Current Trends and Challenges for Rapid SMART Diagnostics at Point-of-Site Testing for Marine Toxins
Abstract
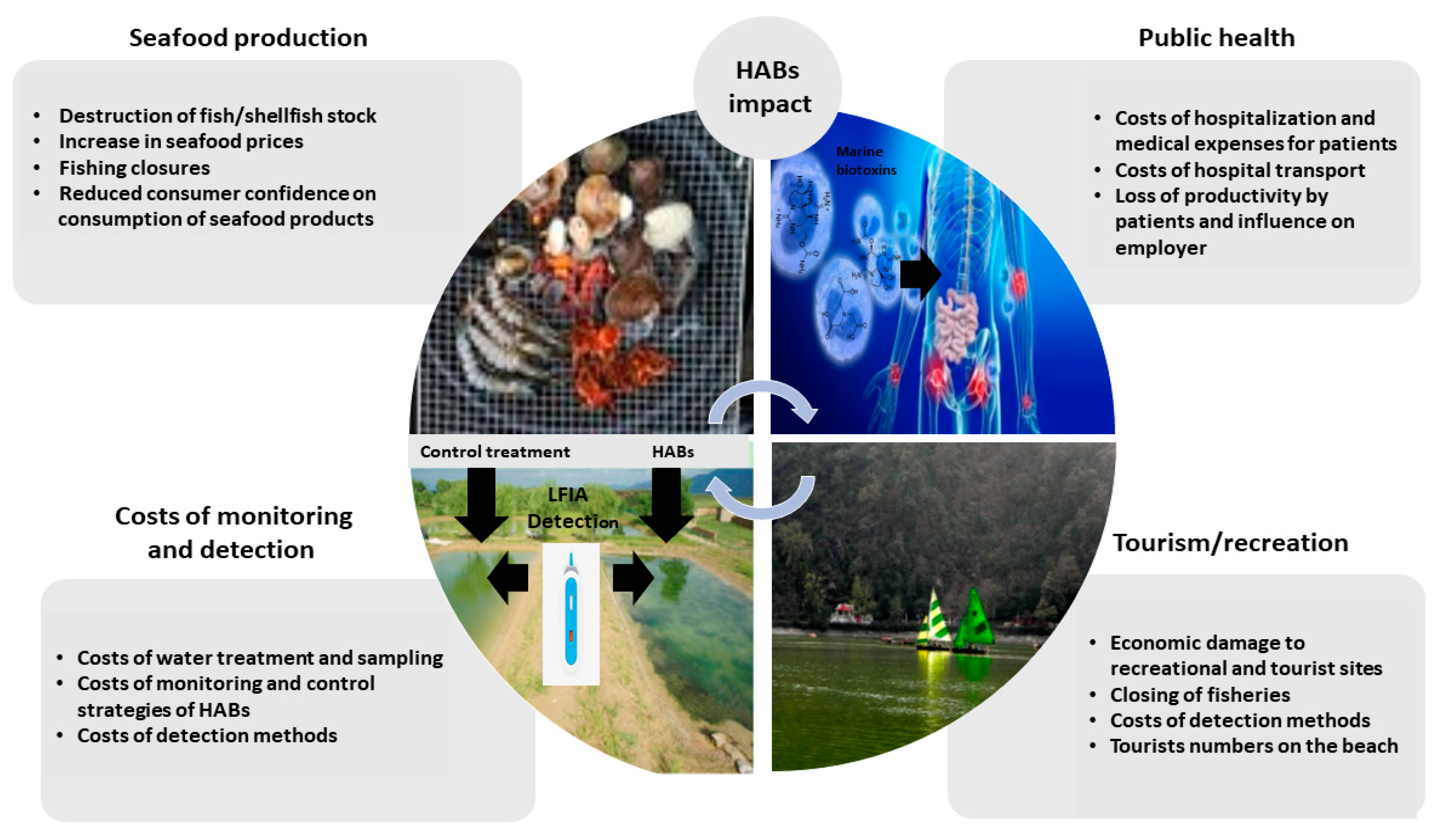
1. Introduction
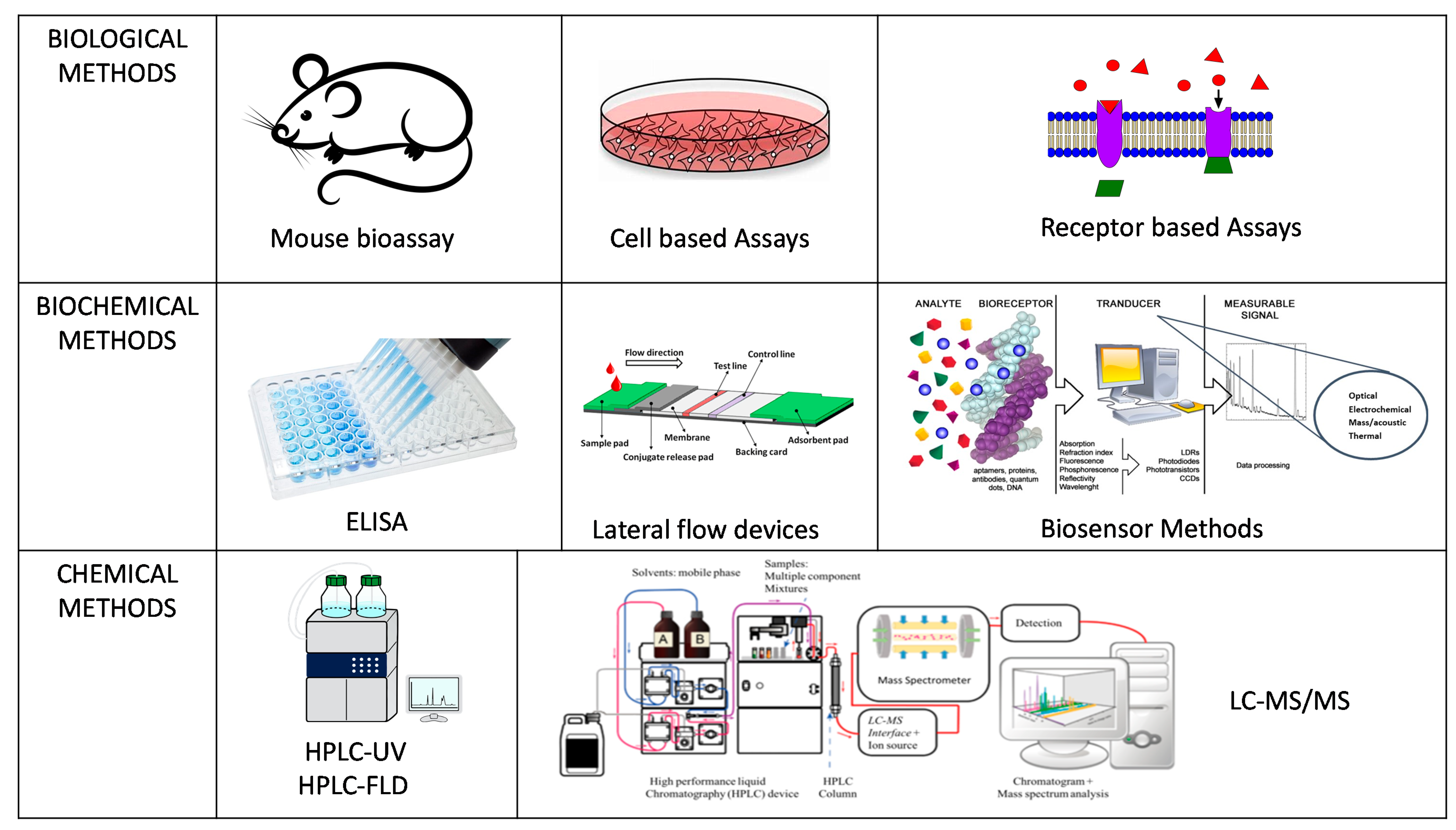
2. Detection Methods for Marine Toxins
3. Methods for Official Control Testing
3.1. Mouse Bioassay (MBA)
3.2. Chemical Methods
3.3. Receptor Binding Assay (RBA)
4. Methods for End-Product Testing (EPT)
4.1. Immunoassays
4.1.1. Enzyme-Linked Immunosorbent Assays (ELISA)
4.1.2. Lateral Flow Immunoassay (LFIA)
5. Proof of Concept Biosensors
5.1. Antibody-Based Biosensors
5.1.1. Flow-Through Microarrays
5.1.2. Fluorometric Assays
5.1.3. Surface Plasmon Resonance (SPR)
5.1.4. Electrochemical Biosensors
5.1.5. Planar Waveguide Cartridges
5.2. Enzyme Inhibition-Based Biosensors
5.3. Aptamers-Based Biosensor
6. Prospective Trends and Technologies
7. Challenges for Sample Preparation
8. Procedural Practicalities for End User Needs
9. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Sustainability in action. In The State of World Fishery and Aquaculture 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Simoes, A.J.G.; Haldogo, C.A. The Economic Complexity Observatory: An Analytical Tool for Understanding the Dynamics of Economic Development. 2017. Available online: https://oec.world/en/profile/hs92/0307/#Exporters (accessed on 1 December 2020).
- Food and Agriculture Organization of the United Nations. GLOBEFISH—Information and Analysis on World Fish Trade. Available online: http://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/1176312/ (accessed on 1 December 2020).
- CDC. Available online: https://www.cdc.gov/habs/general.html (accessed on 1 December 2020).
