Nanostructures in Hydrogen Peroxide Sensing
Abstract
1. Introduction
2. Materials Used for Electrocatalytic Hydrogen Peroxide Sensing
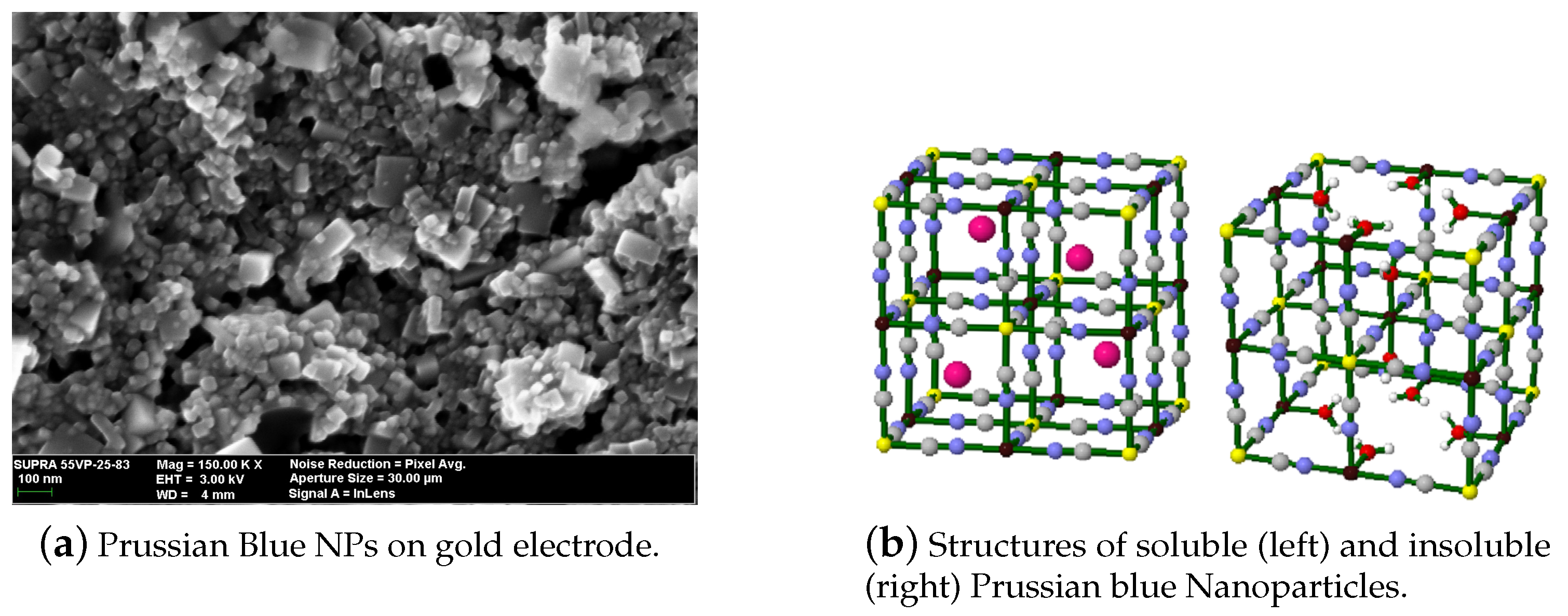
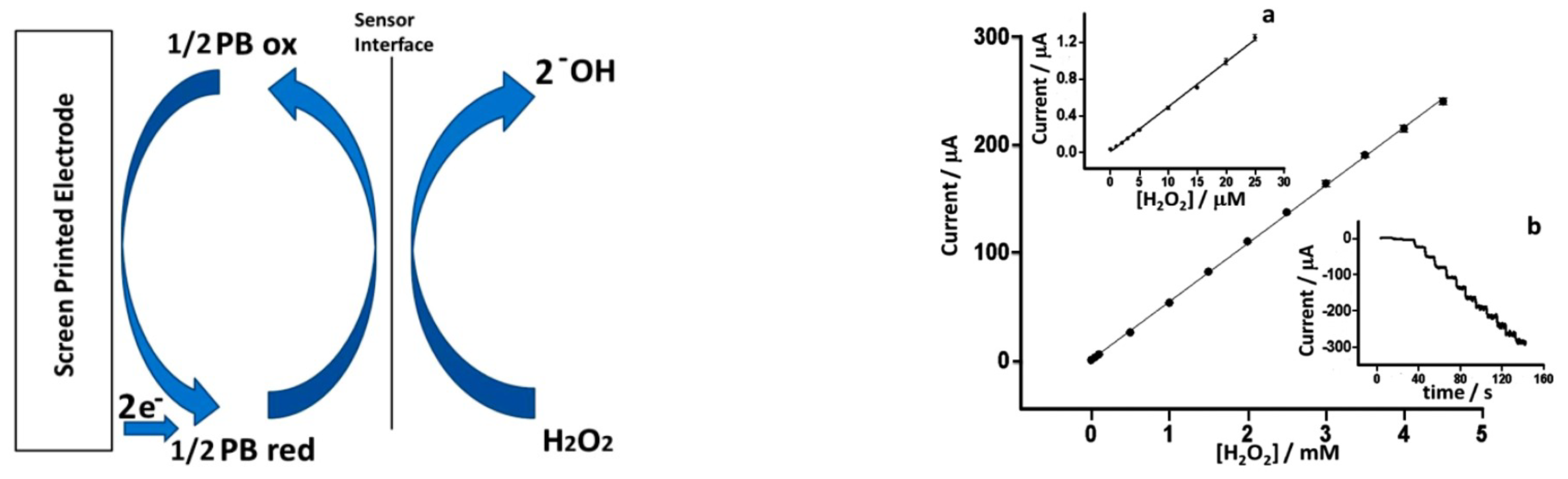
2.1. Metal Hexacyanoferrates
Other Metal Hexacyanoferrates
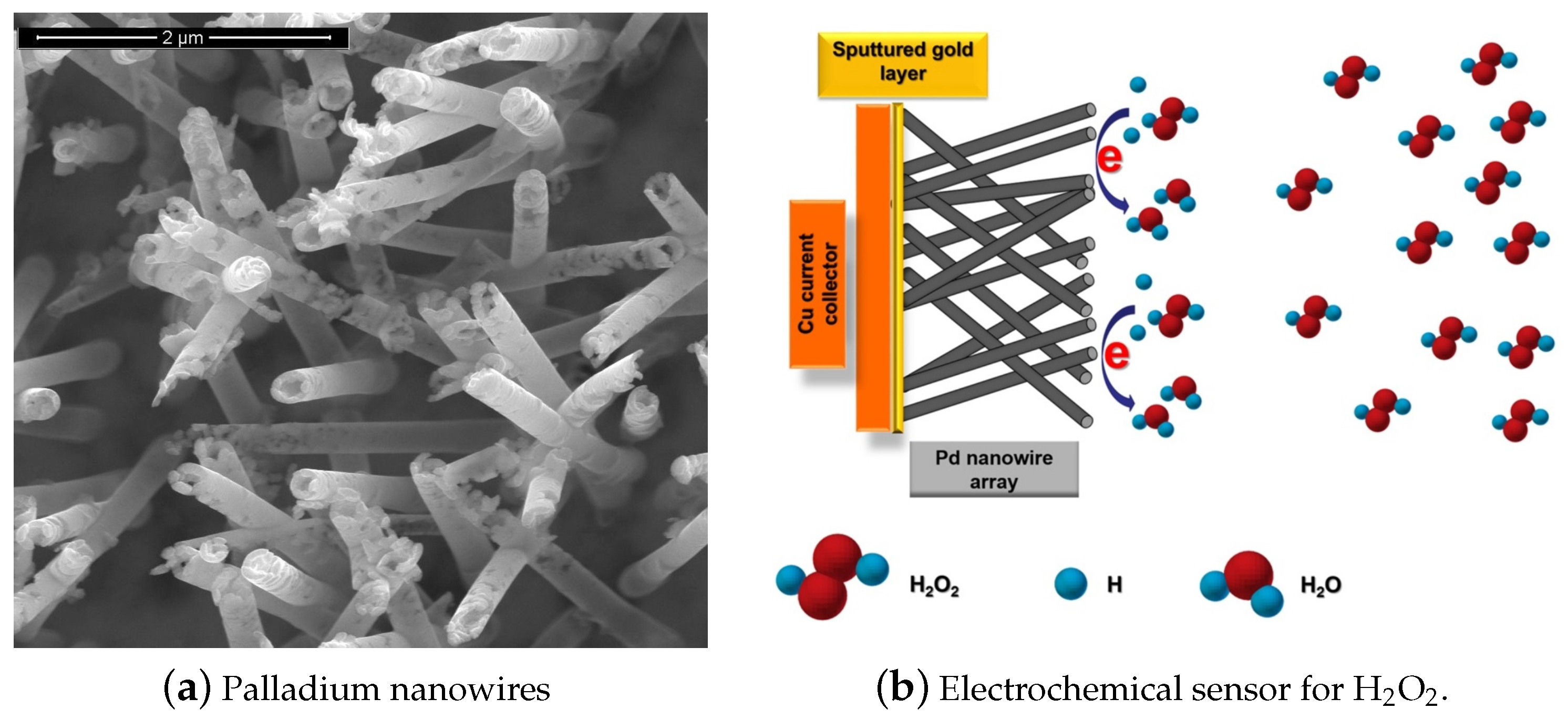
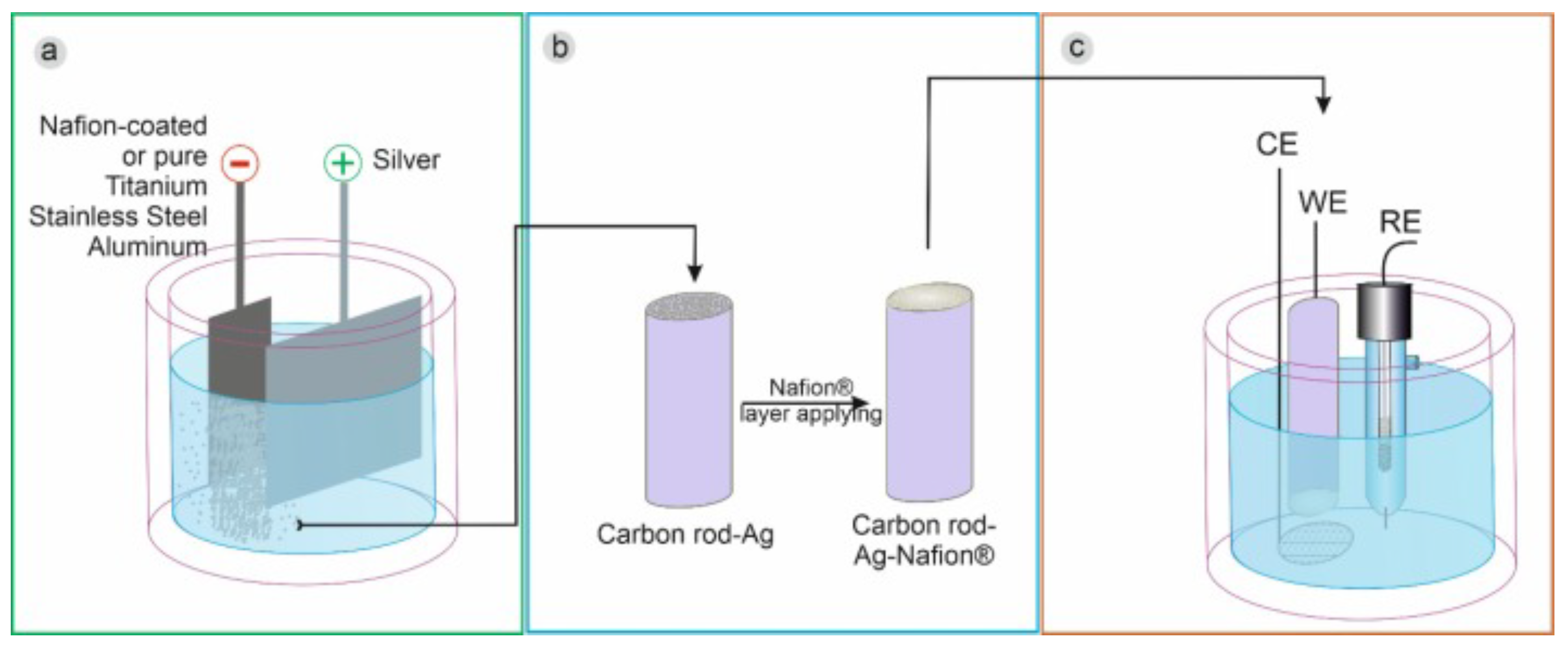
2.2. Metallic Nanostructures
2.3. Metal Oxide Nanostructures
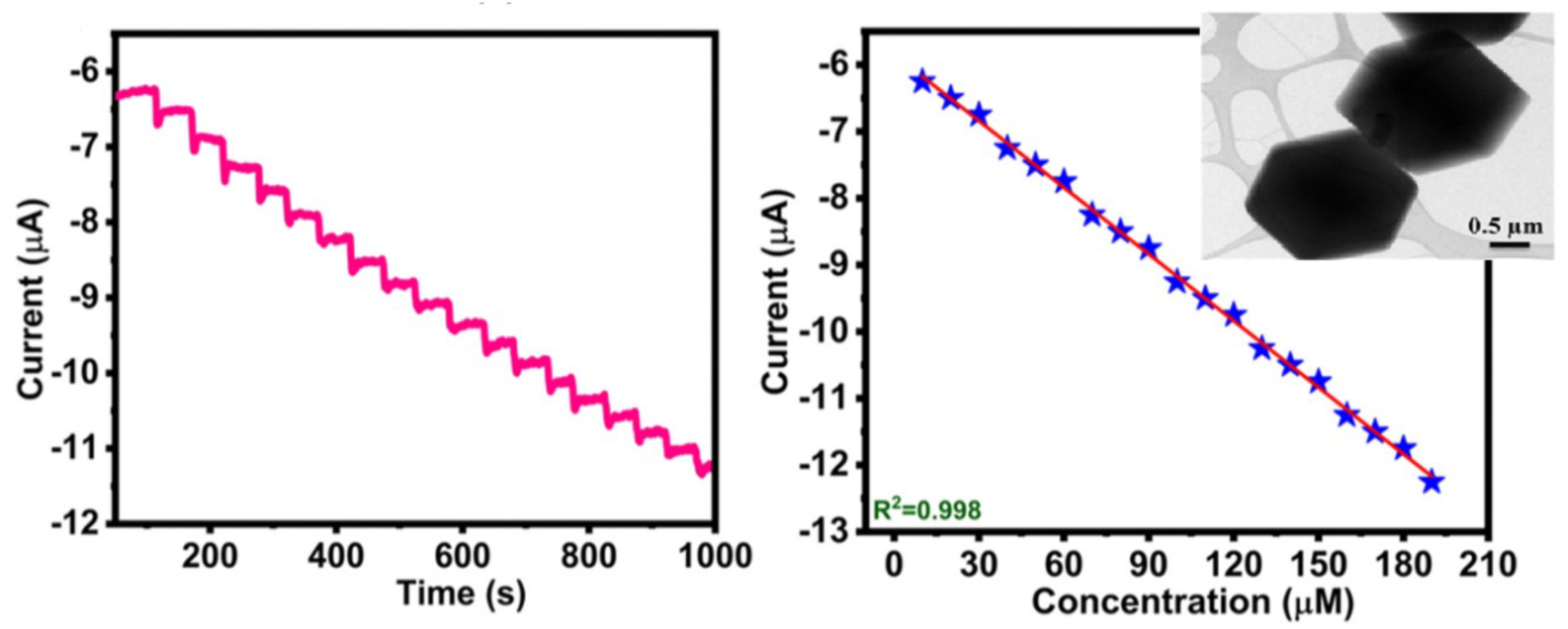
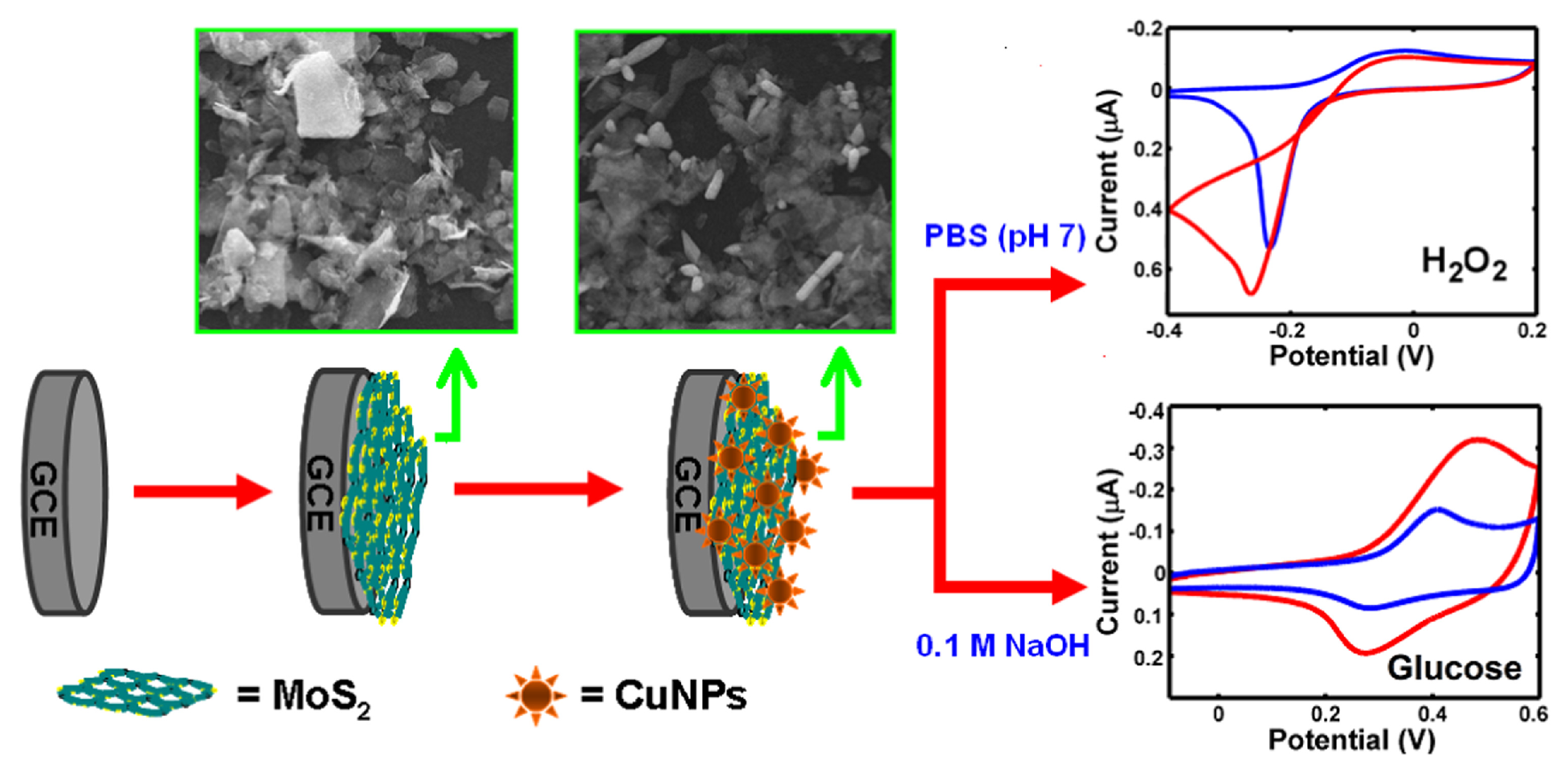
2.4. Mixed Nanostructures
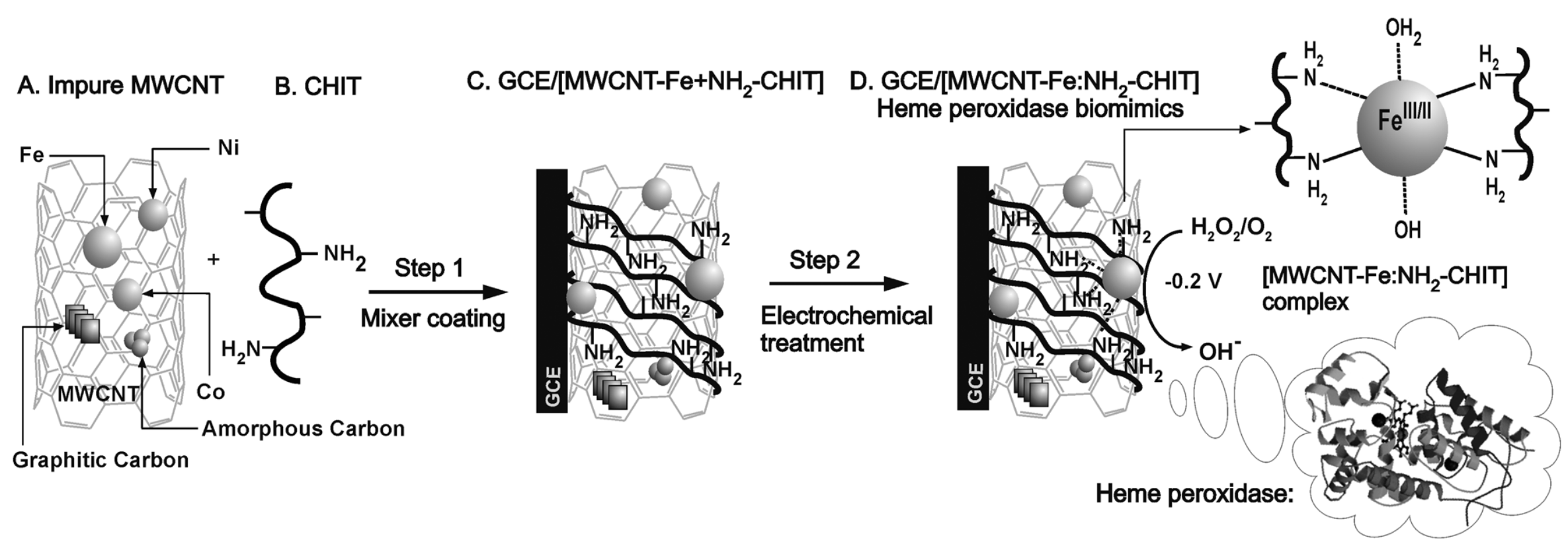
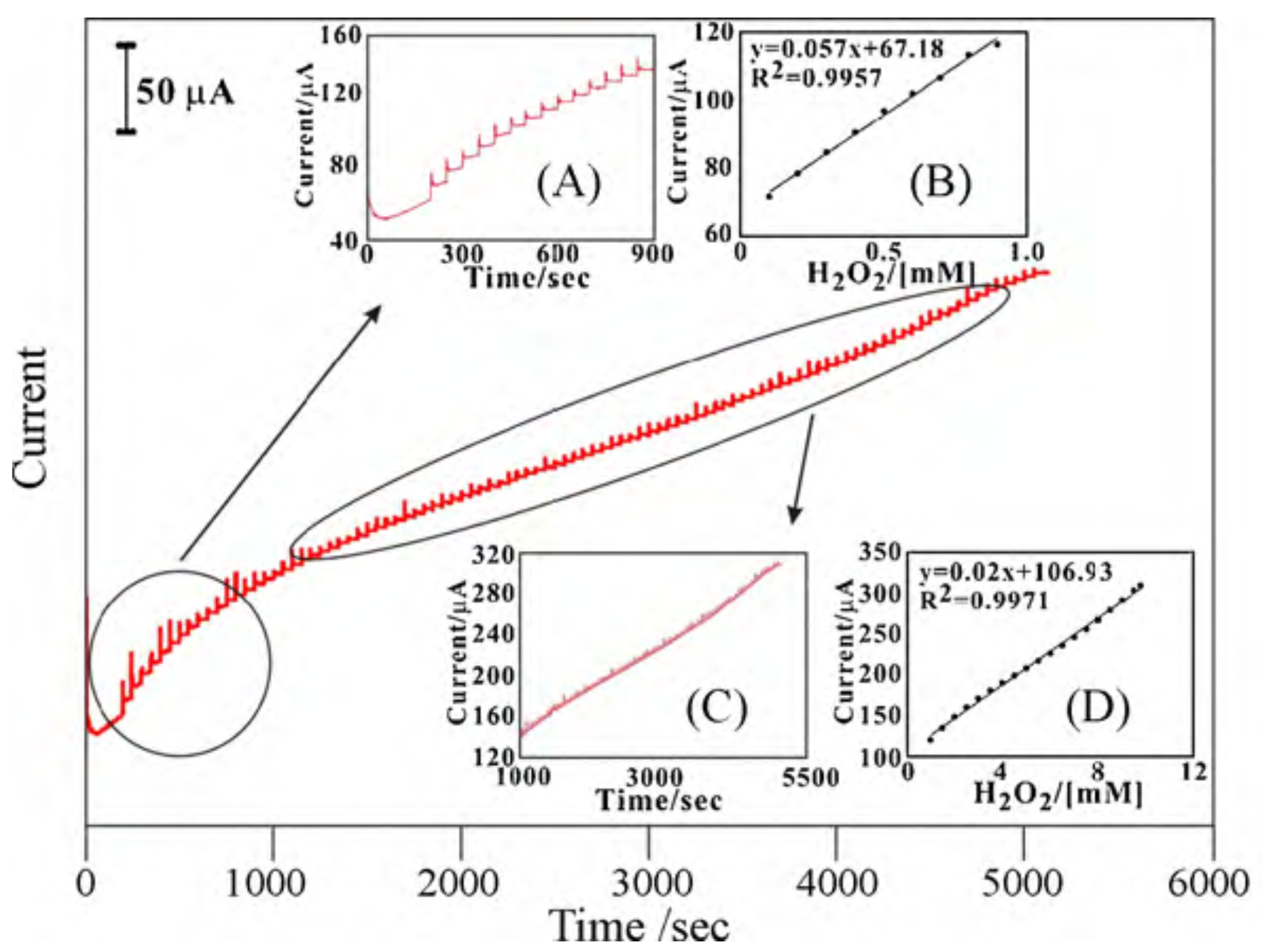
2.5. Biomolecules
| Nanomaterial | Transd. Princp. | Sensitivity A·mM·cm | LR [mM] | LOD M | Ref |
|---|---|---|---|---|---|
| Biom. (AgNPs) | CV | 3 × 10 | 0.05–20 × 10 | 0.02 | [107] |
| HRP-Osmium polymer | Amp | 470 × 10 | 1–500 × 10 | 0.3 | [110] |
| Mixed (SG) | – | 202 × 10 | – | 651.5 | [98] |
| Metal Hex. (PB@PtNPs/GF) | Amp | 40.9 × 10 | – | 1.2 × 10 | [25] |
| Biom. (Hb/FeO@Pt) | Amp. | 12 × 10 | 0.125 × 10–0.16 | 0.03 | [108] |
| Metalic (Ag NWs) | Amp | 4.705 × 10 | 50 × 10–10.35 | 10 | [9] |
| Metalic (nano Pd) | Amp | 1.42 × 10 | 1–14 × 10 | 1 | [50] |
| Metal Ox. (CoO NWs) | Amp | 1.14 × 10 | 0.015 to 0.675 | 2.4 | [72] |
| Metal Hex. (PBNPs) | Amp | 0.762 × 10 | 0–4.5 | 0.2 | [29] |
| Metal Ox. (-MnO/CNTs) | Amp | 243.9 | 0.05 to 22 | 1 | [71] |
| Mixed (CVDG) | – | 173 | – | 15.1 | [98] |
| Cyt c/Graphene FET | Amp | – | 100 × 10–100 × 10 | 100 × 10 | [111] |
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurdis, E.C.; Romeyn, H. Accuracy of Determination of Hydrogen Peroxide by Cerate Oxidimetry. Anal. Chem. 1954, 26, 320–325. [Google Scholar] [CrossRef]
