silver compounds. J. Electron. Spectros. Relat. Phenomena 1991, 56, 273–277. [Google Scholar] [CrossRef]
- Battocchio, C.; Fratoddi, I.; Fontana, L.; Bodo, E.; Porcaro, F.; Meneghini, C.; Pis, I.; Nappini, S.; Mobilio, S.; Russo, M.V.; et al. Silver nanoparticles linked by a Pt-containing organometallic dithiol bridge: Study of local structure and interface by XAFS and SR-XPS. Phys. Chem. Chem. Phys. 2014, 16, 11719–11728. [Google Scholar] [CrossRef]
- Jing, X.; Wang, H.; Chen, H.; Huang, J.; Li, Q.; Sun, D. Biosynthesized Ag/α-Al2O3 catalyst for ethylene epoxidation: The influence of silver precursors. RSC Adv. 2014, 4, 27597–27603. [Google Scholar] [CrossRef]
- Boesel, L.F. Effect of plasticizers on the barrier and mechanical properties of biomimetic composites of chitosan and clay. Carbohydr. Polym. 2015, 115, 356–363. [Google Scholar] [CrossRef]
- Long, Z.; Liang, Y.; Feng, L.; Zhang, H.; Liu, M.; Xu, T. Low-cost and high sensitivity glucose sandwich detection using a plasmonic nanodisk metasurface. Nanoscale 2020, 12, 10809–10815. [Google Scholar] [CrossRef]
- Giovannini, G.; Ardini, M.; Maccaferri, N.; Zambrana-Puyalto, X.; Panella, G.; Angelucci, F.; Ippoliti, R.; Garoli, D.; De Angelis, F. Bio-Assisted Tailored Synthesis of Plasmonic Silver Nanorings and Site-Selective Deposition on Graphene Arrays. Adv. Opt. Mater. 2020, 8, 1901583. [Google Scholar] [CrossRef]
- Chen, K.; Dao, T.D.; Ishii, S.; Aono, M.; Nagao, T. Infrared Aluminum Metamaterial Perfect Absorbers for Plasmon-Enhanced Infrared Spectroscopy. Adv. Funct. Mater. 2015, 25, 6637–6643. [Google Scholar] [CrossRef]
- Ponzellini, P.; Giovannini, G.; Cattarin, S.; Proietti Zaccaria, R.; Marras, S.; Prato, M.; Schirato, A.; D’Amico, F.; Calandrini, E.; De Angelis, F.; et al. Metallic Nanoporous Aluminum–Magnesium Alloy for UV-Enhanced Spectroscopy. J. Phys. Chem. C 2019, 123, 20287–20296. [Google Scholar] [CrossRef]
- Bortolotti, A.; Wong, Y.H.; Korsholm, S.S.; Bahring, N.H.B.; Bobone, S.; Tayyab, S.; van de Weert, M.; Stella, L. On the purported “backbone fluorescence” in protein three-dimensional fluorescence spectra. RSC Adv. 2016, 6, 112870–112876. [Google Scholar] [CrossRef]
- EMEA—European Medicines Agency. European Medicines Agency: An unacceptable choice. Prescrire Int. 2011, 20, 278. [Google Scholar]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Trigueiro, P.; Pedetti, S.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.G.; Gougeon, R.; Fonseca, M.G.; Georgelin, T.; Jaber, M. Going through the wine fining: Intimate dialogue between organics and clays. Colloids Surf. B Biointerfaces 2018, 166, 79–88. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Calandra, P.; Lombardo, D.; Magazù, S. Thermal investigation of montmorillonite/BSA by fourier transform infrared spectroscopy measurements. AIMS Biophys. 2020, 7, 248–266. [Google Scholar] [CrossRef]
- Hsieh, S.R.; Reddy, P.M.; Chang, C.J.; Kumar, A.; Wu, W.C.; Lin, H.Y. Exploring the behavior of bovine serum albumin in response to changes in the chemical composition of responsive polymers: Experimental and simulation studies. Polymers 2016, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Do, V.Q.; Seo, Y.-S.; Duong, M.T.H.; Ahn, H.-C.; Huh, H.J.; Lee, M.-Y. Application of Fisetin to the Quantitation of Serum Albumin. J. Clin. Med. 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Yang, L.; Qu, Y.; Hua, J. A new diketopyrrolopyrrole-based near-infrared (NIR) fluorescent biosensor for BSA detection and AIE-assisted bioimaging. Tetrahedron Lett. 2014, 55, 6998–7001. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Chowdhury, H.M.; Lakowicz, R.J. Metal-Enhanced Single-Molecule Fluorescence on Silver Particle Monomer and Dimer: Coupling Effect between Metal Particles. Nano Lett. 2007, 7, 2101–2107. [Google Scholar] [CrossRef]
