The Impact of Short-Term Exposure to Air Pollution on the Exhaled Breath of Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Exposures
2.3.1. Exposure Parameters
2.3.2. PNC Sources
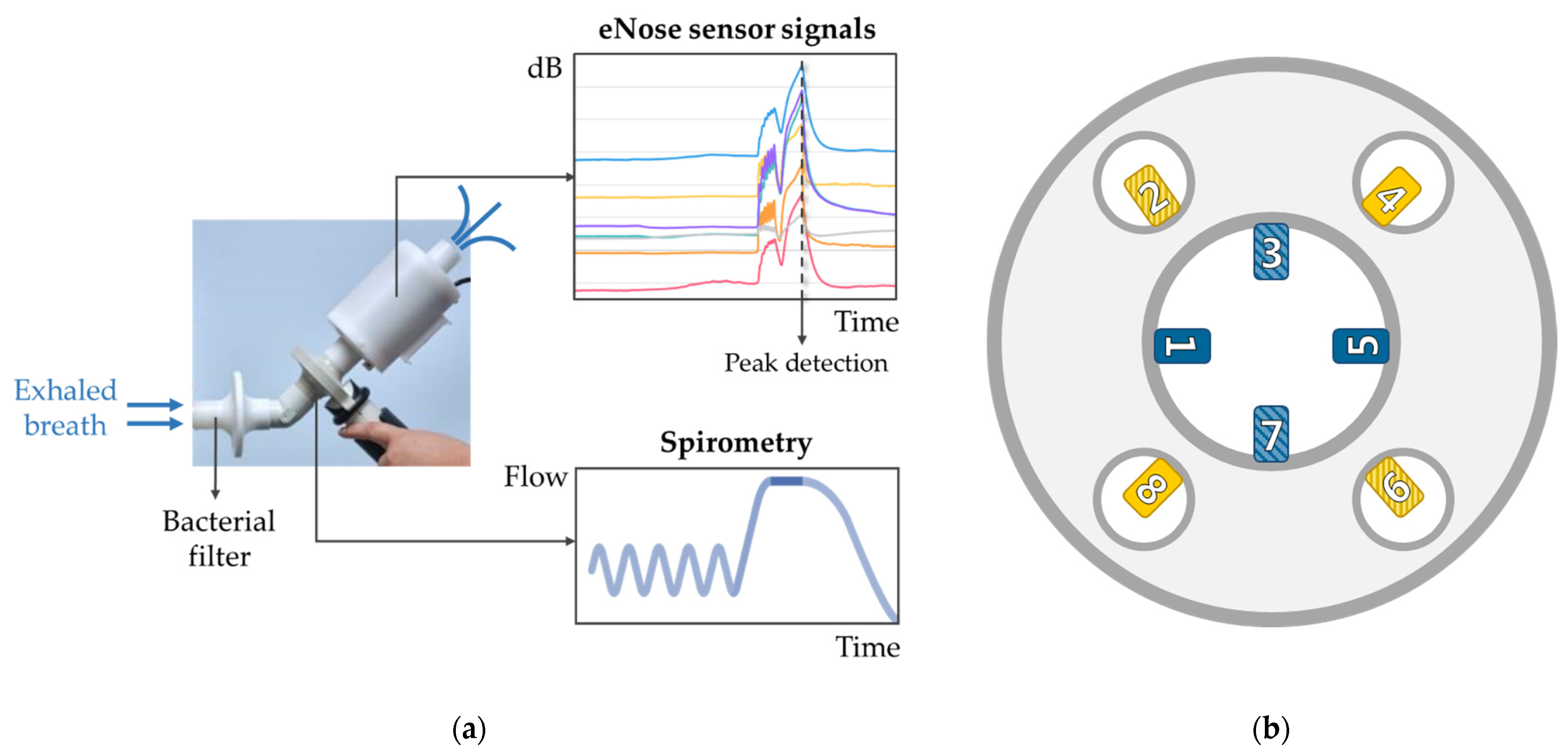
2.4. Exhaled Breath Analysis
2.4.1. eNose Measurements
2.4.2. Data Processing
2.5. Statistical Analysis
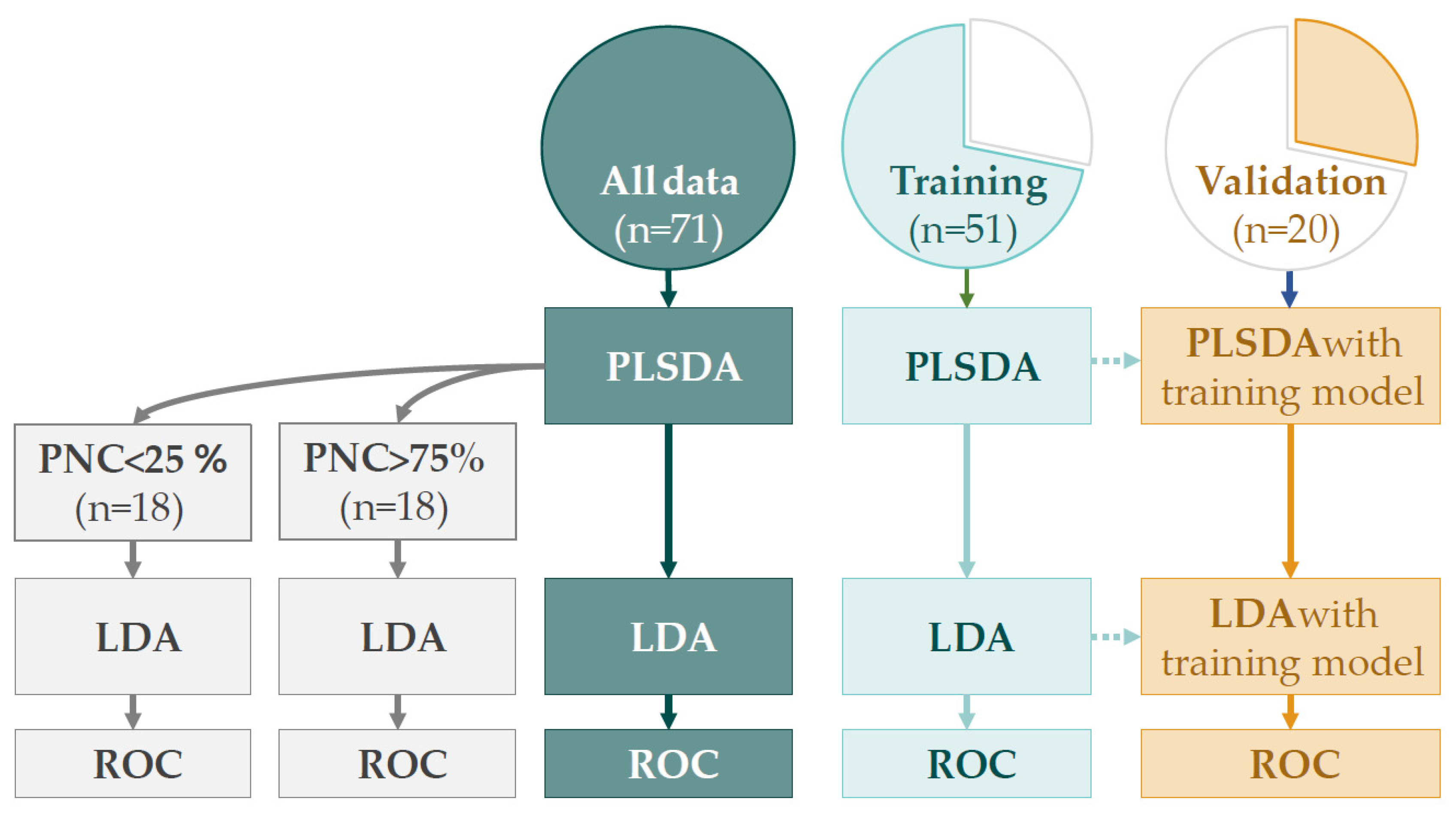
2.5.1. Discriminant Analysis
2.5.2. Linear Mixed Effect Models
3. Results
3.1. Participants
3.2. Exposures
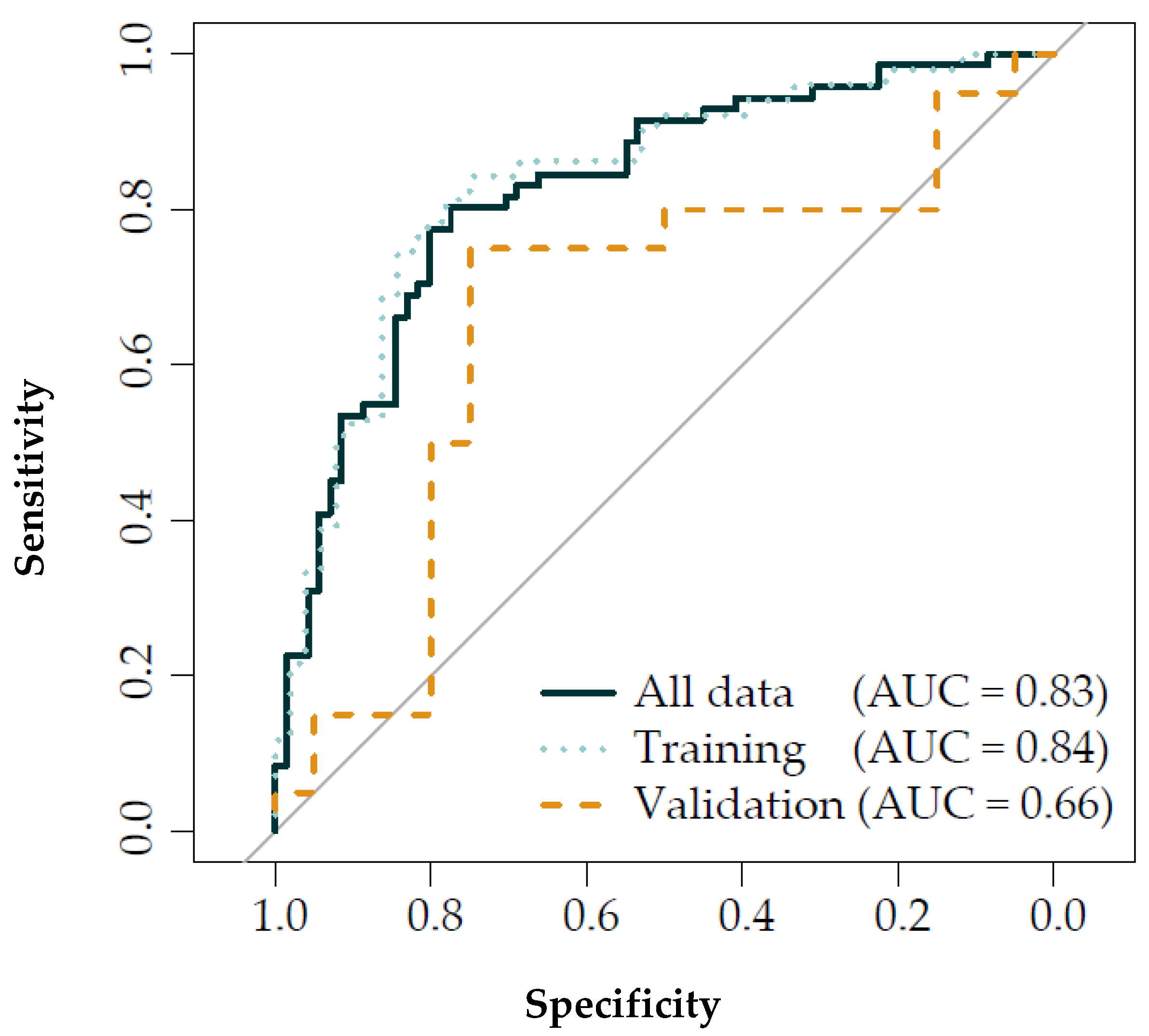
3.3. Discriminant Analysis
3.4. Linear Mixed Effect Models
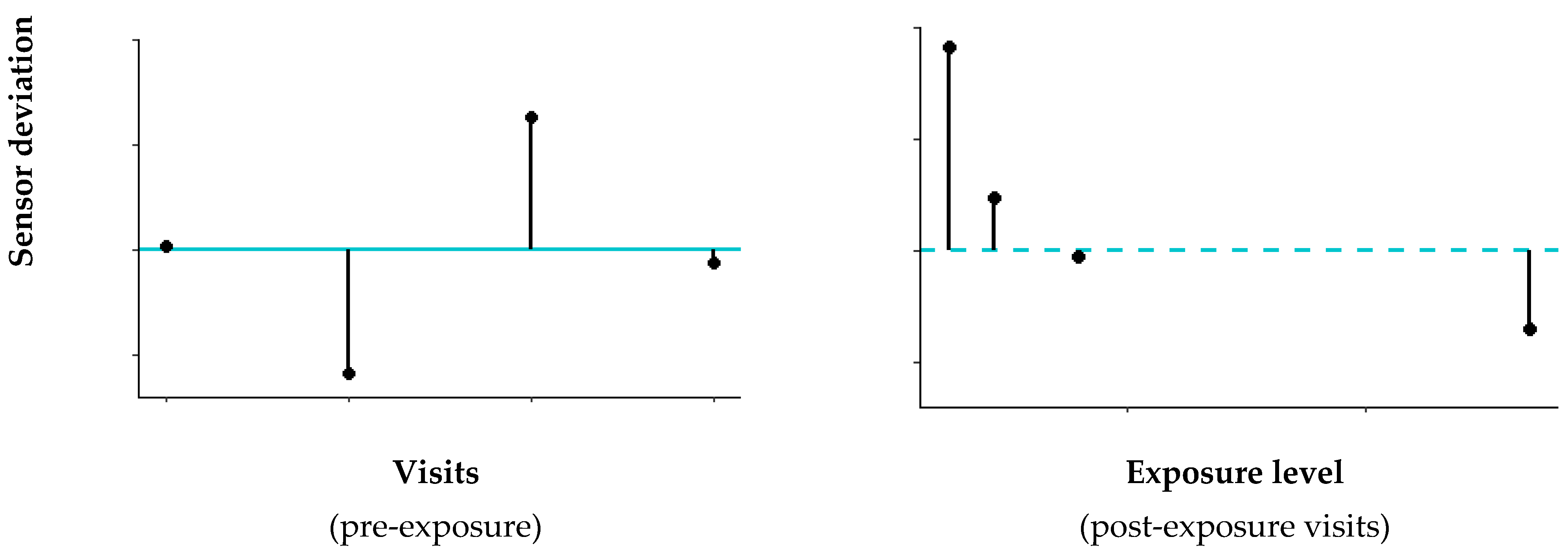
3.4.1. Pre-Exposure Variability eNose Deviations
3.4.2. Associations eNose Deviations and Pollutants
3.4.3. Associations eNose Deviations and PNC Sources
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lammers, A.; van Bragt, J.J.M.H.; Brinkman, P.; Neerincx, A.H.; Bos, L.D.; Vijverberg, S.J.H.; Maitland-van der Zee, A.H. Breathomics in Chronic Airway Diseases. Syst. Med. 2021, 1, 244–255. [Google Scholar] [CrossRef]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, P.; Van Der Zee, A.H.M.; Wagener, A.H. Breathomics and treatable traits for chronic airway diseases. Curr. Opin. Pulm. Med. 2019, 25, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Habre, R.; Zhou, H.; Eckel, S.P.; Enebish, T.; Fruin, S.; Bastain, T.; Rappaport, E.; Gilliland, F. Short-term effects of airport-associated ultrafine particle exposure on lung function and inflammation in adults