- Ralston, E.P.; Kite-Powell, H.; Beet, A. An estimate of the cost of acute health effects from food- and water-borne marine pathogens and toxins in the USA. J. Water Health 2011, 9, 680–694. [Google Scholar] [CrossRef]
- Anderson, D. HABs in a changing world: A perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climatic and environmental change. In Proceedings of the 15th International Conference on Harmful Algae, Changwon, Gyeongnam, Korea, 29 October–2 November 2012; pp. 3–17. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26640829%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4667985 (accessed on 1 December 2020).
- Botana, L.M. (Ed.) Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 2nd ed.; Food Science and Technology: New York, NY, USA, 2008. [Google Scholar]
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of Tetrodotoxin Shellfish Poisoning (TSP) Toxins and Causative Factors in Bivalve Molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef]
- Davidson, K.; Baker, C.; Higgins, C.; Higman, W.; Swan, S.; Veszelovszki, A.; Turner, A.D. Potential Threats Posed by New or Emerging Marine Biotoxins in UK Waters and Examination of Detection Methodologies Used for Their Control: Cyclic Imines. Mar. Drugs 2015, 13, 7087–7112. [Google Scholar] [CrossRef]
- Reynolds, D.A.; Yoo, M.-J.; Dixson, D.L.; Ross, C. Exposure to the Florida red tide dinoflagellate, Karenia brevis, and its associated brevetoxins induces ecophysiological and proteomic alterations in Porites astreoides. PLoS ONE 2020, 15, e0228414. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M. Palytoxins: A still haunting Hawaiian curse. Phytochem. Rev. 2010, 9, 491–500. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L. LC-MS of palytoxin and its analogues: State of the art and future perspectives. Toxicon 2011, 57, 376–389. [Google Scholar] [CrossRef]
- Ramos, V.; Vasconcelos, V. Palytoxin and Analogs: Biological and Ecological Effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef] [PubMed]
- Ajani, P.; Harwood, D.T.; Murray, S.A. Recent Trends in Marine Phycotoxins from Australian Coastal Waters. Mar. Drugs 2017, 15, 33. [Google Scholar] [CrossRef]
- Tartaglione, L.; Iacovo, E.D.; Mazzeo, A.; Casabianca, S.; Ciminiello, P.; Penna, A.; Dell’Aversano, C. Variability in Toxin Profiles of the Mediterranean Ostreopsis cf. ovata and in Structural Features of the Produced Ovatoxins. Environ. Sci. Technol. 2017, 51, 13920–13928. [Google Scholar] [CrossRef]
- Amar, M.; Aráoz, R.; Iorga, B.I.; Yasumoto, T.; Servent, D.; Molgó, J. Prorocentrolide-A from Cultured Prorocentrum lima Dinoflagellates Collected in Japan Blocks Sub-Types of Nicotinic Acetylcholine Receptors. Toxins 2018, 10, 97. [Google Scholar] [CrossRef]
- Moreiras, G.; Leão, J.M.; Gago-Martínez, A. Analysis of Cyclic Imines in Mussels (Mytilus galloprovincialis) from Galicia (NW Spain) by LC-MS/MS. Int. J. Environ. Res. Public Health 2019, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Asencio, L.; Clausing, R.J.; Vandersea, M.; Chamero-Lago, D.; Gómez-Batista, M.; Hernández-Albernas, J.I.; Chomérat, N.; Rojas-Abrahantes, G.; Litaker, R.W.; Tester, P.; et al. Ciguatoxin Occurrence in Food-Web Components of a Cuban Coral Reef Ecosystem: Risk-Assessment Implications. Toxins 2019, 11, 722. [Google Scholar] [CrossRef]
- Farrell, H.; Murray, S.A.; Zammit, A.; Edwards, A.W. Management of Ciguatoxin Risk in Eastern Australia. Toxins 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Boente-Juncal, A.; Álvarez, M.; Antelo, Á.; Rodríguez, I.; Calabro, K.; Vale, C.; Thomas, O.P.; Botana, L.M. Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus. Toxins 2019, 11, 79. [Google Scholar] [CrossRef]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Doucette, G.J.; Medlin, L.K.; McCarron, P.; Hess, P. Detection and Surveillance of Harmful Algal Bloom Species and Toxins. Harmful Algal Blooms 2018, 39–114. [Google Scholar] [CrossRef]
- Medlin, L.K.; Orozco, J. Molecular Techniques for the Detection of Organisms in Aquatic Environments, with Emphasis on Harmful Algal Bloom Species. Sensors 2017, 17, 1184. [Google Scholar] [CrossRef]
- Medlin, L.; Gamella, M.; Mengs, G.; Serafín, V.; Campuzano, S.; Pingarrón, J.M. Advances in the Detection of Toxic Algae Using Electrochemical Biosensors. Biosensors 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Blondeau-Patissier, D.; Gower, J.F.; Dekker, A.G.; Phinn, S.R.; Brando, V.E. A review of ocean color remote sensing methods and statistical techniques for the detection, mapping and analysis of phytoplankton blooms in coastal and open oceans. Prog. Oceanogr. 2014, 123, 123–144. [Google Scholar] [CrossRef]
- Malthus, T.J.; Lehmann, E.; Ho, X.; Botha, E.; Anstee, J. Implementation of a Satellite Based Inland Water Algal Bloom Alerting System Using Analysis Ready Data. Remote. Sens. 2019, 11, 2954. [Google Scholar] [CrossRef]
- Malthus, T.J.; Ohmsen, R.; Van Der Woerd, H.J. An Evaluation of Citizen Science Smartphone Apps for Inland Water Quality Assessment. Remote. Sens. 2020, 12, 1578. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 853/2004 of the European Parlamient and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for on the Hygiene of Foodstuffs; Official Journal of the European Union, L: Luxembourg, 2004; Volume 139, p. 55. [Google Scholar]
- European Commission. Regulation (EC) No 2074/2005: Laying down Implementing Measures for Certain Products under Regulation (EC) No 853/2004 of the European Parliament and of the Council and for the Organisation of Official Controls under Regulation (EC) No 854/2004 of the European Parliament and of the Council and Regulation (EC) No 882/2004 of the European Parliament and of the Council, Derogating from Regulation (EC) No 852/2004 of the European Parliament and of the Council and Amending Regulations (EC) No 853/2004 and (EC) No 854/2004; Official Journal of the European Union, L: Luxembourg, 2005; Volume 338, pp. 27–59. [Google Scholar]
- European Commission. Regulation (EC) No 1664/2006: Amending Regulation 2074/2005 with Regards to Measures for Certain Products, in Particular the Testing Method for Paralytic Shellfish Poison (PSP); Official Journal of the European Union, L: Luxembourg, 2006; Volume 139, p. 55. [Google Scholar]
- European Commission. Commission Regulation (EU) No 15/2011 of 10 January 2011 amending Regulation (EC) No 2074/2005 as Regards Recognised Testing Methods for Detecting Marine Biotoxins in Live Bivalve Molluscs; Official Journal of the European Union, L: Luxembourg, 2011; pp. 3–6. Available online: https://doi.org/10.3000/17252555.L_2011.006.eng (accessed on 1 December 2020).
- European Commission. Commission Regulation (EU) 2017/1980 of 31 October 2017 amending Annex III to Regulation (EC) No 2074/2005 as Regards Paralytic Shellfish Poison (PSP) Detection Method; Official Journal of the European Union, L: Luxembourg, 2017; pp. 8–9. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R1980&from=EN (accessed on 1 December 2020).
- Food and Agriculture Organization of the United Nations and World Health Organization. Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs. Available online: https://apps.who.int/iris/bitstream/handle/10665/250663/9789241511483-eng.pdf;jsessionid=8C3485B7D71998D8D0E870446619B97D?sequence=1 (accessed on 1 December 2020).
- Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Nebbia, C.S.; Oswald, I.P.; Rose, M.; Roudot, A.-C.; Schwerdtle, T.; Vleminckx, C.; Vollmer, G.; et al. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, 4752. [Google Scholar] [CrossRef]
- Gobler, C.J.; Koch, F.; Kang, Y.; Berry, D.L.; Tang, Y.Z.; Lasi, M.; Walters, L.; Hall, L.; Miller, J.D. Expansion of harmful brown tides caused by the pelagophyte, Aureoumbra lagunensis DeYoe et Stockwell, to the US east coast. Harmful Algae 2013, 27, 29–41. [Google Scholar] [CrossRef]
- Koch, F.; Kang, Y.; Villareal, T.A.; Anderson, D.M.; Gobler, C.J. A Novel Immunofluorescence Flow Cytometry Technique Detects the Expansion of Brown Tides Caused by Aureoumbra lagunensis to the Caribbean Sea. Appl. Environ. Microbiol. 2014, 80, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Kudela, R.M.; Gobler, C.J. Harmful dinoflagellate blooms caused by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 2012, 14, 71–86. [Google Scholar] [CrossRef]
- McCarthy, M.; Bane, V.; García-Altares, M.; van Pelt, F.N.; Furey, A.; O’Halloran, J. Assessment of emerging biotoxins (pinnatoxin G and spirolides) at Europe’s first marine reserve: Lough Hyne. Toxicon 2015, 108, 202–209. [Google Scholar] [CrossRef]
- Rhodes, L. World-wide occurrence of the toxic dinoflagellate genus Ostreopsis Schmidt. Toxicon 2011, 57, 400–407. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Qiu, L.-M.; Yu, R.-C.; Kong, F.-Z.; Wang, Y.-F.; Yan, T.; Gobler, C.J.; Zhou, M.-J. Emergence of brown tides caused by Aureococcus anophagefferens Hargraves et Sieburth in China. Harmful Algae 2012, 19, 117–124. [Google Scholar] [CrossRef]
- Campbell, K.; Vilariño, N.; Botana, L.M.; Elliott, C.T. A European perspective on progress in moving away from the mouse bioassay for marine-toxin analysis. TrAC Trends Anal. Chem. 2011, 30, 239–253. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Ofuji, K.; Satake, M.; McMahon, T.; James, K.J.; Naoki, H.; Oshima, Y.; Yasumoto, T. Structures of Azaspiracid Analogs, Azaspiracid-4 and Azaspiracid-5, Causative Toxins of Azaspiracid Poisoning in Europe. Biosci. Biotechnol. Biochem. 2001, 65, 740–742. [Google Scholar] [CrossRef]
- Rehmann, N.; Hess, P.; Quilliam, M.A. Discovery of new analogs of the marine biotoxin azaspiracid in blue mussels (Mytilus edulis) by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Dell’Aversano, C.; Krock, B.; Ciminiello, P.; Percopo, I.; Tillmann, U.; Soprano, V.; Zingone, A. Mediterranean Azadinium dexteroporum (Dinophyceae) produces six novel azaspiracids and azaspiracid-35: A structural study by a multi-platform mass spectrometry approach. Anal. Bioanal. Chem. 2017, 409, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.R.; Tan, J.Y.C.; Ugalde, S.C.; Hallegraeff, G.M.; Campbell, K.; Harwood, D.T.; Dorantes-Aranda, J.J. Single-Laboratory Validation of the Neogen Qualitative Lateral Flow Immunoassay for the Detection of Paralytic Shellfish Toxins in Mussels and Oysters. J. AOAC Int. 2018, 101, 480–489. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Amending Regulation (EC) No 401/2006 as Regards Methods of Sampling of Large Lots, Spices and Food Supplements, Performance Criteria for T-2, HT-2 Toxin and Citrinin and Screening Methods of Analysis; Commission Regulation (EU) No 519/2014 of 16 May 2014; Official Journal of the European Union, L: Luxembourg, 2014; Volume L147, pp. 29–43. Available online: https://www.legislation.gov.uk/eur/2014/519 (accessed on 1 December 2020).
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Nippon Suisan Gakkaishi 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Hollingworth, T.; Wekell, M.M. Official Methods of Analysis of the AOAC, 15th ed.; Hellrich, K., Ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- EU Directive. Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes; Official Journal of the European Union, L: Luxembourg, 2010. Available online: https://www.legislation.gov.uk/eudr/2010/63 (accessed on 1 December 2020).
- Botana, L.M.; Vilariño, N.; Alfonso, A.; Vale, C.; Louzao, C.; Elliott, C.T.; Campbell, K.; Botana, A.M. The problem of toxicity equivalent factors in developing alternative methods to animal bioassays for marine-toxin detection. TrAC Trends Anal. Chem. 2010, 29, 1316–1325. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 97/61/EC of 20 October 1997 Amending the Annex to Directive 91/492/EEC Laying down the Health Conditions for the Production and Placing on the Market of Live Bivalve Molluscs; Official Journal of the European Communities: Luxembourg, 1997; pp. 35–36. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31997L0061&qid=1588259655574&from=EN (accessed on 1 December 2020).
- EU-RL-MB. EU-Harmonised Standard Operating Procedure for Determination of Domoic Acid in Shellfish and Finfish by RP-HPLC Using UV Detection. 12; 2008. Available online: http://aesan.msssi.gob.es/CRLMB/docs/docs/procedimientos/EU-Harmonised-SOP-ASP-HPLC-UV_Version1.pdf (accessed on 1 December 2020).
- EU-RL-MB. EU-Harmonised Standard Operating Procedure for Determination of Lipophilic Marine Biotoxins in Molluscs by LC-MS/MS. 31; 2015. Available online: https://doi.org/www.aesan.msps.es/en/CRLMB/web/home.shtml (accessed on 1 December 2020).
- Lawrence, J.F.; Niedzwiadek, B. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish by Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2001, 84, 1099–1108. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C.; De Astudillo, L.R.; Biré, R.; A Burdaspal, P.; Ceredi, A.; Davis, B.; Dias, E.; Eaglesham, G.; et al. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection: Collaborative Study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; Van De Riet, J.M.; Burns, B.G.; Thomas, K.M.; A Quilliam, M. Rapid Postcolumn Methodology for Determination of Paralytic Shellfish Toxins in Shellfish Tissue. J. AOAC Int. 2008, 91, 589–597. [Google Scholar] [CrossRef]
- Suzuki, T.; Uchida, H.; Watanabe, R. LC/MS Analysis of Marine Toxins. Advances in Ion Mobility-Mass Spectrometry: Fundamentals, Instrumentation and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 137–192. [Google Scholar]
- Rodríguez, I.; Alfonso, A.; González-Jartín, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography–mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- Quilliam, M.A.; Xie, M.; Hardstaff, W.R. Rapid Extraction and Cleanup for Liquid Chromatographic Determination of Domoic Acid in Unsalted Seafood. J. AOAC Int. 1995, 78, 543–554. [Google Scholar] [CrossRef]
- These, A.; Scholz, J.; Preiss-Weigert, A. Sensitive method for the determination of lipophilic marine biotoxins in extracts of mussels and processed shellfish by high-performance liquid chromatography–tandem mass spectrometry based on enrichment by solid-phase extraction. J. Chromatogr. A 2009, 1216, 4529–4538. [Google Scholar] [CrossRef] [PubMed]
- Gerssen, A.; McElhinney, M.A.; Mulder, P.P.J.; Bire, R.; Hess, P.; De Boer, J. Solid phase extraction for removal of matrix effects in lipophilic marine toxin analysis by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009, 394, 1213–1226. [Google Scholar] [CrossRef]
- Botana, L.M.; Alfonso, A.; Botana, A.; Vieytes, M.R.; Vale, C.; Vilariño, N.; Louzao, C. Functional assays for marine toxins as an alternative, high-throughput-screening solution to animal tests. TrAC Trends Anal. Chem. 2009, 28, 603–611. [Google Scholar] [CrossRef]
- Doucette, G.J.; Logan, M.M.; Ramsdell, J.S.; Van Dolah, F.M. Development and preliminary validation of a microtiter plate-based receptor binding assay for paralytic shellfish poisoning toxins. Toxicon 1997, 35, 625–636. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; A Leighfield, T.; Doucette, G.J.; Bean, L.; Niedzwiadek, B.; Rawn, D.F.K. Single-laboratory validation of the microplate receptor binding assay for paralytic shellfish toxins in shellfish. J. Assoc. Off. Anal. Chem. 2010, 92, 1705–1713. [Google Scholar]
- Van Dolah, F.M.; E Fire, S.; A Leighfield, T.; Mikulski, C.M.; Doucette, G.J. Determination of Paralytic Shellfish Toxins in Shellfish by Receptor Binding Assay: Collaborative Study. J. AOAC Int. 2012, 95, 795–812. [Google Scholar] [CrossRef]
- Turner, A.D.; Broadwater, M.; Van Dolah, F. Use of the receptor binding assay for determination of paralytic shellfish poisoning toxins in bivalve molluscs from Great Britain and the assessment of method performance in oysters. Toxicon 2018, 148, 155–164. [Google Scholar] [CrossRef]
- Alonso, E.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Evaluation of toxicity equivalent factors of paralytic shellfish poisoning toxins in seven human sodium channels types by an automated high throughput electrophysiology system. Arch. Toxicol. 2015, 90, 479–488. [Google Scholar] [CrossRef]
- Bialojan, C.; Takai, A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem. J. 1988, 256, 283–290. [Google Scholar] [CrossRef]
- Zeulab. Okatest. 2020. Available online: https://www.zeulab.com/en/producto/water-and-marine-toxins/enzymatic-water-and-marine-toxins/okatest/ (accessed on 1 December 2020).
