Current Development and Applications of Super-Resolution Ultrasound Imaging
Abstract
1. Introduction
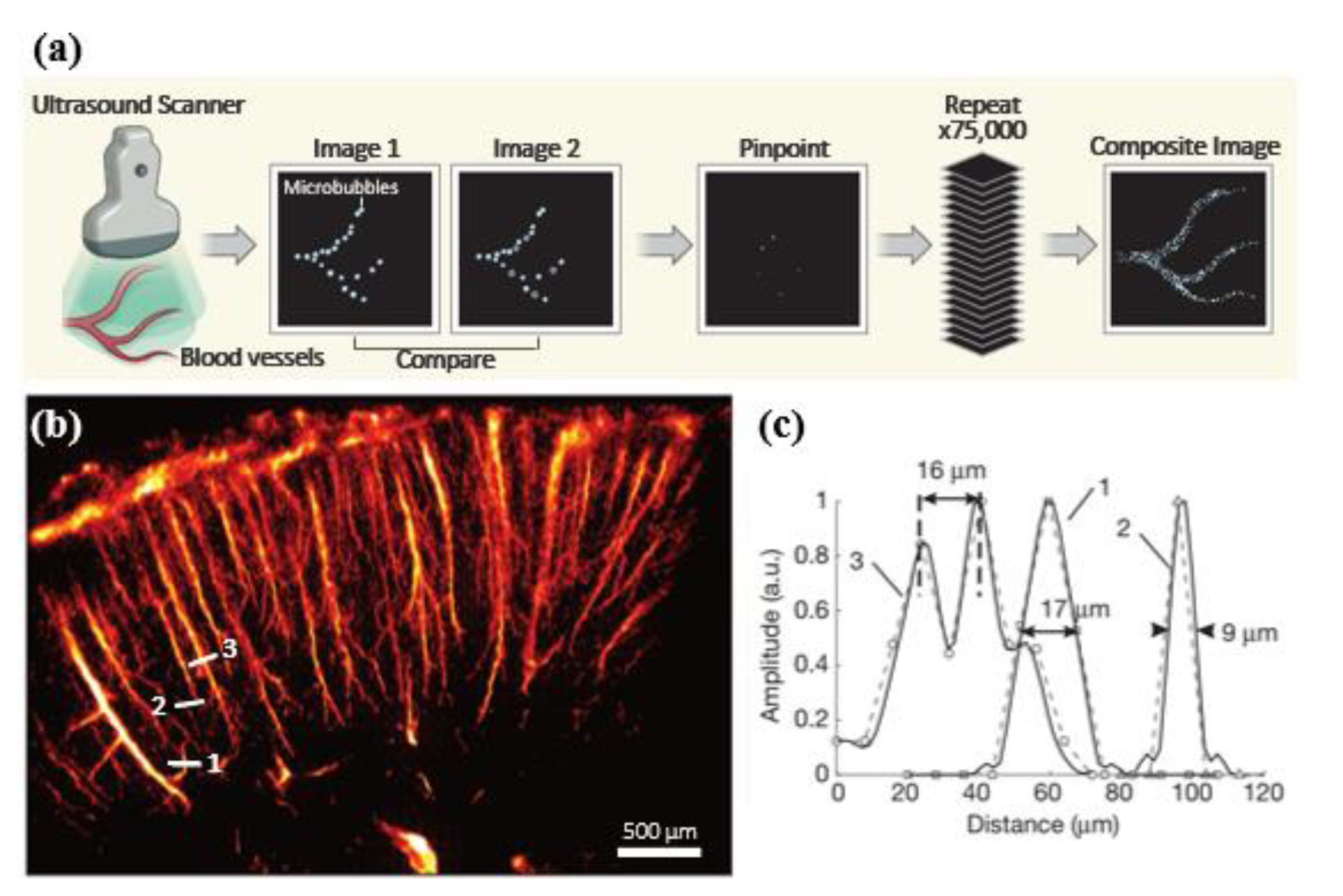
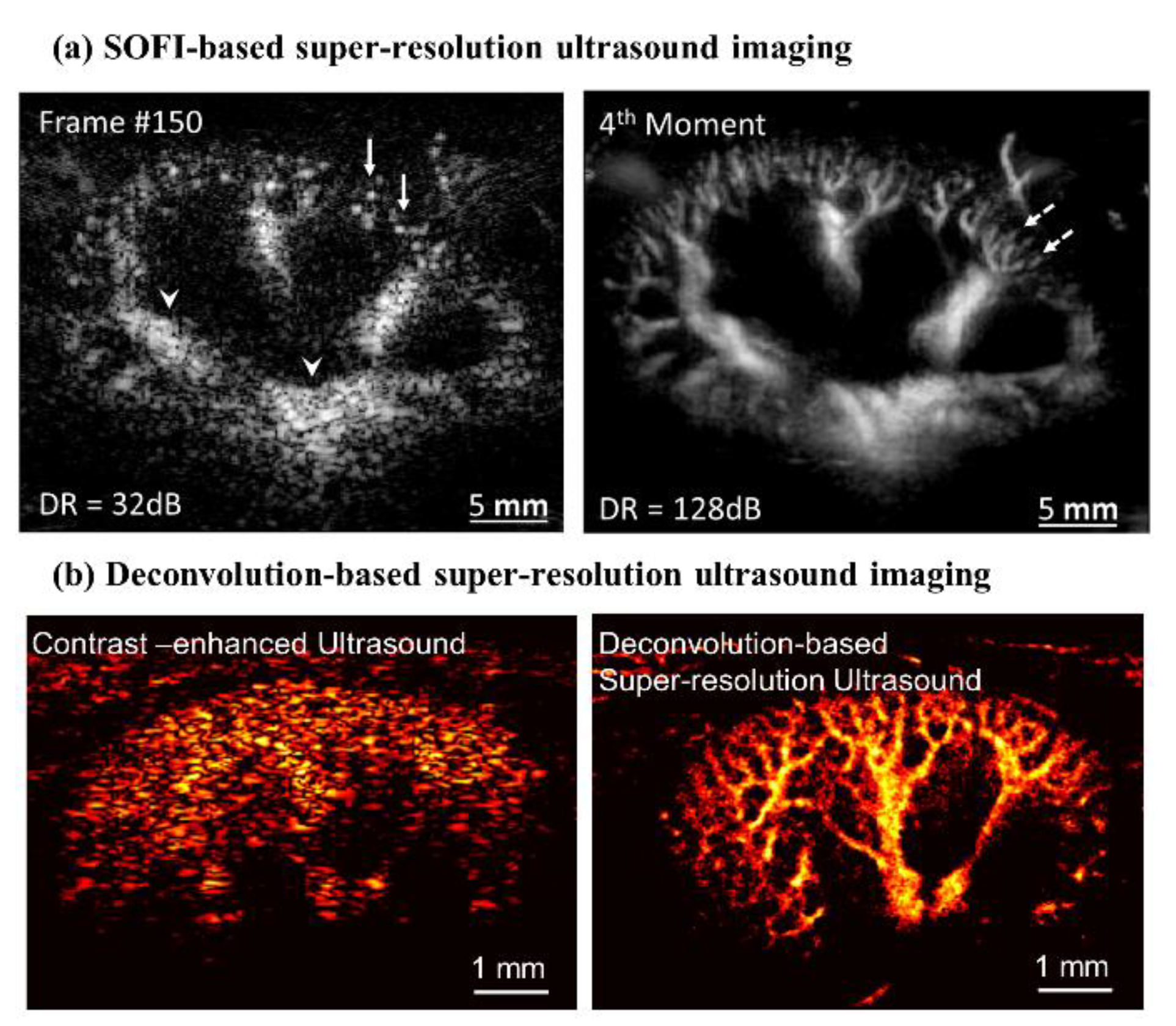
2. General Technical Components of Super-Resolution Ultrasound Imaging
3. Deep Learning-Based Super-Resolution Ultrasound Imaging
4. Current Biomedical Applications of Super-Resolution Ultrasound Imaging
4.1. Cancer
4.2. Kidney
4.3. Other Applications
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bullitt, E.; Lin, N.U.; Ewend, M.G.; Zeng, D.; Winer, E.P.; Carey, L.A.; Smith, J.K. Tumor therapeutic response and vessel tortuosity: Preliminary report in metastatic breast cancer. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4191, pp. 561–568. [Google Scholar]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; ADQI XIII Work Group. Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Hörbelt, M.; Lee, S.-Y.; Mang, H.E.; Knipe, N.L.; Sado, Y.; Kribben, A.; Sutton, T.A. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am. J. Physiol. Physiol. 2007, 293, F688–F695. [Google Scholar] [CrossRef] [PubMed]
- Molitoris, B.A. Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J. Clin. Investig. 2014, 124, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Kramann, R.; Tanaka, M.; Humphreys, B.D. Fluorescence Microangiography for Quantitative Assessment of Peritubular Capillary Changes after AKI in Mice. J. Am. Soc. Nephrol. 2014, 25, 1924–1931. [Google Scholar] [CrossRef]
- Ritman, E.L.; Lerman, A. The Dynamic Vasa Vasorum. Cardiovasc. Res. 2007, 75, 649–658. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Shi, G.-P. Vasa vasorum in atherosclerosis and clinical significance. Int. J. Mol. Sci. 2015, 16, 11574–11608. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Purushothaman, K.R.; Fuster, V.; Echeverri, D.; Truszczynska, H.; Sharma, S.K.; Badimon, J.J.; O’Connor, W.N. Plaque Neovascularization Is Increased in Ruptured Atherosclerotic Lesions of Human Aorta. Circulation 2004, 110, 2032–2038. [Google Scholar] [CrossRef]