- Matsubara, C.; Kawamoto, N.; Takamura, K. Oxo[5, 10, 15, 20-tetra(4-pyridyl)porphyrinato]titanium(IV): An ultra-high sensitivity spectrophotometric reagent for hydrogen peroxide. Analyst 1992, 117, 1781. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Xu, C.; Wang, L. Chemiluminescence flow biosensor for hydrogen peroxide using DNAzyme immobilized on eggshell membrane as a thermally stable biocatalyst. Biosens. Bioelectron. 2009, 24, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Tommons, C.; Bailey, R.T.; Tedford, M.C.; Crilly, P.J. Reversible, fluorescence-based optical sensor for hydrogen peroxide. Analyst 2007, 132, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Wada, M.; Kuroda, N.; Akiyama, S.; Imai, K. High-Performance Liquid Chromatographic Determination of Hydrogen Peroxide with Peroxyoxalate Chemiluminescence Detection. J. Liq. Chromatogr. 1994, 17, 2111–2126. [Google Scholar] [CrossRef]
- Garguilo, M.G.; Huynh, N.; Proctor, A.; Michael, A.C. Amperometric sensors for peroxide, choline, and acetylcholine based on electron transfer between horseradish peroxidase and a redox polymer. Anal. Chem. 1993, 65, 523–528. [Google Scholar] [CrossRef]
- Ahammad, A.J.S. Hydrogen Peroxide Biosensors Based on Horseradish Peroxidase and Hemoglobin. J. Biosens. Bioelectron. 2013, 1–11. [Google Scholar] [CrossRef]
- Patella, B.; Inguanta, R.; Piazza, S.; Sunseri, C. A nanostructured sensor of hydrogen peroxide. Sens. Actuators B Chem. 2017, 245, 44–54. [Google Scholar] [CrossRef]
- Hsiao, W.H.; Chen, H.Y.; Cheng, T.M.; Huang, T.K.; Chen, Y.L.; Lee, C.Y.; Chiu, H.T. Urchin-like Ag nanowires as non-enzymatic hydrogen peroxide sensor. J. Chin. Chem. Soc. 2012, 59, 500–506. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. Prussian-Blue-based amperometric biosensors in flow-injection analysis. Talanta 1996, 43, 1597–1606. [Google Scholar] [CrossRef]