- Smienk, H.G.F.; Calvo, D.; Razquin, P.; Domínguez, E.; Mata, L. Single Laboratory Validation of A Ready-to-Use Phosphatase Inhibition Assay for Detection of Okadaic Acid Toxins. Toxins 2012, 4, 339–352. [Google Scholar] [CrossRef]
- Smienk, H.; Domínguez, E.; Rodríguez-Velasco, M.L.; Clarke, D.; Kapp, K.; Katikou, P.; Cabado, A.G.; Otero, A.; Vieites, J.M.; Razquin, P.; et al. Quantitative Determination of the Okadaic Acid Toxins Group by a Colorimetric Phosphatase Inhibition Assay: Interlaboratory Study. J. AOAC Int. 2013, 96, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Aráoz, R.; Nghiêm, H.-O.; Rippka, R.; Palibroda, N.; De Marsac, N.T.; Herdman, M. Neurotoxins in axenic oscillatorian cyanobacteria: Coexistence of anatoxin-a and homoanatoxin-a determined by ligand-binding assay and GC/MS. Microbiology 2005, 151, 1263–1273. [Google Scholar] [CrossRef]
- Aráoz, R.; Vilariño, N.; Botana, L.M.; Molgó, J. Ligand-binding assays for cyanobacterial neurotoxins targeting cholinergic receptors. Anal. Bioanal. Chem. 2010, 397, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Fonfría, E.S.; Vilariño, N.; Espiña, B.; Louzao, M.C.; Alvarez, M.; Molgó, J.; Aráoz, R.; Botana, L.M. Feasibility of gymnodimine and 13-desmethyl C spirolide detection by fluorescence polarization using a receptor-based assay in shellfish matrixes. Anal. Chim. Acta 2010, 657, 75–82. [Google Scholar] [CrossRef]
- Rodríguez, L.P.; Vilariño, N.; Molgó, J.; Araoz, R.; Botana, L.M. High-throughput receptor-based assay for the detection of spirolides by chemiluminescence. Toxicon 2013, 75, 35–43. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; McCall, J.R.; Bourdelais, A.J.; Baden, D.G.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Quintana, H.A.F.; et al. Fluorescent Receptor Binding Assay for Detecting Ciguatoxins in Fish. PLoS ONE 2016, 11, e0153348. [Google Scholar] [CrossRef]
- Pelin, M.; Sosa, S.; Brovedani, V.; Fusco, L.; Poli, M.; Tubaro, A. A Novel Sensitive Cell-Based Immunoenzymatic Assay for Palytoxin Quantitation in Mussels. Toxins 2018, 10, 329. [Google Scholar] [CrossRef]
- Garthwaite, I. Keeping shellfish safe to eat: A brief review of shellfish toxins, and methods for their detection. Trends Food Sci. Technol. 2000, 11, 235–244. [Google Scholar] [CrossRef]
- Dubois, M.; Demoulin, L.; Charlier, C.; Singh, G.; Godefroy, S.; Campbell, K.; Elliott, C.; Delahaut, P. Development of ELISAs for detecting domoic acid, okadaic acid, and saxitoxin and their applicability for the detection of marine toxins in samples collected in Belgium. Food Addit. Contam. Part A 2010, 27, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, I.; Ross, K.M.; O Miles, C.; Briggs, L.R.; Towers, N.R.; Borrell, T.; Busby, P. Integrated Enzyme-Linked Immunosorbent Assay Screening System for Amnesic, Neurotoxic, Diarrhetic, and Paralytic Shellfish Poisoning Toxins Found in New Zealand. J. AOAC Int. 2001, 84, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.; Burrell, S.; Holland, P. Review of the Currently Available Field Methods for Detection of Marine Biotoxins in Shellfish Flesh. 86; September 2015. Available online: http://www.foodstandards.gov.scot/review-currently-available-field-methods-detection-marine-biotoxins-shellfish-flesh (accessed on 1 December 2020).
- Johnson, H.M.; Frey, P.A.; Angelotti, R.; Campbell, J.E.; Lewis, K.H. Haptenic properties of paralytic shellfish poison conjugated to proteins by formaldehyde treatment. Proc. Soc. Exp. Biol. Med. 1964, 117, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Cembella, A.D.; Parent, Y.; Jones, D.; Lamoureux, G. Specificity and cross-reactivity of an absorption-inhibition enzyme-linked immunoassay for the detection of paralytic shellfish toxins. In Proceedings of the 4th International Conference on Toxic Marine Phytoplankton, Lund, Sweden, 26–30 June 1989. [Google Scholar]
- Chu, F.S.; Fan, T.S.L. Indirect Enzyme-Linked Immunosorbent Assay for Saxitoxin in Shellfish. J. Assoc. Off. Anal. Chem. 1985, 68, 13–16. [Google Scholar] [CrossRef]
- Renz, V.; Terplan, G. En enzymimmunologischer nachweis von saxitoxin. Arch. Fur Lebensm. 1988, 39, 25–33. [Google Scholar]
- Chu, F.S.; Hsu, K.-H.; Huang, X.; Barrett, R.; Allison, C. Screening of Paralytic Shellfish Posioning Toxins in Naturally Occurring Samples with Three Different Direct Competitive Enzyme-Linked Immunosorbent Assays. J. Agric. Food Chem. 1996, 44, 4043–4047. [Google Scholar] [CrossRef]
- Huang, X.; Hsu, K.-H.; Chu, F.S. Direct Competitive Enzyme-Linked Immunosorbent Assay for Saxitoxin and Neosaxitoxin. J. Agric. Food Chem. 1996, 44, 1029–1035. [Google Scholar] [CrossRef]
- McCall, J.R.; Holland, W.C.; Keeler, D.M.; Hardison, D.R.; Litaker, R.W. Improved Accuracy of Saxitoxin Measurement Using an Optimized Enzyme-Linked Immunosorbent Assay. Toxins 2019, 11, 632. [Google Scholar] [CrossRef]
- Harrison, K.; Johnson, S.; Turner, A.D. Application of rapid test kits for the determination of paralytic shellfish poisoning (PSP) toxins in bivalve molluscs from Great Britain. Toxicon 2016, 119, 352–361. [Google Scholar] [CrossRef]
- Carmody, E.P.; James, K.J.; Kelly, S.S. Diarrhetic Shellfish Poisoning: Evaluation of Enzyme-Linked Immunosorbent Assay Methods for Determination of Dinophyslstoxin-2. J. AOAC Int. 1995, 78, 1403–1407. [Google Scholar] [CrossRef]
- Chin, J.D.; A Quilliam, M.; Fremy, J.M.; Mohapatra, S.K.; Skorska, H.M. Screening for Okadaic Acid by Immunoassay. J. AOAC Int. 1995, 78, 508–513. [Google Scholar] [CrossRef]
- Draisci, R.; Croci, L.; Giannetti, L.; Cozzi, L.; Lucentini, L.; De Medici, D.; Stacchini, A. Comparison of mouse bioassay, HPLC and enzyme immunoassay methods for determining diarrhetic shellfish poisoning toxins in mussels. Toxicon 1994, 32, 1379–1384. [Google Scholar] [CrossRef]
- Morton, S.L.; Donald, R. Determination of okadaic acid content of dinoflagellate cells: A comparison of the HPLC-fluorescent method and two monoclonal antibody ELISA test kits. Toxicon 1996, 34, 947–954. [Google Scholar] [CrossRef]
- Tubaro, A.; Sosa, S.; Bruno, M.; Gucci, P.M.B.; Volterra, L.; Della Loggia, R. Diarrhoeic shellfish toxins in Adriatic Sea mussels evaluated by an ELISA method. Toxicon 1992, 30, 673–676. [Google Scholar] [CrossRef]
- Matsuura, S.; Kita, H.; Takagaki, Y. Specificity of Mouse Monoclonal Anti-Okadaic Acid Antibodies to Okadaic Acid and Its Analogs among Diarrhetic Shellfish Toxins. Biosci. Biotechnol. Biochem. 1994, 58, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ota, H.; Yamasaki, M. Direct evidence of transformation of dinophysistoxin-1 to 7-O-acyl-dinophysistoxin-1 (dinophysistoxin-3) in the scallop Patinopecten yessoensis. Toxicon 1999, 37, 187–198. [Google Scholar] [CrossRef]
- Johnson, S.; Harrison, K.; Turner, A.D. Application of rapid test kits for the determination of Amnesic Shellfish Poisoning in bivalve molluscs from Great Britain. Toxicon 2016, 117, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Harrison, K.; Turner, A.D. Application of rapid test kits for the determination of Diarrhetic Shellfish Poisoning (DSP) toxins in bivalve molluscs from Great Britain. Toxicon 2016, 111, 121–129. [Google Scholar] [CrossRef]
- Turner, A.D.; Goya, A.B. Comparison of four rapid test kits for the detection of okadaic acid-group toxins in bivalve shellfish from Argentina. Food Control. 2016, 59, 829–840. [Google Scholar] [CrossRef]
- Garthwaite, I.; Ross, K.M.; Miles, C.O.; Hansen, R.P.; Foster, D.; Wilkins, A.L.; Towers, N.R. Polyclonal antibodies to domoic acid, and their use in immunoassays for domoic acid in sea water and shellfish. Nat. Toxins 1998, 6, 93–104. [Google Scholar] [CrossRef]
- Kawatsu, K.; Hamano, Y.; Noguchi, T. Production and characterization of a monoclonal antibody against domoic acid and its application to enzyme immunoassay. Toxicon 1999, 37, 1579–1589. [Google Scholar] [CrossRef]
- Newsome, H.; Truelove, J.; Hierlihy, L.; Collins, P. Determination of domoic acid in serum and urine by immunochemical analysis. Bull. Environ. Contam. Toxicol. 1991, 47, 329–334. [Google Scholar] [CrossRef]