- Hyafil, F.; Cornily, J.-C.; Feig, J.E.; Gordon, R.; Vucic, E.; Amirbekian, V.; Fisher, E.A.; Fuster, V.; Feldman, L.J.; Fayad, Z.A. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat. Med. 2007, 13, 636–641. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Glover, D.K.; Lanza, G.M.; Fayad, Z.A.; Johnson, L.L. Imaging Atherosclerosis and Vulnerable Plaque. J. Nucl. Med. 2010, 51, 51S–65S. [Google Scholar] [CrossRef]
- Kubo, T.; Imanishi, T.; Takarada, S.; Kuroi, A.; Ueno, S.; Yamano, T.; Tanimoto, T.; Matsuo, Y.; Masho, T.; Kitabata, H.; et al. Assessment of Culprit Lesion Morphology in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2007, 50, 933–939. [Google Scholar] [CrossRef]
- Jang, I.-K.; Tearney, G.J.; MacNeill, B.; Takano, M.; Moselewski, F.; Iftima, N.; Shishkov, M.; Houser, S.; Aretz, H.T.; Halpern, E.F.; et al. In Vivo Characterization of Coronary Atherosclerotic Plaque by Use of Optical Coherence Tomography. Circulation 2005, 111, 1551–1555. [Google Scholar] [CrossRef]
- Winter, P.M.; Morawski, A.M.; Caruthers, S.D.; Fuhrhop, R.W.; Zhang, H.; Williams, T.A.; Allen, J.S.; Lacy, E.K.; Robertson, J.D.; Lanza, G.M.; et al. Molecular Imaging of Angiogenesis in Early-Stage Atherosclerosis with αvβ3-Integrin–Targeted Nanoparticles. Circulation 2003, 108, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Caruthers, S.D.; Huang, W.; Williams, T.A.; Zhang, H.; Wickline, S.A.; Lanza, G.M.; Winter, P.M. MR molecular imaging of aortic angiogenesis. JACC. Cardiovasc. Imaging 2010, 3, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, W.; Hooker, A.; Spilker, M.; Vicini, P.; Ferguson, M.; Hatsukami, T.; Yuan, C. Quantitative Magnetic Resonance Imaging Analysis of Neovasculature Volume in Carotid Atherosclerotic Plaque. Circulation 2003, 107, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Molan, M.P.; Hornsey, E.; Bellomo, R. Measurement of renal blood flow by phase-contrast magnetic resonance imaging during septic acute kidney injury. Crit. Care Med. 2012, 40, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kozawa, E.; Okada, H.; Inukai, K.; Watanabe, S.; Kikuta, T.; Watanabe, Y.; Takenaka, T.; Katayama, S.; Tanaka, J.; et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J. Am. Soc. Nephrol. 2011, 22, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Faubel, S.; Patel, N.U.; Lockhart, M.E.; Cadnapaphornchai, M.A. Renal relevant radiology: Use of ultrasonography in patients with AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 382–394. [Google Scholar] [CrossRef]