- Li, N.; Su, X.; Lu, Y. Nanomaterial-based biosensors using dual transducing elements for solution phase detection. Analyst 2015, 140, 2916–2943. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. Assoc. Clin. Biochem. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar]
- Nossol, E.; Zarbin, A.J.G. A simple and innovative route to prepare a novel carbon nanotube/prussian blue electrode and its utilization as a highly sensitive H2O2 amperometric sensor. Adv. Funct. Mater. 2009, 19, 3980–3986. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhang, D.; Wu, Q.; Zheng, J.; Tang, H. Electrodeposition of Prussian blue nanoparticles on polyaniline coated halloysite nanotubes for nonenzymatic hydrogen peroxide sensing. Anal. Methods 2015, 7, 6896–6903. [Google Scholar] [CrossRef]
- Mattos, I.L.D.; Orton, L.G.; Uzgas, T.R.; Aryakin, A.A.K.; Química, D.D.; Ciências, F.D.; Box, P.O. Sensor for Hydrogen Peroxide Based on Prussian Blue Modified Electrode : Improvement of the Operational Stability. Anal. Sci. 2000, 16, 795–798. [Google Scholar] [CrossRef]
- Karyakin, A. Prussian blue-based first-generation biosensor. A sensitive amperometric electrode for glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Millward, R.C.; Madden, C.E.; Sutherland, I.; Mortimer, R.J.; Fletcher, S.; Marken, F. Directed assembly of multilayers—The case of Prussian Blue. Chem. Commun. 2001, 2, 1994–1995. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M. Prussian Blue Nanoparticles and its Analogues as New-Generation T1-Weighted MRI Contrast Agents for Cellular Imaging. Master’s Thesis, Kent State University, Kent, OH, USA, 2010. [Google Scholar]