- Osada, M.; Marks, L.; Stewart, J. Determination of domoic acid by two different versions of a competitive enzyme-linked immunosorbent assay (ELISA). Bull. Environ. Contam. Toxicol. 1995, 54, 797–804. [Google Scholar] [CrossRef]
- Rhodes, L.; Scholin, C.; Garthwaite, I. Pseudo-nitzschia in New Zealand and the role of DNA probes and immunoassays in refining marine biotoxin monitoring programmes. Nat. Toxins 1998, 6, 105–111. [Google Scholar] [CrossRef]
- Saeed, A.F.U.H.; Ling, S.; Yuan, J.; Wang, S. The Preparation and Identification of a Monoclonal Antibody against Domoic Acid and Establishment of Detection by Indirect Competitive ELISA. Toxins 2017, 9, 250. [Google Scholar] [CrossRef]
- Sanchis, A.; Bosch-Orea, C.; Salvador, J.-P.; Marco, M.-P.; Farré, M. Development and validation of a multianalyte immunoassay for the quantification of environmental pollutants in seawater samples from the Catalonia coastal area. Anal. Bioanal. Chem. 2019, 411, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Kitts, D. A competitive enzyme-linked immunoassay for domoic acid determination in human body fluids. Food Chem. Toxicol. 1994, 32, 1147–1154. [Google Scholar] [CrossRef]
- Tsao, Z.-J.; Liao, Y.-C.; Liu, B.-H.; Su, C.-C.; Yu, F.-Y. Development of a Monoclonal Antibody against Domoic Acid and Its Application in Enzyme-Linked Immunosorbent Assay and Colloidal Gold Immunostrip. J. Agric. Food Chem. 2007, 55, 4921–4927. [Google Scholar] [CrossRef]
- Yu, F.-Y.; Liu, B.-H.; Wu, T.-S.; Chi, T.-F.; Su, M.-C. Development of a Sensitive Enzyme-Linked Immunosorbent Assay for the Determination of Domoic Acid in Shellfish. J. Agric. Food Chem. 2004, 52, 5334–5339. [Google Scholar] [CrossRef]
- Kleivdal, H.; Kristiansen, S.-I.; Nilsen, M.V.; Briggs, L. Single-Laboratory Validation of the Biosense Direct Competitive Enzyme-Linked Immunosorbent Assay (ELISA) for Determination of Domoic Acid Toxins in Shellfish. J. AOAC Int. 2007, 90, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Kleivdal, H.; Kristiansen, S.-I.; Nilsen, M.V.; Goksyr, A.; Briggs, L.; Holland, P.; McNabb, P.; Aasheim, A.; Aune, T.; Bates, S.; et al. Determination of Domoic Acid Toxins in Shellfish by Biosense ASP ELISAA Direct Competitive Enzyme-Linked Immunosorbent Assay: Collaborative Study. J. AOAC Int. 2007, 90, 1011–1027. [Google Scholar] [CrossRef]
- Shaw, I.; O’Reilly, A.; Charleton, M.; Kane, M. Development of a High-Affinity Anti-Domoic Acid Sheep scFv and its Use in Detection of the Toxin in Shellfish. Anal. Chem. 2008, 80, 3205–3212. [Google Scholar] [CrossRef]
- Ling, S.; Xiao, S.; Xie, C.; Wang, R.; Zeng, L.; Wang, K.; Zhang, D.; Li, X.; Wang, S. Preparation of Monoclonal Antibody for Brevetoxin 1 and Development of Ic-ELISA and Colloidal Gold Strip to Detect Brevetoxin 1. Toxins 2018, 10, 75. [Google Scholar] [CrossRef]
- Briggs, L.R.; Miles, C.O.; Fitzgerald, J.M.; Ross, K.M.; Garthwaite, I.; Towers, N.R. Enzyme-Linked Immunosorbent Assay for the Detection of Yessotoxin and Its Analogues. J. Agric. Food Chem. 2004, 52, 5836–5842. [Google Scholar] [CrossRef]
- Samdal, I.A.; Løvberg, K.E.; Briggs, L.R.; Kilcoyne, J.; Xu, J.; Forsyth, C.J.; Miles, C.O. Development of an ELISA for the Detection of Azaspiracids. J. Agric. Food Chem. 2015, 63, 7855–7861. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; De La Iglesia, P.; Del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of Tetrodotoxins in Puffer Fish by a Self-Assembled Monolayer-Based Immunoassay and Comparison with Surface Plasmon Resonance, LC-MS/MS, and Mouse Bioassay. Anal. Chem. 2015, 87, 10839–10847. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Rambla-Alegre, M.; Leonardo, S.; Bellés, C.; Campbell, K.; Elliott, C.T.; Gerssen, A.; Klijnstra, M.D.; Diogène, J.; Campàs, M. Development and validation of a maleimide-based enzyme-linked immunosorbent assay for the detection of tetrodotoxin in oysters and mussels. Talanta 2018, 176, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Tsumuraya, T.; Sato, T.; Hirama, M.; Fujii, I. Highly Sensitive and Practical Fluorescent Sandwich ELISA for Ciguatoxins. Anal. Chem. 2018, 90, 7318–7324. [Google Scholar] [CrossRef]
- CRLMB (Community Reference Laboratory for Marine Biotoxins). Report on Toxicology Working Group Meeting. 2005. Available online: http://www.aesan.msps.es/en/CRLMB/web/home.shtml (accessed on 1 December 2020).
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- U.S. FDA (United States Food and Drug Administration). Fish and Fishery Products Hazards and Controls Guidance (Fourth). 2020. Available online: https://www.fda.gov/media/80637/download (accessed on 1 December 2020).
- Boscolo, S.; Pelin, M.; De Bortoli, M.; Fontanive, G.; Barreras, A.; Berti, F.; Sosa, S.; Chaloin, O.; Bianco, A.; Yasumoto, T.; et al. Sandwich ELISA Assay for the Quantitation of Palytoxin and Its Analogs in Natural Samples. Environ. Sci. Technol. 2013, 47, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, W.; Campbell, K.; Melville, K.; Holmes, S.J.; Rice, J.; Elliott, C.T. Development and Validation of a Novel Lateral Flow Immunoassay (LFIA) for the Rapid Screening of Paralytic Shellfish Toxins (PSTs) from Shellfish Extracts. Anal. Chem. 2015, 87, 5324–5332. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Tan, J.Y.C.; Hallegraeff, G.M.; Campbell, K.; Ugalde, S.C.; Harwood, D.T.; Bartlett, J.K.; Campàs, M.; Crooks, S.; Gerssen, A.; et al. Detection of Paralytic Shellfish Toxins in Mussels and Oysters Using the Qualitative Neogen Lateral-Flow Immunoassay: An Interlaboratory Study. J. AOAC Int. 2018, 101, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, W.; Meneely, J.; Campbell, K.; Hooper, M.; Melville, K.; Holmes, S.; Rice, J.; Elliott, C. Development and validation of the first high performance-lateral flow immunoassay (HP-LFIA) for the rapid screening of domoic acid from shellfish extracts. Talanta 2013, 116, 663–669. [Google Scholar] [CrossRef]
- Jawaid, W.; Meneely, J.P.; Campbell, K.; Melville, K.; Holmes, S.J.; Rice, J.; Elliott, C.T. Development and Validation of a Lateral Flow Immunoassay for the Rapid Screening of Okadaic Acid and All Dinophysis Toxins from Shellfish Extracts. J. Agric. Food Chem. 2015, 63, 8574–8583. [Google Scholar] [CrossRef] [PubMed]
- Laycock, M.V.; Jellett, J.F.; Belland, E.R.; Bishop, P.C.; Thériault, B.; Russell-Tattrie, A.L.; Quilliam, M.A.; Cembella, A.D.; Richards, R.C. MIST AlertTM: A Rapid Assay for Paralytic Shellfish Poisoning Toxins. In Intergovernmental Panel on Climate Change; Harmful Algal Blooms 2000; Cambridge University Press: Cambridge, UK, 2001; pp. 254–256. [Google Scholar] [CrossRef]
- Laycock, M.V.; Jellett, J.F.; Easy, D.J.; Donovan, M.A. First report of a new rapid assay for diarrhetic shellfish poisoning toxins. Harmful Algae 2006, 5, 74–78. [Google Scholar] [CrossRef]
- Turner, A.D.; Tarnovius, S.; Johnson, S.; Higman, W.A.; Algoet, M. Testing and application of a refined rapid detection method for paralytic shellfish poisoning toxins in UK shellfish. Toxicon 2015, 100, 32–41. [Google Scholar] [CrossRef]
- Shen, H.; Xu, F.; Xiao, M.; Fu, Q.; Cheng, Z.; Zhang, S.; Huang, C.; Tang, Y. A new lateral-flow immunochromatographic strip combined with quantum dot nanobeads and gold nanoflowers for rapid detection of tetrodotoxin. Analyst 2017, 142, 4393–4398. [Google Scholar] [CrossRef] [PubMed]
- Nelis, J.L.D.; Tsagkaris, A.; Zhao, Y.; Lou-Franco, J.; Nolan, P.; Zhou, H.; Cao, C.; Rafferty, K.; Hajslova, J.; Elliott, C.; et al. The end user sensor tree: An end-user friendly sensor database. Biosens. Bioelectron. 2019, 130, 245–253. [Google Scholar] [CrossRef]
- Szkola, A.; Campbell, K.; Elliott, C.T.; Niessner, R.; Seidel, M. Automated, high performance, flow-through chemiluminescence microarray for the multiplexed detection of phycotoxins. Anal. Chim. Acta 2013, 787, 211–218. [Google Scholar] [CrossRef]
- Fraga, M.; Vilariño, N.; Louzao, M.C.; Rodriguez, P.; Campbell, K.; Elliott, C.T.; Botana, L.M. Multidetection of Paralytic, Diarrheic, and Amnesic Shellfish Toxins by an Inhibition Immunoassay Using a Microsphere-Flow Cytometry System. Anal. Chem. 2013, 85, 7794–7802. [Google Scholar] [CrossRef]