with asthma. Environ. Int. 2018, 118, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, K.M.; Brostrøm, A.; Koivisto, A.J.; Koponen, I.; Berthing, T.; Bertram, N.; Kling, K.I.; Dal Maso, M.; Kangasniemi, O.; Poikkimäki, M.; et al. Airport emission particles: Exposure characterization and toxicity following intratracheal instillation in mice. Part. Fibre Toxicol. 2019, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Bello, D.; Gaines, P.; Martin, J.; Pal, A.K.; Gore, R.; Woskie, S. Nanoparticles from photocopiers induce oxidative stress and upper respiratory tract inflammation in healthy volunteers. Nanotoxicology 2013, 7, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Du, X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur. Respir. J. 2009, 34, 559–567. [Google Scholar] [CrossRef]
- He, R.W.; Shirmohammadi, F.; Gerlofs-Nijland, M.E.; Sioutas, C.; Cassee, F.R. Pro-inflammatory responses to PM 0.25 from airport and urban traffic emissions. Sci. Total Environ. 2018, 640–641, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Beswick, P.H.; Gilmour, P.S. Free radical activity associated with the surface of particles: A unifying factor in determining biological activity? Toxicol. Lett. 1996, 88, 293–298. [Google Scholar] [CrossRef]
- Albrecht, F.W.; Maurer, F.; Müller-Wirtz, L.M.; Schwaiblmair, M.H.; Hüppe, T.; Wolf, B.; Sessler, D.I.; Volk, T.; Kreuer, S.; Fink, T. Exhaled volatile organic compounds during inflammation induced by TNF-α in ventilated rats. Metabolites 