- Cao, W.; Cui, S.; Yang, L.; Wu, C.; Liu, J.; Yang, F.; Liu, Y.; Bin, J.; Hou, F.F. Contrast-Enhanced Ultrasound for Assessing Renal Perfusion Impairment and Predicting Acute Kidney Injury to Chronic Kidney Disease Progression. Antioxid. Redox Signal. 2017, 27, 1397–1411. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A.; Hoyt, K. New Ultrasound Techniques Promise Further Advances in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Staub, D.; Schinkel, A.F.L.; Coll, B.; Coli, S.; van der Steen, A.F.W.; Reed, J.D.; Krueger, C.; Thomenius, K.E.; Adam, D.; Sijbrands, E.J.; et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: From early atherosclerosis to the identification of unstable plaques. JACC. Cardiovasc. Imaging 2010, 3, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Moguillansky, D.; Leng, X.; Carson, A.; Lavery, L.; Schwartz, A.; Chen, X.; Villanueva, F.S. Quantification of plaque neovascularization using contrast ultrasound: A histologic validation. Eur. Heart J. 2011, 32, 646–653. [Google Scholar] [CrossRef]
- Magnoni, M.; Coli, S.; Marrocco-Trischitta, M.M.; Melisurgo, G.; De Dominicis, D.; Cianflone, D.; Chiesa, R.; Feinstein, S.B.; Maseri, A. Contrast-enhanced ultrasound imaging of periadventitial vasa vasorum in human carotid arteries. Eur. J. Echocardiogr. 2008, 10, 260–264. [Google Scholar] [CrossRef]
- Hess, S.T.; Girirajan, T.P.K.; Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef]
- Errico, C.; Pierre, J.; Pezet, S.; Desailly, Y.; Lenkei, Z.; Couture, O.; Tanter, M. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 2015, 527, 499–502. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Hynynen, K. A super-resolution ultrasound method for brain vascular mapping. Med. Phys. 2013, 40. [Google Scholar] [CrossRef]
- Viessmann, O.M.; Eckersley, R.J.; Christensen-Jeffries, K.; Tang, M.X.; Dunsby, C. Acoustic super-resolution with ultrasound and microbubbles. Phys. Med. Biol. 2013, 58, 6447–6458. [Google Scholar] [CrossRef]