- Buleandra, M.; Rabinca, A.A.; Mihailciuc, C.; Balan, A.; Nichita, C.; Stamatin, I.; Ciucu, A.A. Screen-printed Prussian Blue modified electrode for simultaneous detection of hydroquinone and catechol. Sens. Actuators B Chem. 2014, 203, 824–832. [Google Scholar] [CrossRef]
- Lin, M.; Yang, J.; Cho, M.; Lee, Y. Hydrogen peroxide detection using a polypyrrole/Prussian blue nanowire modified electrode. Macromol. Res. 2011, 19, 673–678. [Google Scholar] [CrossRef]
- Ricci, F.; Palleschi, G.; Yigzaw, Y.; Gorton, L.; Ruzgas, T.; Karyakin, A. Investigation of the effect of different glassy carbon materials on the performance of Prussian Blue based sensors for hydrogen peroxide. Electroanalysis 2003, 15, 175–182. [Google Scholar] [CrossRef]
- Garjonyte, R.; Malinauskas, A. Operational stability of amperometric hydrogen peroxide sensors, based on ferrous and copper hexacyanoferrates. Sens. Actuators B Chem. 1999, 56, 93–97. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem. Commun. 1999, 1, 78–82. [Google Scholar] [CrossRef]
- Malinauskas, A.; Araminaite, R.; Mickevičiute, G.; Garjonyte, R. Evaluation of operational stability of Prussian blue- and cobalt hexacyanoferrate-based amperometric hydrogen peroxide sensors for biosensing application. Mater. Sci. Eng. C 2004, 24, 513–519. [Google Scholar] [CrossRef]
- Han, L.; Tricard, S.; Fang, J.; Zhao, J.; Shen, W. Prussian blue @ platinum nanoparticles/graphite felt nanocomposite electrodes: Application as hydrogen peroxide sensor. Biosens. Bioelectron. 2013, 43, 120–124. [Google Scholar] [CrossRef]