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, P.; Wagener, A.H.; Hekking, P.P.; Bansal, A.T.; Maitland-van der Zee, A.H.; Wang, Y.; Weda, H.; Knobel, H.H.; Vink, T.J.; Rattray, N.J.; et al. Identification and prospective stability of electronic nose (eNose)–derived inflammatory phenotypes in patients with severe asthma. J. Allergy Clin. Immunol. 2019, 143, 1811–1820.e7. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; De Nijs, S.B.; Peters, S.; Dekker, T.; Knobel, H.H.; Vink, T.J.; Willard, N.P.; Zwinderman, A.H.; Krouwelse, F.H.; Janssen, H.G.; et al. Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur. Respir. J. 2011, 38, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.; Janssen, N.A.H.; Boere, A.J.F.; Berger, M.; Longo, C.; Vijverberg, S.J.H.; Neerincx, A.H.; Maitland-van der Zee, A.H.; Cassee, F.R. Effects of short-term exposures to ultrafine particles near an airport in healthy subjects. Environ. Int. 2020, 141, 105779. [Google Scholar] [CrossRef] [PubMed]
- Pirhadi, M.; Mousavi, A.; Sowlat, M.H.; Janssen, N.A.H.; Cassee, F.R.; Sioutas, C. Relative contributions of a major international airport activities and other urban sources to the particle number concentrations (PNCs) at a nearby monitoring site. Environ. Pollut. 2020, 260, 114027. [Google Scholar] [CrossRef]
- De Vries, R.; Dagelet, Y.W.F.; Spoor, P.; Snoey, E.; Jak, P.M.C.; Brinkman, P.; Dijkers, E.; Bootsma, S.K.; Elskamp, F.; de Jongh, F.H.C.; et al. Clinical and inflammatory phenotyping by breathomics in chronic airway diseases irrespective of the diagnostic label. Eur. Respir. J. 2018, 51, 1701817. [Google Scholar] [CrossRef]