- Desailly, Y.; Couture, O.; Fink, M.; Tanter, M. Sono-activated ultrasound localization microscopy. Appl. Phys. Lett. 2013, 103, 174107. [Google Scholar] [CrossRef]
- Christensen-Jeffries, K.; Browning, R.J.; Tang, M.-X.; Dunsby, C.; Eckersley, R.J. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 2015, 34, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Demené, C.; Deffieux, T.; Pernot, M.; Osmanski, B.F.; Biran, V.; Gennisson, J.L.; Sieu, L.A.; Bergel, A.; Franqui, S.; Correas, J.M.; et al. Spatiotemporal Clutter Filtering of Ultrafast Ultrasound Data Highly Increases Doppler and fUltrasound Sensitivity. IEEE Trans. Med. Imaging 2015, 34, 2271–2285. [Google Scholar] [CrossRef]
- Yu, J.; Lavery, L.; Kim, K. Super-resolution ultrasound imaging method for microvasculature in vivo with a high temporal accuracy. Sci. Rep. 2018, 8, 13918. [Google Scholar] [CrossRef]
- Song, P.; Trzasko, J.D.; Manduca, A.; Huang, R.; Kadirvel, R.; Kallmes, D.F.; Chen, S. Improved Super-Resolution Ultrasound Microvessel Imaging with Spatiotemporal Nonlocal Means Filtering and Bipartite Graph-Based Microbubble Tracking. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Christensen-Jeffries, K.; Couture, O.; Dayton, P.A.; Eldar, Y.C.; Hynynen, K.; Kiessling, F.; O’Reilly, M.; Pinton, G.F.; Schmitz, G.; Tang, M.X.; et al. Super-resolution Ultrasound Imaging. Ultrasound Med. Biol. 2020, 46, 865–891. [Google Scholar] [CrossRef]
- Van Sloun, R.J.G.; Solomon, O.; Bruce, M.; Khaing, Z.Z.; Eldar, Y.C.; Mischi, M. Deep Learning for Super-resolution Vascular Ultrasound Imaging. In Proceedings of the International Conference on Acoustics, Speech and Signal Processing, Brighton, UK, 12–17 May 2019; pp. 1055–1059. [Google Scholar]
- Liu, X.; Zhou, T.; Lu, M.; Yang, Y.; He, Q.; Luo, J. Deep Learning for Ultrasound Localization Microscopy. IEEE Trans. Med. Imaging 2020, 39, 3064–3078. [Google Scholar] [CrossRef]
- Shi, W.; Caballero, J.; Huszár, F.; Totz, J.; Aitken, A.P.; Bishop, R.; Rueckert, D.; Wang, Z. Real-Time Single Image and Video Super-Resolution Using an Efficient Sub-Pixel Convolutional Neural Network; IEEE Computer Society: Washington, DC, USA; pp. 1874–1883.