- Zen, J.M.; Senthil Kumar, A.; Chung, C.R. A glucose biosensor employing a stable artificial peroxidase based on ruthenium purple anchored cinder. Anal. Chem. 2003, 75, 2703–2709. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Zhai, Y.; Liu, H.; Li, L. Prussian Blue/Reduced Graphene Oxide Composite for the Amperometric Determination of Dopamine and Hydrogen Peroxide. Anal. Lett. 2015, 48, 2786–2798. [Google Scholar] [CrossRef]
- Ni, P.; Zhang, Y.; Sun, Y.; Shi, Y.; Dai, H.; Hu, J.; Li, Z. Facile synthesis of Prussian blue @ gold nanocomposite for nonenzymatic detection of hydrogen peroxide. RSC Adv. 2013, 3, 15987. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Killard, A.J. Development of a hydrogen peroxide sensor based on screen-printed electrodes modified with inkjet-printed Prussian blue nanoparticles. Sensors 2014, 14, 14222–14234. [Google Scholar] [CrossRef]
- Pandey, S. Applications of Ionic Liquids in Spectroscopy. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 1–12. [Google Scholar] [CrossRef]
- Abbot, A.P.; Ryder, K.; Peter, L.; Taylor, A.W. Ionic Liquids Completely UnCOILed: Critical Expert Overviews. In Ionic Liquids Completely UnCOILed: Critical Expert Overviews, 1st ed.; Plechkova, N.V., Seddon, K.R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Zhu, X.; Niu, X.; Zhao, H.; Lan, M. Doping ionic liquid into Prussian blue-multiwalled carbon nanotubes modified screen-printed electrode to enhance the nonenzymatic H2O2 sensing performance. Sens. Actuators B Chem. 2014, 195, 274–280. [Google Scholar] [CrossRef]
- Tria, S.A.; Lopez-Ferber, D.; Gonzalez, C.; Bazin, I.; Guiseppi-Elie, A. Microfabricated biosensor for the simultaneous amperometric and luminescence detection and monitoring of Ochratoxin A. Biosens. Bioelectron. 2016, 79, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, D.M.; Hocevar, S.B.; McAdams, E.T.; Ogorevc, B.; Zhang, X. Nickel hexacyanoferrate modified screen-printed carbon electrode for sensitive detection of ascorbic acid and hydrogen peroxide. Front. Biosci. 