- Rodríguez, L.P.; Vilariño, N.; Louzao, M.C.; Dickerson, T.J.; Nicolaou, K.C.; Frederick, M.O.; Botana, L.M. Microsphere-based immunoassay for the detection of azaspiracids. Anal. Biochem. 2014, 447, 58–63. [Google Scholar] [CrossRef]
- Rodríguez, L.P.; Vilariño, N.; Molgó, J.; Araoz, R.; Louzao, M.C.; Taylor, P.; Talley, T.; Botana, L.M. Development of a Solid-Phase Receptor-Based Assay for the Detection of Cyclic Imines Using a Microsphere-Flow Cytometry System. Anal. Chem. 2013, 85, 2340–2347. [Google Scholar] [CrossRef]
- Campbell, K.; Stewart, L.D.; Doucette, G.J.; Fodey, T.L.; Haughey, S.A.; Vilariño, N.; Kawatsu, K.; Elliott, C.T. Assessment of Specific Binding Proteins Suitable for the Detection of Paralytic Shellfish Poisons Using Optical Biosensor Technology. Anal. Chem. 2007, 79, 5906–5914. [Google Scholar] [CrossRef]
- Campbell, K.; Rawn, D.; Niedzwiadek, B.; Elliott, C. Paralytic shellfish poisoning (PSP) toxin binders for optical biosensor technology: Problems and possibilities for the future: A review. Food Addit. Contam. Part A 2011, 28, 711–725. [Google Scholar] [CrossRef]
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Haughey, S.A.; Top, H.V.D.; Van Egmond, H.; Vilariño, N.; Botana, L.M.; Elliott, C.T. Single Laboratory Validation of a Surface Plasmon Resonance Biosensor Screening method for Paralytic Shellfish Poisoning Toxins. Anal. Chem. 2010, 82, 2977–2988. [Google Scholar] [CrossRef]
- Campbell, K.; Huet, A.-C.; Charlier, C.; Higgins, C.; Delahaut, P.; Elliott, C.T. Comparison of ELISA and SPR biosensor technology for the detection of paralytic shellfish poisoning toxins. J. Chromatogr. B 2009, 877, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Haughey, S.A.; Campbell, K.; Yakes, B.J.; Prezioso, S.M.; DeGrasse, S.L.; Kawatsu, K.; Elliott, C.T. Comparison of biosensor platforms for surface plasmon resonance based detection of paralytic shellfish toxins. Talanta 2011, 85, 519–526. [Google Scholar] [CrossRef]
- Prado, E.; Colas, F.; Laurent, S.; Tardivel, M.; Evrard, J.; Forest, B.; Boche, A.; Rouxel, J. Toward a SPR imaging in situ system to detect marine biotoxin. In Proceedings of the SPIE 11361, Biophotonics in Point-of-Care, 113610J, Online Only. 13 April 2020. [Google Scholar] [CrossRef]
- Van Den Top, H.J.; Elliott, C.T.; Haughey, S.A.; Vilariño, N.; Van Egmond, H.P.; Botana, L.M.; Campbell, K. Surface Plasmon Resonance Biosensor Screening Method for Paralytic Shellfish Poisoning Toxins: A Pilot Interlaboratory Study. Anal. Chem. 2011, 83, 4206–4213. [Google Scholar] [CrossRef]
- Yakes, B.J.; DeGrasse, S.L.; Poli, M.; Deeds, J.R. Antibody characterization and immunoassays for palytoxin using an SPR biosensor. Anal. Bioanal. Chem. 2011, 400, 2865–2869. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Chen, S.; Taylor, A.D.; Homola, J.; Hock, B.; Jiang, S. Detection of low-molecular-weight domoic acid using surface plasmon resonance sensor. Sens. Actuators B Chem. 2005, 107, 193–201. [Google Scholar] [CrossRef]
- Fonfría, E.S.; Vilariño, N.; Campbell, K.; Elliott, C.; Haughey, S.A.; Ben-Gigirey, B.; Vieites, J.M.; Kawatsu, A.K.; Botana, L.M. Paralytic Shellfish Poisoning Detection by Surface Plasmon Resonance-Based Biosensors in Shellfish Matrixes. Anal. Chem. 2007, 79, 6303–6311. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.C.; Soelberg, S.D.; Eberhart, B.-T.L.; Spencer, S.; Wekell, J.C.; Chinowsky, T.; Trainer, V.L.; Furlong, C.E. Detection of the toxin domoic acid from clam extracts using a portable surface plasmon resonance biosensor. Harmful Algae 2007, 6, 166–174. [Google Scholar] [CrossRef]
- Llamas, N.M.; Stewart, L.; Fodey, T.; Higgins, H.C.; Velasco, M.L.R.; Botana, L.M.; Elliott, C.T. Development of a novel immunobiosensor method for the rapid detection of okadaic acid contamination in shellfish extracts. Anal. Bioanal. Chem. 2007, 389, 581–587. [Google Scholar] [CrossRef] [PubMed]
- McNamee, S.E.; Elliott, C.T.; Delahaut, P.; Campbell, K. Multiplex biotoxin surface plasmon resonance method for marine biotoxins in algal and seawater samples. Environ. Sci. Pollut. Res. 2012, 20, 6794–6807. [Google Scholar] [CrossRef]
- Campbell, K.; McNamee, S.E.; Huet, A.-C.; Delahaut, P.; Vilariño, N.; Botana, L.M.; Poli, M.; Elliott, C.T. Evolving to the optoelectronic mouse for phycotoxin analysis in shellfish. Anal. Bioanal. Chem. 2014, 406, 6867–6881. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Karapetis, S.; Tzamtzis, N. Development of an Electrochemical Biosensor for the Rapid Detection of Saxitoxin Based on Air Stable Lipid Films with Incorporated Anti-STX Using Graphene Electrodes. Electroanalysis 2017, 29, 990–997. [Google Scholar] [CrossRef]
- Leonardo, S.; Kilcoyne, J.; Samdal, I.A.; Miles, C.O.; O’Sullivan, C.K.; Diogene, J.; Campàs, M. Detection of azaspiracids in mussels using electrochemical immunosensors for fast screening in monitoring programs. Sens. Actuators B Chem. 2018, 262, 818–827. [Google Scholar] [CrossRef]
- Zamolo, V.A.; Valenti, G.; Venturelli, E.; Chaloin, O.; Marcaccio, M.; Boscolo, S.; Castagnola, V.; Sosa, S.; Berti, F.; Fontanive, G.; et al. Highly Sensitive Electrochemiluminescent Nanobiosensor for the Detection of Palytoxin. ACS Nano 2012, 6, 7989–7997. [Google Scholar] [CrossRef] [PubMed]
- McNamee, S.E.; Elliott, C.T.; Greer, B.; Lochhead, M.; Campbell, K. Development of a Planar Waveguide Microarray for the Monitoring and Early Detection of Five Harmful Algal Toxins in Water and Cultures. Environ. Sci. Technol. 2014, 48, 13340–13349. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Campàs, M.; Yakes, B.J.; Deeds, J.R.; Katikou, P.; Kawatsu, K.; Lochhead, M.; Elliott, C.T.; Campbell, K. Tetrodotoxin detection in puffer fish by a sensitive planar waveguide immunosensor. Sens. Actuators B Chem. 2017, 253, 967–976. [Google Scholar] [CrossRef]
- Campàs, M.; Marty, J.-L. Enzyme sensor for the electrochemical detection of the marine toxin okadaic acid. Anal. Chim. Acta 2007, 605, 87–93. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, X.; Su, K.; Xu, G.; Wang, P. Disposable poly (o-aminophenol)-carbon nanotubes modified screen print electrode-based enzyme sensor for electrochemical detection of marine toxin okadaic acid. Sens. Actuators B Chem. 2016, 235, 170–178. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhang, W.; Zhu, M.; Liu, Z.; Kong, Y.; Liu, S. Marine Toxins Detection by Biosensors Based on Aptamers. Toxins 2019, 12, 1. [Google Scholar] [CrossRef]
- Gao, S.; Hu, B.; Zheng, X.; Cao, Y.; Liu, D.; Sun, M.; Jiao, B.; Wang, L. Gonyautoxin 1/4 aptamers with high-affinity and high-specificity: From efficient selection to aptasensor application. Biosens. Bioelectron. 2016, 79, 938–944. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, X.; Hu, B.; Sun, M.; Wu, J.; Jiao, B.; Wang, L. Enzyme-linked, aptamer-based, competitive biolayer interferometry biosensor for palytoxin. Biosens. Bioelectron. 2017, 89, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, X.; Wu, J. A biolayer interferometry-based competitive biosensor for rapid and sensitive detection of saxitoxin. Sens. Actuators B Chem. 2017, 246, 169–174. [Google Scholar] [CrossRef]
- Chinnappan, R.; AlZabn, R.; Fataftah, A.K.; Alhoshani, A.; Zourob, M. Probing high-affinity aptamer binding region and development of aptasensor platform for the detection of cylindrospermopsin. Anal. Bioanal. Chem. 2020, 412, 4691–4701. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, Y.; Guo, X.; Gao, Y.; Han, Y.; Sun, J.; Han, L. A rapid and ultrasensitive colorimetric biosensor based on aptamer functionalized Au nanoparticles for detection of saxitoxin. RSC Adv. 2020, 10, 15293–15298. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.; Dillon, M.; Hajslova, J.; Elliott, C. Smartphone-based optical assays in the food safety field. TrAC Trends Anal. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef]
- Fang, J.; Qiu, X.; Wan, Z.; Zou, Q.; Su, K.; Hu, N.; Wang, P. A sensing smartphone and its portable accessory for on-site rapid biochemical detection of marine toxins. Anal. Methods 2016, 8, 6895. [Google Scholar] [CrossRef]