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 26 June–1 July 2016; IEEE Computer Society: Washington, DC, USA, 2016; pp. 770–778. [Google Scholar]
- Bar-zion, A.; Tremblay-darveau, C.; Solomon, O.; Adam, D.; Eldar, Y.C. Fast VascularUltrasound Imaging with Enhanced Spatial Resolution and Background Rejection. IEEE Trans. Med. Imaging 2017, 36, 169–180. [Google Scholar] [CrossRef]
- Cox, B.; Beard, P. Super-resolution ultrasound. Nature 2015, 527, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, J.; Rush, B.M.; Stocker, S.D.; Tan, R.J.; Kim, K. Ultrasound super-resolution imaging provides a noninvasive assessment of renal microvasculature changes during mouse acute kidney injury. Kidney Int. 2020, 98, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C. The benefits of 3D-4D fetal echocardiography. Maedica 2010, 5, 45–50. [Google Scholar]
- Nehme, E.; Weiss, L.E.; Michaeli, T.; Shechtman, Y. Deep-STORM: Super-resolution single-molecule microscopy by deep learning. Optica 2018, 5, 458–464. [Google Scholar] [CrossRef]
- Van Sloun, R.J.G.; Solomon, O.; Bruce, M.; Khaing, Z.Z.; Wijkstra, H.; Eldar, Y.C.; Mischi, M. Super-resolution Ultrasound Localization Microscopy through Deep Learning. IEEE Trans. Med Imaging 2021. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-net: Convolutional networks for biomedical image segmentation. In Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2015; Volume 9351, pp. 234–241. [Google Scholar]
- Youn, J.; Ommen, M.L.; Stuart, M.B.; Thomsen, E.V.; Larsen, N.B.; Jensen, J.A. Detection and Localization of Ultrasound Scatterers Using Convolutional Neural Networks. IEEE Trans. Med. Imaging 2020, 39, 3855–3867. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.G.; Ghosh, D.; Hoyt, K. Deep Learning of Spatiotemporal Filtering for Fast Super-Resolution Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1820–1829. [Google Scholar] [CrossRef]
- Cancer. Available online: http://www.who.int/en/news-room/fact-sheets/detail/cancer (accessed on 30 September 2020).
- WHO|Early Detection of Cancer. Available online: https://www.who.int/cancer/detection/en/ (accessed on 30 September 2020).
- Folkman, J. Tumor angiogenesis. Adv. Cancer Res. 1974, 19, 331–358. [Google Scholar] [CrossRef]
- Augustin, H.G. Commentary on folkman: How is blood vessel growth regulated in normal and neoplastic tissue? Cancer Res. 2016, 76, 2854–2856. [Google Scholar] [CrossRef]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef]
- Ehling, J.; Theek, B.; Gremse, F.; Baetke, S.; Möckel, D.; Maynard, J.; Ricketts, S.A.; Grüll, H.; Neeman, M.; Knuechel, R.; et al. Micro-CT imaging of tumor angiogenesis: Quantitative measures describing micromorphology and vascularization. Am. J. Pathol. 2014, 184, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.P.; Jin, M.G.; Hyun, J.L.; Kim, M.A.; Kim, H.C.; Kwang, G.K.; Chang, H.L.; Im, J.G. FN13762 murine breast cancer: Region-by-region correlation of first-pass perfusion CT indexes with histologic vascular parameters. Radiology 2009, 251, 721–730. [Google Scholar] [CrossRef]
- Gessner, R.C.; Frederick, C.B.; Foster, F.S.; Dayton, P.A. Acoustic angiography: A new imaging modality for assessing microvasculature architecture. Int. J. Biomed. Imaging 2013, 2013. [Google Scholar] [CrossRef]
- Gessner, R.C.; Aylward, S.R.; Dayton, P.A. Mapping microvasculature with acoustic angiography yields quantifiable differences between healthy and tumor-bearing tissue volumes in a rodent model. Radiology 2012, 264, 733–740. [Google Scholar] [CrossRef]
- Shelton, S.E.; Lee, Y.Z.; Lee, M.; Cherin, E.; Foster, F.S.; Aylward, S.R.; Dayton, P.A. Quantification of microvascular tortuosity during tumor evolution using acoustic angiography. Ultrasound Med. Biol. 2015, 41, 1896–1904. [Google Scholar] [CrossRef]