2005, 10, 483–491. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Q.; Li, H. A novel photoelectrochemical hydrogen peroxide sensor based on nickel(II)-potassium hexacyanoferrate-graphene hybrid materials modified n-silicon electrode. J. Solid State Electrochem. 2016, 20, 1565–1573. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, G.; Zhang, X.; Fang, B. Amperometric detection of hydrogen peroxide using glassy carbon electrodes modified with chromium hexacyanoferrate/single-walled carbon nanotube nanocomposites. Electroanalysis 2009, 21, 179–183. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Wang, G.; Zhao, J.; Hu, M.; Qu, L. A novel nonenzymatic H2O2 sensor based on cobalt hexacyanoferrate nanoparticles and graphene composite modified electrode. Sens. Actuators B Chem. 2015, 208, 593–599. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.; Tricard, S.; Liu, J.; Fang, J.; Zhao, J.; Shen, W. Amperometric detection of hydrogen peroxide utilizing synergistic action of cobalt hexacyanoferrate and carbon nanotubes chemically modified with platinum nanoparticles. RSC Adv. 2013, 3, 281–287. [Google Scholar] [CrossRef]
- De Mattos, I.L.; Gorton, L.; Laurell, T.; Malinauskas, A.; Karyakin, A.A. Development of biosensors based on hexacyanoferrates. Talanta 2000, 52, 791–799. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Ghica, M.E.; Gouveia-Caridade, C.; Oliveira, O.N.; Brett, C.M.A. Enzyme immobilisation on electroactive nanostructured membranes (ENM): Optimised architectures for biosensing. Talanta 2008, 76, 922–928. [Google Scholar] [CrossRef]