- Cheng, Z.; Javed, N.M.; O’Carroll, D. Optical and Electrical Properties of Organic Semiconductor Thin Films on Aperiodic Plasmonic Metasurfaces. ACS Appl. Mater. Interfaces 2020, 12, 35579–35587. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Zhang, G.; Peng, Z.; Yao, L.; Wang, Q.; Cao, Z.; Mu, Q.; Xuan, L. Distance-dependent metal enhanced fluorescence by flowerlike silver nanostructures fabricated in liquid crystalline phase. Opt. Mater. 2017, 72, 289–294. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Wei, H.; Xu, H. Hot spots in different metal nanostructures for plasmon-enhanced Raman spectroscopy. Nanoscale 2013, 5, 10794–10805. [Google Scholar] [CrossRef]
- Huang, J.-A.; Mousavi, M.Z.; Giovannini, G.; Zhao, Y.; Hubarevich, A.; Soler, M.A.; Rocchia, W.; Garoli, D.; De Angelis, F. Multiplexed Discrimination of Single Amino Acid Residues in Polypeptides in a Single SERS Hot Spot. Angew. Chem. Int. Ed. 2020, 59, 11423–11431. [Google Scholar] [CrossRef] [PubMed]
- Hubarevich, A.; Huang, J.-A.; Giovannini, G.; Schirato, A.; Zhao, Y.; Maccaferri, N.; De Angelis, F.; Alabastri, A.; Garoli, D. λ-DNA through Porous Materials—Surface-Enhanced Raman Scattering in a Simple Plasmonic Nanopore. J. Phys. Chem. C 2020, 124, 22663–22670. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Yin, H.; Zhu, Q.; Yao, G.; Gu, N. Hierarchical Fabrication of Plasmonic Superlattice Membrane by Aspect-Ratio Controllable Nanobricks for Label-Free Protein Detection. Front. Chem. 2020, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Kandakkathara, A.; Utkin, I.; Fedosejevs, R. Surface-Enhanced Raman Scattering (SERS) Detection of Low Concentrations of Tryptophan Amino Acid in Silver Colloid. Appl. Spectrosc. 2011, 65, 507–513. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Chen, Y.-T. Raman scattering of L-tryptophan enhanced by surface plasmon of silver nanoparticles: Vibrational assignment and structural determination. J. Raman Spectrosc. 2009, 40, 150–156. [Google Scholar] [CrossRef]
- Maiti, N.; Thomas, S.; Jacob, J.A.; Chadha, R.; Mukherjee, T.; Kapoor, S. DFT and surface-enhanced Raman scattering study of tryptophan–silver complex. J. Colloid Interface Sci. 2012, 380, 141–149. [Google Scholar] [CrossRef] [PubMed]






| Sample | Si | O | Al | Mg | Na | Ag | C |
|---|---|---|---|---|---|---|---|
| MMT | 15.4 | 60.3 | 7.2 | 1.5 | 2.0 | - | 13.6 |
| Ag_MMT | 16.2 | 63.0 | 7.0 | 1.8 | - | 0.1 | 11.9 |
| Al_MMT | 15.6 | 62.9 | 7.7 | 1.9 | - | - | 11.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, G.; Garoli, D.; Rupper, P.; Neels, A.; Rossi, R.M.; Boesel, L.F. Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection. Sensors 2021, 21, 2655. https://doi.org/10.3390/s21082655
Giovannini G, Garoli D, Rupper P, Neels A, Rossi RM, Boesel LF. Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection. Sensors. 2021; 21(8):2655. https://doi.org/10.3390/s21082655
Chicago/Turabian StyleGiovannini, Giorgia, Denis Garoli, Patrick Rupper, Antonia Neels, René M. Rossi, and Luciano F. Boesel. 2021. "Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection" Sensors 21, no. 8: 2655. https://doi.org/10.3390/s21082655
APA StyleGiovannini, G., Garoli, D., Rupper, P., Neels, A., Rossi, R. M., & Boesel, L. F. (2021). Metal-Modified Montmorillonite as Plasmonic Microstructure for Direct Protein Detection. Sensors, 21(8), 2655. https://doi.org/10.3390/s21082655