- Shelton, S.E.; Lindsey, B.D.; Tsuruta, J.K.; Foster, F.S.; Dayton, P.A. Molecular Acoustic Angiography: A New Technique for High-resolution Superharmonic Ultrasound Molecular Imaging. Ultrasound Med. Biol. 2016, 42, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Shelton, S.E.; Dayton, P.A. The “Fingerprint” of Cancer Extends Beyond Solid Tumor Boundaries: Assessment with a Novel Ultrasound Imaging Approach. IEEE Trans. Biomed. Eng. 2016, 63, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, B.D.; Shelton, S.E.; Foster, F.S.; Dayton, P.A. Assessment of Molecular Acoustic Angiography for Combined Microvascular and Molecular Imaging in Preclinical Tumor Models. Mol. Imaging Biol. 2017, 19, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Rojas, J.D.; Dayton, P.A. Super resolution contrast ultrasound imaging: Analysis of imaging resolution and application to imaging tumor angiogenesis. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016. [Google Scholar]
- Lin, F.; Tsuruta, J.K.; Rojas, J.D.; Dayton, P.A. Optimizing Sensitivity of Ultrasound Contrast-Enhanced Super-Resolution Imaging by Tailoring Size Distribution of Microbubble Contrast Agent. Ultrasound Med. Biol. 2017, 43, 2488–2493. [Google Scholar] [CrossRef]
- Lin, F.; Shelton, S.E.; Espíndola, D.; Rojas, J.D.; Pinton, G.; Dayton, P.A. 3-D ultrasound localization microscopy for identifying microvascular morphology features of tumor angiogenesis at a resolution beyond the diffraction limit of conventional ultrasound. Theranostics 2017, 7, 196–204. [Google Scholar] [CrossRef]
- Opacic, T.; Dencks, S.; Theek, B.; Piepenbrock, M.; Ackermann, D.; Rix, A.; Lammers, T.; Stickeler, E.; Delorme, S.; Schmitz, G.; et al. Motion model ultrasound localization microscopy for preclinical and clinical multiparametric tumor characterization. Nat. Commun. 2018, 9, 1527. [Google Scholar] [CrossRef]
- Chacón, R.D.; Costanzo, M.V. Triple-negative breast cancer. Breast Cancer Res. 2010, 12, S3. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Xiong, F.; Sirsi, S.R.; Mattrey, R.; Brekken, R.; Kim, J.W.; Hoyt, K. Monitoring early tumor response to vascular targeted therapy using super-resolution ultrasound imaging. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Dencks, S.; Piepenbrock, M.; Opacic, T.; Krauspe, B.; Stickeler, E.; Kiessling, F.; Schmitz, G. Clinical Pilot Application of Super-Resolution US Imaging in Breast Cancer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 517–526. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. What Is Chronic Kidney Disease?|NIDDK. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd/what-is-chronic-kidney-disease (accessed on 12 October 2020).
- Division of Diabetes Translation; National Center for Chronic Disease Prevention and Health Promotion; Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet 2017; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Heung, M.; Chawla, L.S. Acute Kidney Injury: Gateway to Chronic Kidney Disease. Nephron Clin. Pract. 2014, 127, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Donohoe, D.; Roethe, K.; Osborn, J.L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Physiol. 2001, 281, F887–F899. [Google Scholar] [CrossRef]
- Tsuruoka, K.; Yasuda, T.; Koitabashi, K.; Yazawa, M.; Shimazaki, M.; Sakurada, T.; Shirai, S.; Shibagaki, Y.; Kimura, K.; Tsujimoto, F. Evaluation of renal microcirculation by contrast-enhanced ultrasound with sonazoidTM as a contrast agent: Comparison between normal subjects and patients with chronic kidney disease. Int. Heart J. 2010, 51, 176–182. [Google Scholar] [CrossRef]
- Couture, O.; Hingot, V.; Heiles, B.; Muleki-Seya, P.; Tanter, M. Ultrasound Localization Microscopy and Super-Resolution: A State of the Art. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1304–1320. [Google Scholar] [CrossRef]
- Foiret, J.; Zhang, H.; Ilovitsh, T.; Mahakian, L.; Tam, S.; Ferrara, K.W. Ultrasound localization microscopy to image and assess microvasculature in a rat kidney. Sci. Rep. 2017, 7, 13662. [Google Scholar] [CrossRef]
- Tang, S.; Song, P.; Trzasko, J.D.; Lowerison, M.; Huang, C.; Gong, P.; Lok, U.W.; Manduca, A.; Chen, S. Kalman Filter-Based Microbubble Tracking for Robust Super-Resolution Ultrasound Microvessel Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1738–1751. [Google Scholar] [CrossRef]
- Yang, Y.; He, Q.; Zhang, H.; Qiu, L.; Qian, L.; Lee, F.-F.; Liu, Z.; Luo, J. Assessment of Diabetic Kidney Disease Using Ultrasound Localization Microscopy: An In Vivo Feasibility Study in Rats. In Proceedings of the 2018 IEEE International Ultrasonics Symposium, Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Andersen, S.B.; Hoyos, C.A.V.; Taghavi, I.; Gran, F.; Hansen, K.L.; Sorensen, C.M.; Jensen, J.A.; Nielsen, M.B. Super-Resolution Ultrasound Imaging of Rat Kidneys before and after Ischemia-Reperfusion. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019. [Google Scholar]