- Mayorga Martinez, C.C.; Treo, E.F.; Madrid, R.E.; Felice, C.C. Evaluation of chrono-impedance technique as transduction method for a carbon paste/glucose oxidase (CP/GOx) based glucose biosensor. Biosens. Bioelectron. 2010, 26, 1239–1244. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, W.; Zeng, J.; Liu, X.; Zeng, X. Fabrication of a copper nanoparticle/chitosan/carbon nanotube-modified glassy carbon electrode for electrochemical sensing of hydrogen peroxide and glucose. Microchim. Acta 2008, 160, 253–260. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Wang, M.; Chatrathi, M. Carbon-nanotube/copper composite electrodes for capillary electrophoresis microchip detection of carbohydrates. Analyst 2004, 129, 512–515. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Lu, X.; Zhou, Z.; Xiao, D. All solid-state pH electrode based on titanium nitride sensitive film. Electroanalysis 2006, 18, 1493–1498. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Gu, L.; Zhang, L.; Zhou, X.; Liu, Z.; Han, P.; Xu, H.; Yao, J.; Zhang, X.; et al. A biocompatible titanium nitride nanorods derived nanostructured electrode for biosensing and bioelectrochemical energy conversion. Biosens. Bioelectron. 2011, 26, 4088–4094. [Google Scholar] [CrossRef]
- Hrapovic, S.; Liu, Y.; Male, K.B.; Luong, J.H.T. Electrochemical Biosensing Platforms Using Platinum Nanoparticles and Carbon Nanotubes. Anal. Chem. 2004, 76, 1083–1088. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, L.; Cai, Q. An electro-catalytic biosensor fabricated with Pt-Au nanoparticle-decorated titania nanotube array. Bioelectrochemistry 2008, 74, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, Z.; Yang, Y.; Zhang, W.; Wang, Q. Low-potential sensitive hydrogen peroxide detection based on nanotubular TiO2 and platinum composite electrode. Electroanalysis 2008, 20, 970–975. [Google Scholar] [CrossRef]