- Su, K.; Qiu, X.; Fang, J.; Zou, Q.; Wang, P. An improved efficient biochemical detection method to marine toxins with a smartphone-based portable system—Bionic e-Eye. Sens. Actuators B Chem. 2017, 238, 1165–1172. [Google Scholar] [CrossRef]
- Chinowsky, T.M.; Soelberg, S.D.; Baker, P.; Swanson, N.R.; Kauffman, P.; Mactutis, A.; Grow, M.S.; Atmar, R.; Yee, S.S.; Furlong, C.E. Portable 24-analyte surface plasmon resonance instruments for rapid, versatile biodetection. Biosens. Bioelectron. 2007, 22, 2268–2275. [Google Scholar] [CrossRef]
- Rahimi, F.; Chatzimichail, S.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. A Review of Portable High-Performance Liquid Chromatography: The Future of the Field? Chromatographia 2020, 83, 1–31. [Google Scholar] [CrossRef]
- Jensen, P.A.; Dougherty, B.V.; Moutinho, T.J.; Papin, J.A. Miniaturized Plate Readers for Low-Cost, High-Throughput Phenotypic Screening. J. Lab. Autom. 2015, 20, 51–55. [Google Scholar] [CrossRef][Green Version]
- Berg, B.; Cortazar, B.; Tseng, D.; Ozkan, H.; Feng, S.; Wei, Q.; Chan, R.Y.-L.; Burbano, J.; Farooqui, Q.; Lewinski, M.; et al. Cellphone-Based Hand-Held Microplate Reader for Point-of-Care Testing of Enzyme-Linked Immunosorbent Assays. ACS Nano 2015, 9, 7857–7866. [Google Scholar] [CrossRef]
- McElhiney, J.; Drever, M.; Lawton, L.A.; Porter, A.J. Rapid Isolation of a Single-Chain Antibody against the Cyanobacterial Toxin Microcystin-LR by Phage Display and Its Use in the Immunoaffinity Concentration of Microcystins from Water. Appl. Environ. Microbiol. 2002, 68, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- McElhiney, J.; Lawton, L.A.; Porter, A.J. Detection and quantification of microcystins (cyanobacterial hepatotoxins) with recombinant antibody fragments isolated from a naïve human phage display library. FEMS Microbiol. Lett. 2000, 193, 83–88. [Google Scholar] [CrossRef]
- Hara, Y.; Dong, J.; Ueda, H. Open-sandwich immunoassay for sensitive and broad-range detection of a shellfish toxin gonyautoxin. Anal. Chim. Acta 2013, 793, 107–113. [Google Scholar] [CrossRef]
- Peltomaa, R.; Benito-Peña, E.; Barderas, R.; Moreno-Bondi, M.C. Phage Display in the Quest for New Selective Recognition Elements for Biosensors. ACS Omega 2019, 4, 11569–11580. [Google Scholar] [CrossRef] [PubMed]
- Shriver-Lake, L.C.; Liu, J.L.; Lee, P.A.B.; Goldman, E.R.; Dietrich, R.; Märtlbauer, E.; Anderson, G.P. Integrating scFv into xMAP Assays for the Detection of Marine Toxins. Toxins 2016, 8, 346. [Google Scholar] [CrossRef]
- Maguire, I.; Fitzgerald, J.; Heery, B.; Nwankire, C.; O’Kennedy, R.; Ducrée, J.; Regan, F. Novel Microfluidic Analytical Sensing Platform for the Simultaneous Detection of Three Algal Toxins in Water. ACS Omega 2018, 3, 6624–6634. [Google Scholar] [CrossRef] [PubMed]
- Cunha, I.; Biltes, R.; Sales, M.; Vasconcelos, V. Aptamer-Based Biosensors to Detect Aquatic Phycotoxins and Cyanotoxins. Sensors 2018, 18, 2367. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Tavares, A.C.; Zourob, M. Selection and Identification of DNA Aptamers against Okadaic Acid for Biosensing Application. Anal. Chem. 2013, 85, 11794–11801. [Google Scholar] [CrossRef]
- Gu, H.; Duan, N.; Wu, S.; Hao, L.; Xia, Y.; Ma, X.; Wang, Z. Graphene oxide-assisted non-immobilized SELEX of okdaic acid aptamer and the analytical application of aptasensor. Sci. Rep. 2016, 6, 21665. [Google Scholar] [CrossRef]
- Handy, S.M.; Yakes, B.J.; DeGrasse, J.A.; Campbell, K.; Elliott, C.T.; Kanyuck, K.M.; DeGrasse, S.L. First report of the use of a saxitoxin–protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 2013, 61, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Nelis, J.L.D.; Migliorelli, D.; Jafari, S.; Generelli, S.; Lou-Franco, J.; Salvador, J.P.; Marco, M.P.; Cao, C.; Elliott, C.T.; Campbell, K. The benefits of carbon black, gold and magnetic nanomaterials for point-of-harvest electrochemical quantification of domoic acid. Mikrochim. Acta 2020, 187, 164. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Migliorelli, D.; Mühlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215. [Google Scholar] [CrossRef]
- Gholami, M.D.; Sonar, P.; Ayoko, G.A.; Izake, E.L. A highly sensitive SERS quenching nanosensor for the determination of tumor necrosis factor alpha in blood. Sens. Actuators B Chem. 2020, 310, 127867. [Google Scholar] [CrossRef]
- Lawrence, J.E.; Cembella, A.D.; Ross, N.W.; Wright, J. Cross-reactivity of an anti-okadaic acid antibody to dinophysistoxin-4 (DTX-4), dinophysistoxin-5 (DTX-5), and an okadaic acid diol ester. Toxicon 1998, 36, 1193–1196. [Google Scholar] [CrossRef]
- Shestowsky, W.S.; Holmes, C.; Hu, T.; Marr, J.; Wright, J.; Chin, J.; Sikorska, H. An Anti-okadaic Acid-Anti-idiotypic Antibody Bearing an Internal Image of Okadaic Acid Inhibits Protein Phosphatase PP1 and PP2A Catalytic Activity. Biochem. Biophys. Res. Commun. 1993, 192, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Shestowsky, W.S.; Quilliam, M.A.; Sikorska, H.M. An idiotypic-anti-idiotypic competitive immunoassay for quantitation of okadaic acid. Toxicon 1992, 30, 1441–1448. [Google Scholar] [CrossRef]
- Schulz, K.; Pöhlmann, C.; Dietrich, R.; Märtlbauer, E.; Elßner, T. Electrochemical Biochip Assays Based on Anti-idiotypic Antibodies for Rapid and Automated On-Site Detection of Low Molecular Weight Toxins. Front. Chem. 2019, 7, 31. [Google Scholar] [CrossRef]
- Peacock, M.B.; Gibble, C.M.; Senn, D.B.; Cloern, J.E.; Kudela, R.M. Blurred lines: Multiple freshwater and marine algal toxins at the land-sea interface of San Francisco Bay, California. Harmful Algae 2018, 73, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Curtis, J.M.; Walter, J.A.; McLachlan, J.L.; Wright, J.L. Two new water-soluble dsp toxin derivatives from the dinoflagellate prorocentrum maculosum: Possible storage and excretion products. Tetrahedron Lett. 1995, 36, 9273–9276. [Google Scholar] [CrossRef]
- Li, A.; Chen, H.; Qiu, J.; Lin, H.; Gu, H. Determination of multiple toxins in whelk and clam samples collected from the Chukchi and Bering seas. Toxicon 2016, 109, 84–93. [Google Scholar] [CrossRef]
- Fang, L.; Yao, X.; Wang, L.; Li, J. Solid-Phase Extraction-Based Ultra-Sensitive Detection of Four Lipophilic Marine Biotoxins in Bivalves by High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 373–379. [Google Scholar] [CrossRef]
- Puech, L.; Dragacci, S.; Gleizes, E.; Fremy, J.-M. Use of immunoaffinity columns for clean-up of diarrhetic toxins (okadaic acid and dinophysistoxins) extracts from shellfish prior to their analysis by HPLC/fluorimetry. Food Addit. Contam. 1999, 16, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, L.; Li, Z.; Wang, S.; Li, J.; Cao, W.; Sun, C.; Zheng, L.; Wang, X. Simultaneous screening for lipophilic and hydrophilic toxins in marine harmful algae using a serially coupled reversed-phase and hydrophilic interaction liquid chromatography separation system with high-resolution mass spectrometry. Anal. Chim. Acta 2016, 914, 117–126. [Google Scholar] [CrossRef]
- Devlin, R.; Campbell, K.; Kawatsu, K.; Elliott, C. Studies in the Use of Magnetic Microspheres for Immunoaffinity Extraction of Paralytic Shellfish Poisoning Toxins from Shellfish. Toxins 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Bragg, W.A.; Garrett, A.; I Hamelin, E.; Coleman, R.M.; Campbell, K.; Elliott, C.T.; Johnson, R.C. Quantitation of saxitoxin in human urine using immunocapture extraction and LC–MS. Bioanalysis 2018, 10, 229–239. [Google Scholar] [CrossRef] [PubMed]

| Poisoning Syndrome | Toxin | Major Toxin Producing Species | No. of Analogues | Vector (MRL a µg/kg) | Key Areas of Occurrence | Short Term Health Consequences | Long Term Health Consequences | References |
|---|---|---|---|---|---|---|---|---|
| Amnesic Shellfish Poisoning (ASP) | Domoic Acid (DA) | Pseudo-nitzschia spp. | ~10 | Shellfish (20,000) | United Kingdom, Europe, USA, Mexico, Australia, New Zealand, Canada | Vomiting, diarrhoea, liver inflammation, abdominal pain, confusion, disorientation, memory loss | Anterograde memory deficit, seizures leading to coma and death | [6] |