- Chen, Q.; Kumar, A.; Tan, R.J.; Kim, K. Ultrasound Super-Resolution Imaging Algorithm for a Curved Array Transducer for Human Kidney Imaging. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; p. 1. [Google Scholar]
- Snyder, H.M.; Corriveau, R.A.; Craft, S.; Faber, J.E.; Greenberg, S.M.; Knopman, D.; Lamb, B.T.; Montine, T.J.; Nedergaard, M.; Schaffer, C.B.; et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010, 9, 689–701. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Huang, C.; Lowerison, M.R.; Trzasko, J.D.; Manduca, A.; Bresler, Y.; Tang, S.; Gong, P.; Lok, U.W.; Song, P.; Chen, S. Short Acquisition Time Super-Resolution Ultrasound Microvessel Imaging via Microbubble Separation. Sci. Rep. 2020, 10, 6007. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic Plaque Progression and Vulnerability to Rupture. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Gössl, M.; Versari, D.; Hildebrandt, H.A.; Bajanowski, T.; Sangiorgi, G.; Erbel, R.; Ritman, E.L.; Lerman, L.O.; Lerman, A. Segmental heterogeneity of vasa vasorum neovascularization in human coronary atherosclerosis. JACC. Cardiovasc. Imaging 2010, 3, 32–40. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, J.; Lukashova, L.; Latoche, J.D.; Zhu, J.; Lavery, L.; Verdelis, K.; Anderson, C.J.; Kim, K. Validation of Ultrasound Super-Resolution Imaging of Vasa Vasorum in Rabbit Atherosclerotic Plaques. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Rowland, E.M.; Harput, S.; Riemer, K.; Leow, C.H.; Clark, B.; Cox, K.; Lim, A.; Christensen-Jeffries, K.; Zhang, G.; et al. 3D Super-Resolution US Imaging of Rabbit Lymph Node Vasculature in Vivo by Using Microbubbles. Radiology 2019, 291, 642–650. [Google Scholar] [CrossRef]
- Qian, X.; Kang, H.; Li, R.; Lu, G.; Du, Z.; Shung, K.K.; Humayun, M.S.; Zhou, Q. In vivo Visualization of Eye Vasculature using Super-resolution Ultrasound Microvessel Imaging. IEEE Trans. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Q.; Yang, Y.; Liu, Z.; He, Q.; Wei, L.; Luo, J. Non-rigid Motion Correction for Ultrasound Localization Microscopy of the Liver in vivo. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Harput, S.; Christensen-Jeffries, K.; Brown, J.; Li, Y.; Williams, K.J.; Davies, A.H.; Eckersley, R.J.; Dunsby, C.; Tang, M.-X. Two-Stage Motion Correction for Super-Resolution Ultrasound Imaging in Human Lower Limb. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 803–814. [Google Scholar] [CrossRef]
- Hingot, V.; Errico, C.; Tanter, M.; Couture, O. Subwavelength motion-correction for ultrafast ultrasound localization microscopy. Ultrasonics 2017, 77, 17–21. [Google Scholar] [CrossRef]
- Bar-Zion, A.; Solomon, O.; Tremblay-Darveau, C.; Adam, D.; Eldar, Y.C. Sushi: Sparsity-based ultrasound super-resolution hemodynamic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 2365–2380. [Google Scholar] [CrossRef] [PubMed]
- Heiles, B.; Correia, M.; Hingot, V.; Pernot, M.; Provost, J.; Tanter, M.; Couture, O. Ultrafast 3D Ultrasound Localization Microscopy Using a 32 × 32 Matrix Array. IEEE Trans. Med. Imaging 2019, 38, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Harput, S.; Tortoli, P.; Eckersley, R.J.; Dunsby, C.; Tang, M.X.; Christensen-Jeffries, K.; Ramalli, A.; Brown, J.; Zhu, J.; Zhang, G.; et al. 3-D Super-Resolution Ultrasound Imaging with a 2-D Sparse Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 269–277. [Google Scholar] [CrossRef]
- Jensen, J.A.; Tomov, B.G.; Ommen, M.L.; Øygard, S.H.; Schou, M.; Sams, T.; Stuart, M.B.; Beers, C.; Thomsen, E.V.; Larsen, N.B. Three-Dimensional Super-Resolution Imaging Using a Row-Column Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 538–546. [Google Scholar] [CrossRef]
- Brown, J.; Christensen-Jeffries, K.; Harput, S.; Zhang, G.; Zhu, J.; Dunsby, C.; Tang, M.X.; Eckersley, R.J. Investigation of Microbubble Detection Methods for Super-Resolution Imaging of Microvasculature. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 676–691. [Google Scholar] [CrossRef]