- Ohlwein, S.; Kappeler, R.; Kutlar Joss, M.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef]
- Filipiak, W.; Ruzsanyi, V.; Mochalski, P.; Filipiak, A.; Bajtarevic, A.; Ager, C.; Denz, H.; Hilbe, W.; Jamnig, H.; Hackl, M.; et al. Dependence of exhaled breath composition on exogenous factors, smoking habits and exposure to air pollutants. J. Breath Res. 2012, 6, 036008. [Google Scholar] [CrossRef]
- de Jesus, A.L.; Rahman, M.M.; Mazaheri, M.; Thompson, H.; Knibbs, L.D.; Jeong, C.; Evans, G.; Nei, W.; Ding, A.; Qiao, L.; et al. Ultrafine particles and PM2.5 in the air of cities around the world: Are they representative of each other? Environ. Int. 2019, 129, 118–135. [Google Scholar] [CrossRef]
- Hudda, N.; Simon, M.C.; Zamore, W.; Durant, J.L. Aviation-Related Impacts on Ultrafine Particle Number Concentrations Outside and Inside Residences near an Airport. Environ. Sci. Technol. 2018, 52, 1765–1772. [Google Scholar] [CrossRef]
- Van Nunen, E.; Vermeulen, R.; Tsai, M.Y.; Probst-Hensch, N.; Ineichen, A.; Davey, M.; Imboden, M.; Ducret-Stich, R.; Naccarati, A.; Raffaele, D.; et al. Land Use Regression Models for Ultrafine Particles in Six European Areas. Environ. Sci. Technol. 2017, 51, 3336–3345. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, C.D.; Herrera-Mejía, L.; Roldán-Henao, N.; Isaza, A. Effects of fireworks on particulate matter concentration in a narrow valley: The case of the Medellín metropolitan area. Environ. Monit. Assess. 2020, 192, 6. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C. A review of the impact of fireworks on particulate matter in ambient air. J. Air Waste Manag. Assoc. 2016, 66, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.; Smargiassi, A.; Kosatsky, T.; Fournier, M.; Dabek-Zlotorzynska, E.; Celo, V.; Mathieu, D.; Servranckx, R.; D’amours, R.; Malo, A.; et al. Characterisation of particulate exposure during fireworks displays. Atmos. Environ. 2010, 44, 4325–4329. [Google Scholar] [CrossRef]
- Streiner, D.L.; Norman, G.R. Correction for multiple testing: Is there a resolution? Chest 2011, 140, 16–18. [Google Scholar] [CrossRef] [PubMed]
 &
&  ) and post-exposure (○ & Δ) measurements were larger for high PNC exposures (left) compared to low PNC exposures (right). High levels were defined as PNC levels > 75% percentile (i.e., >71,900 #/cm3) and low < 25% percentile (i.e., <23,800 #/cm3). PLSDA = partial least square discriminant analysis; PNC = particle number concentration
) and post-exposure (○ & Δ) measurements were larger for high PNC exposures (left) compared to low PNC exposures (right). High levels were defined as PNC levels > 75% percentile (i.e., >71,900 #/cm3) and low < 25% percentile (i.e., <23,800 #/cm3). PLSDA = partial least square discriminant analysis; PNC = particle number concentration
 &