| Diarrhetic Shellfish Poisoning (DSP) | Okadaic Acid (OA) & Dinophysistoxins (DTX) | Dinophysis spp. Prorocentrum lima | ~8 | Shellfish (160 b) | Worldwide (United Kingdom, Europe, Scandinavia, North & South America, Asia, Australia & New Zealand) | Nausea, vomiting, diarrhoea, abdominal pain accompanied by chills, headache, fever | Gastrointestinal tumour promoter in laboratory animals | [6] |
| Azaspiracid (AZA) | Azadinium spp. | ~60 | Shellfish (160) | Ireland, Mediterranean, South America | Diarrhoea, neurotoxic effects | Unknown | [7] | |
| Yessotoxin (YTX) | Protoceratium reticulatum Lingulodinium polyedra | ~36 | Shellfish (3750) | China, Japan | Unknown | Unknown | ||
| Pectenotoxin (PTX) | Dinophysis fortii | ~13 | Shellfish (160 b) | China, Japan | ||||
| Paralytic Shellfish Poisoning (PSP) | Saxitoxin (STX) & Gonyautotoxin (GTX) | Alexandrium spp., Gymnodinium catenatum, Pyrodinium spp. | >57 | Shellfish Crustaceans (800 c) | Worldwide (United Kingdom, Europe, Scandinavia, North & South America, Asia, Africa, Australia & New Zealand) | Paraesthesia, drowsiness, incoherent speech, respiratory paralysis leading to death | Unknown | [6] |
| Tetrodotoxin (TTX) * | Marine bacteria spp. | >10 | Gastropods Fish | China, Japan, United Kingdom, Gulf of Mexico, Mediterranean | [8] | |||
| Neurotoxic Shellfish Poisoning | Brevetoxin (BTX/Pbtx) | Karenia spp. | >12 | Shellfish (800) | Florida, Gulf of Mexico, New Zealand | Act on site 5 of the sodium channel receptor. Nausea, diarrhoea, vomiting, numbness of lips, tongue, &throat, muscular aches, fever, chills, abdominal cramping, reduced heart rate, pupil dilation | Unknown | [9,10] |
| Other | Palytoxin (PLTX) & Ostreocin (OSTD) | Ostreopsis spp. | 2 (for PLTX) | Fish Crustacean Shellfish (30 d) | Mediterranean (Italy, Spain) | In vitro binds to the Sodium Potassium ATPase Vomiting, diarrhoea, respiratory distress, death | Unknown | [11,12] |
| Mascarenotoxin (McTX) * | 2 | [13] | ||||||
| Ovatoxins (OVTX) * | 9 | [13,14,15] | ||||||
| Gymnodimine * | Gymnodinium spp. Karenia spp. Alexandrium ostenfeldii Vulcanodinium rugosum | 5 | Shellfish | Scandinavia, United Kingdom, Mediterranean | Not fully known. Similar effects to DSP toxins in mice. Interact with nicotinic acetylcholine receptors Effects in humans have not been reported. | Unknown | [7,16,17] | |
| Spirolides * | 16 | |||||||
| Pinnatoxins (PnTX) * | 8 | |||||||
| Pteriatoxins (PtTX) * | 3 | |||||||
| Prorocentrolides * | Prorocentrum spp. | 6 | ||||||
| Spiroprorocentrimines * | TBD | |||||||
| Ciguatera Fish Poisoning | Ciguatoxin (CTX) * | Gambierdiscus spp. Amphidinum spp. | ~23 | Reef Fish Shellfish Giant claims | Caribbean, Indian and Pacific waters in tropical zone, Spain, Portugal | Act on site 5 of the sodium channel receptor. Nausea, vomiting, diarrhoea, paraesthesia, temperature dysesthesia, pain, weakness, bradycardia, hypotension | Recurrent symptoms from months to years of chronic effects | [7,18,19] |
| Maitotoxin (MTX) | Gambierdiscus spp. Fukuyoa spp. | 4 | Reef fish | Pacific Ocean | Mode of action not fully elucidated. Toxin believed to play a role in CFP. | [14,20,21] |
| Method | Additional Materials Required (Not Included in Main Kit) | Time per Sample (Sample Preparation) | Cost 1 | Complexity 2 | Pathway to Commercialization |
|---|---|---|---|---|---|
| ELISA/RBA | Microtiter plate reader Orbital shaker Clean running water Pipette(s) w. disposable tips Absorbent towels Timer Sample extraction kit 3 | 90 min (15–60 min 4) | ££ | 2 | Commercially available (Table 1) |
| LFD | LFD cassette reader Timer Sample extraction kit 3 | 35–45 min (15–60 min 4) | £ | 1 | Commercially available (Table 1) |
| Flow-through immunoassay | Chemiluminescence imaging Flow-based microarray analysis platform (MCR3) Pipette(s) w. disposable tips Sample extraction kit 3 | 20 min (20 min) | ££ | 2 | Assays must be validated in single- and multi- laboratory studies MCR3 technology is not portable for on-site testing by end-users |
| SPR | Biacore™ Q optical biosensor Pipette(s) w. disposable tips Sample extraction kit 3 | 10 min (60 min) | £££ | 3 | Assays have been validated in single-laboratory studies to AOAC standards SPR technology is suitable for use by novice end-users High cost Wizard driven software for use/maintenance |
| Electrochemical | Electrochemical analyser Pipette(s) w. disposable tips Sample extraction kit 3 | 10–45 min (30–60 min) | ££ | 2 | Assays must be validated in single- and multi- laboratory studies Challenging set-up for end users |
| Planar waveguide | Waveguide reader Pipette(s) w. disposable tips Sample extraction kit 3 | 20 min (60 min) | £ | 1 | Assays must be validated in single- and multi- laboratory studies Software for data analysis not suitable for novice end user |
| Toxin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Company | Product | Type | DA | OA | DTXs | STX | GTXs | TTX | BTXs | CTXs |
| Bioo Scientific | MaxSignal®® Domoic Acid | ELISA | √ ☐ | |||||||
| MaxSignal®® Okadaic Acid | ELISA | √ ☐ | * | |||||||
| MaxSignal®® Saxitoxin | ELISA | √ ☐ | * | |||||||
| Zeulab | DomoTest | ELISA | ||||||||
| OkaTest | RBA | √ ☐ | * | |||||||
| Saxitest | ELISA | |||||||||
| Creative Diagnostics | Domoic Acid Kit | ELISA | √ ☐ | |||||||
| Tetrodotoxin Kit | ELISA | √ ☐ | ||||||||
| Mercury Science | Domoic Acid Kit | ELISA | √ ☐ | |||||||
| Domoic Acid Field Kit | DOT 1 | √ ☐ | ||||||||
| Total Saxitoxin Kit | ELISA | √ ☐ | * | |||||||
| Abraxis | Domoic Acid ELISA Kit | ELISA | √ ☐ | |||||||
| Okadaic acid ELISA Kit | ELISA | √ ☐ | * | |||||||
| Okadaic Acid PP2A Kit | RBA | √ ☐ | √ ☐ | |||||||
| Saxitoxins Shipboard Kit | ELISA | √ ☐ | − | |||||||
| Brevetoxin (NSP) Test | ELISA | √ ☐ | ||||||||
| Marbionc | Brevetoxin ELISA Kit | ELISA | √ ☐ | |||||||
| Brevetoxin/Ciguatoxin Kit | RBA | √ ☐ | √ ☐ | |||||||
| Unibiotest | Tetrodotoxin ELISA Test | ELISA | √ ☐ | |||||||
| Tetrodotoxin Rapid Test | LFIA | √ ☐ | ||||||||
| Beacon | Saxitoxin ELISA kit | ELISA | √ ☐ | √ ☐ | ||||||
| R-Biopharm | EuroProxima Domoic Acid | ELISA | √ ☐ | |||||||
| EuroProxima Okadaic Acid | ELISA | √ ☐ | √ 2 | |||||||
| EuroProxima Saxitoxin | ELISA | √ ☐ | √ ☐ | |||||||
| EuroProxima Tetrodotoxin | ELISA | √ ☐ | ||||||||
| Biosense®® Laboratories | ASP ELISA Kit | ELISA | √ ☐ | |||||||
| DSP ELISA kit | ELISA | √ ☐ | − | |||||||
| PSP ELISA kit | ELISA | √ ☐ | − | |||||||
| Neogen | Reveal®® 2.0 for ASP | LFIA | √ ☐ | |||||||
| Reveal®® 2.0 for DSP | LFIA | √ ☐ | √ ☐ | |||||||
| Reveal®® 2.0 for PSP | LFIA | √ ☐ | * | |||||||
| Scotia | ASP Test | LFIA | √ ☐ | |||||||
| DSP Test | LFIA | √ ☐ | * | |||||||
| PSP Test | LFIA | √ ☐ | * | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dillon, M.; Zaczek-Moczydlowska, M.A.; Edwards, C.; Turner, A.D.; Miller, P.I.; Moore, H.; McKinney, A.; Lawton, L.; Campbell, K. Current Trends and Challenges for Rapid SMART Diagnostics at Point-of-Site Testing for Marine Toxins. Sensors 2021, 21, 2499. https://doi.org/10.3390/s21072499
Dillon M, Zaczek-Moczydlowska MA, Edwards C, Turner AD, Miller PI, Moore H, McKinney A, Lawton L, Campbell K. Current Trends and Challenges for Rapid SMART Diagnostics at Point-of-Site Testing for Marine Toxins. Sensors. 2021; 21(7):2499. https://doi.org/10.3390/s21072499
Chicago/Turabian StyleDillon, Michael, Maja A. Zaczek-Moczydlowska, Christine Edwards, Andrew D. Turner, Peter I. Miller, Heather Moore, April McKinney, Linda Lawton, and Katrina Campbell. 2021. "Current Trends and Challenges for Rapid SMART Diagnostics at Point-of-Site Testing for Marine Toxins" Sensors 21, no. 7: 2499. https://doi.org/10.3390/s21072499
APA StyleDillon, M., Zaczek-Moczydlowska, M. A., Edwards, C., Turner, A. D., Miller, P. I., Moore, H., McKinney, A., Lawton, L., & Campbell, K. (2021). Current Trends and Challenges for Rapid SMART Diagnostics at Point-of-Site Testing for Marine Toxins. Sensors, 21(7), 2499. https://doi.org/10.3390/s21072499