- Bar-Zion, A.; Solomon, O.; Maresca, D.; Shapiro, M.G.; Eldar, Y.C. Towards Vascular Ultrasound Super-Resolution without Contrast Agents. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; p. 1. [Google Scholar]
- Boni, E.; Yu, A.C.H.; Freear, S.; Jensen, J.A.; Tortoli, P. Ultrasound open platforms for next-generation imaging technique development. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1078–1092. [Google Scholar] [CrossRef]
- Burshtein, A.; Birk, M.; Chernyakova, T.; Eilam, A.; Kempinski, A.; Eldar, Y.C. Sub-Nyquist Sampling and Fourier Domain Beamforming in Volumetric Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yoon, H.; Khalifa, Y.M.; Emelianov, S.Y. Design of a Volumetric Imaging Sequence Using a Vantage-256 Ultrasound Research Platform Multiplexed with a 1024-Element Fully Sampled Matrix Array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 248–257. [Google Scholar] [CrossRef]
- Yoon, H.; Song, T.K. Sparse rectangular and spiral array designs for 3D medical ultrasound imaging. Sensors 2020, 20, 173. [Google Scholar] [CrossRef]
- Rasmussen, M.F.; Christiansen, T.L.; Thomsen, E.V.; Jensen, J.A. 3-D imaging using row-column-addressed arrays with integrated apodization -Part i: Apodization design and line element beamforming. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 947–958. [Google Scholar] [CrossRef]
- O’Donnell, M. Coded excitation system for improving the penetration of real-time phased-array imaging systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, A.; Secomski, W.; Trots, I.; Litniewski, J. Extending penetration depth using coded ultrasonography. Bull. Pol. Acad. Sci. 2004, 52, 215–220. [Google Scholar]
- Principles, S.I.B.; Shen, J.; Member, S.; Ebbini, E.S. A New Coded-Excitation Ultrasound Imaging. Ultrason. Ferroelectr. Freq. Control IEEE Trans. 1996, 43, 131–140. [Google Scholar]
- Chiao, R.Y.; Hao, X. Coded excitation for diagnostic ultrasound: A system developer’s perspective. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 160–170. [Google Scholar] [CrossRef] [PubMed]






| Localization [32] | SOFI [43] | Deconvolution [36] | |
|---|---|---|---|
| Spatial Resolution * | High (9~17 µm) | Low (227.3 ± 9.0 µm) | Middle (41 µm) |
| Numbers of Frames for Reconstruction | 75,000 frames | 150 frames | 300 frames |
| Temporal Resolution | 150 s @ 500 Hz | 0.3 s @ 500 Hz | 0.6 s @ 500 Hz |
| Microbubble Concentration | Low (Diluted, 2 × 108 MBs/mL, Bolus Injection of 1.5 mL) | High (1.2 × 1010 MBs/mL, Bolus Injection of 0.5 mL) | High (1.2 × 1010 MBs/mL, Bolus Injection of 0.2 mL) |
| Application | Brain | Kidney | Atherosclerosis, Kidney |
| Deep-ULM [39] | CNN Based Network for Multiple Target Detection [50] | mSPCN-ULM [40] | Deep 3D CNN for Spatiotemporal Filtering [51] | |
|---|---|---|---|---|
| Target | Localization from the dense microbubbles | Localization from the dense microbubbles | Localization from the dense microbubbles | Microbubble extraction |
| Microbubble Concentration of Synthetic Data for Training | High (~2.6 MBs/) | High (~2.44 MBs/) | Very high (~6.4 MBs/) | N/A * |
| Network Type | U-net | Convolutional neural network | Modified subpixel convolutional neural network | 3-D convolutional neural network |
| Training Dataset | Synthetic data and unique data generated for each iteration | 10,240 synthetic data | 10,000 synthetic data | 9000 frames acquired from five subjects |
| Spatial Resolution | ~30 μm | 27~46 μm | 24~28 μm | 25 μm |
| Applied Activation Function | Leaky rectified linear unit (ReLU) | Leaky ReLU | ReLU | ReLU |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Song, H.; Yu, J.; Kim, K. Current Development and Applications of Super-Resolution Ultrasound Imaging. Sensors 2021, 21, 2417. https://doi.org/10.3390/s21072417
Chen Q, Song H, Yu J, Kim K. Current Development and Applications of Super-Resolution Ultrasound Imaging. Sensors. 2021; 21(7):2417. https://doi.org/10.3390/s21072417
Chicago/Turabian StyleChen, Qiyang, Hyeju Song, Jaesok Yu, and Kang Kim. 2021. "Current Development and Applications of Super-Resolution Ultrasound Imaging" Sensors 21, no. 7: 2417. https://doi.org/10.3390/s21072417
APA StyleChen, Q., Song, H., Yu, J., & Kim, K. (2021). Current Development and Applications of Super-Resolution Ultrasound Imaging. Sensors, 21(7), 2417. https://doi.org/10.3390/s21072417