&  ) and post-exposure (○ & Δ) measurements were larger for high PNC exposures (left) compared to low PNC exposures (right). High levels were defined as PNC levels > 75% percentile (i.e., >71,900 #/cm3) and low < 25% percentile (i.e., <23,800 #/cm3). PLSDA = partial least square discriminant analysis; PNC = particle number concentration
) and post-exposure (○ & Δ) measurements were larger for high PNC exposures (left) compared to low PNC exposures (right). High levels were defined as PNC levels > 75% percentile (i.e., >71,900 #/cm3) and low < 25% percentile (i.e., <23,800 #/cm3). PLSDA = partial least square discriminant analysis; PNC = particle number concentration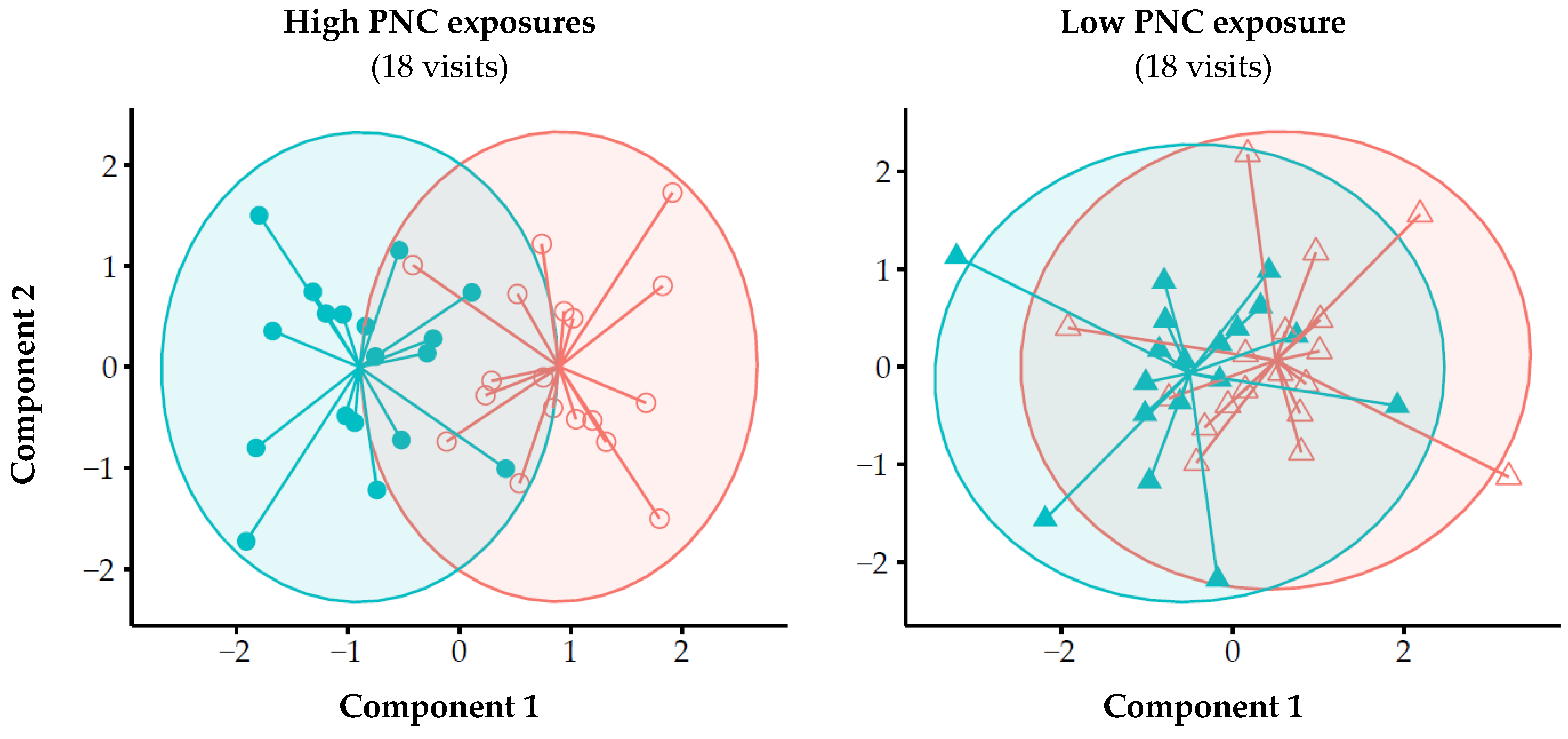
| Exposure Days (n = 26) | |||
|---|---|---|---|
| Mean | 5–95th Percentile | Range | |
| Pollutant | |||
| PNC (#/cm3) | 53,100 | 19,300–138,200 | 12,600–173,200 |
| PM (µg/m3) | 24.6 | 14.9–41.3 | 13.6–47.5 |
| BC (µg/m3) | 0.59 | 0.21–1.26 | 0.13–1.55 |
| NO2 (µg/m3) | 27 | 13–44 | 12–48 |
| CO (µg/m3) | 634 | 516–782 | 494–830 |
| PNC sources | |||
| Total aviation (#/cm3) | 26,100 | 3500–84,400 | 3200–101,800 |
| Take-off (#/cm3) | 16,600 | 700–58,800 | 500–62,000 |
| Landing (#/cm3) | 9500 | 1500–26,500 | 400–42,300 |
| Total traffic (#/cm3) | 9100 | 3600–16,700 | 1700–33,100 |
| Airport traffic (#/cm3) | 2400 | 500–4900 | 100–6800 |
| Road traffic (#/cm3) | 6700 | 2000–14,600 | 600–31,100 |
| Weather conditions | |||
| Temperature (°C) | 24 | 20–27 | 19–29 |
| Relative humidity (%) | 54 | 43–66 | 40–66 |
| Sensor | Deviation Percentage (%) Median (IQR) |
|---|---|
| 1 | 6.8 (3.3–8.8) |
| 3 | 3.9 (2.7–4.9) |
| 4 | 4.3 (2.5–6.9) |
| 5 | 6.4 (3.6–10.8) |
| 6 | 4.7 (2.6–6.5) |
| 7 | 13.2 (8.4–17.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lammers, A.; Neerincx, A.H.; Vijverberg, S.J.H.; Longo, C.; Janssen, N.A.H.; Boere, A.J.F.; Brinkman, P.; Cassee, F.R.; van der Zee, A.H.M. The Impact of Short-Term Exposure to Air Pollution on the Exhaled Breath of Healthy Adults. Sensors 2021, 21, 2518. https://doi.org/10.3390/s21072518
Lammers A, Neerincx AH, Vijverberg SJH, Longo C, Janssen NAH, Boere AJF, Brinkman P, Cassee FR, van der Zee AHM. The Impact of Short-Term Exposure to Air Pollution on the Exhaled Breath of Healthy Adults. Sensors. 2021; 21(7):2518. https://doi.org/10.3390/s21072518
Chicago/Turabian StyleLammers, Ariana, Anne H. Neerincx, Susanne J. H. Vijverberg, Cristina Longo, Nicole A. H. Janssen, A. John F. Boere, Paul Brinkman, Flemming R. Cassee, and Anke H. Maitland van der Zee. 2021. "The Impact of Short-Term Exposure to Air Pollution on the Exhaled Breath of Healthy Adults" Sensors 21, no. 7: 2518. https://doi.org/10.3390/s21072518
APA StyleLammers, A., Neerincx, A. H., Vijverberg, S. J. H., Longo, C., Janssen, N. A. H., Boere, A. J. F., Brinkman, P., Cassee, F. R., & van der Zee, A. H. M. (2021). The Impact of Short-Term Exposure to Air Pollution on the Exhaled Breath of Healthy Adults. Sensors, 21(7), 2518. https://doi.org/10.3390/s21072518