- Chakraborty, S.; Retna Raj, C. Pt nanoparticle-based highly sensitive platform for the enzyme-free amperometric sensing of H2O2. Biosens. Bioelectron. 2009, 24, 3264–3268. [Google Scholar] [CrossRef]
- Gutes, A.; Laboriante, I.; Carraro, C.; Maboudian, R. Palladium nanostructures from galvanic displacement as hydrogen peroxide sensor. Sens. Actuators B Chem. 2010, 147, 681–686. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Cai, D.; Zhang, S.; Zhang, B.; Tang, J.; Wu, Z. A hydrogen peroxide sensor based on Ag nanoparticles electrodeposited on natural nano-structure attapulgite modified glassy carbon electrode. Talanta 2011, 86, 266–270. [Google Scholar] [CrossRef]
- Lu, W.; Chang, G.; Luo, Y.; Liao, F.; Sun, X. Method for effective immobilization of Ag nanoparticles/graphene oxide composites on single-stranded DNA modified gold electrode for enzymeless H2O2 detection. J. Mater. Sci. 2011, 46, 5260–5266. [Google Scholar] [CrossRef]
- Zhang, Y.; Janyasupab, M.; Liu, C.W.; Lin, P.Y.; Wang, K.W.; Xu, J.; Liu, C.C. Improvement of amperometric biosensor performance for H2O2 detection based on Bbimetallic PtM (M = Ru, Au, and Ir) nanoparticles. Int. J. Electrochem. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Lee, J.H.; Huynh-Nguyen, B.C.; Ko, E.; Kim, J.H.; Seong, G.H. Fabrication of flexible, transparent silver nanowire electrodes for amperometric detection of hydrogen peroxide. Sens. Actuators B Chem. 2016, 224, 789–797. [Google Scholar] [CrossRef]
- Brzozka, A.; Brudzisz, A.; Jele, A.; Wes, J.; Iwaniec, M.; Sulka, G.D. A comparative study of electrocatalytic reduction of hydrogen peroxide at carbon rod electrodes decorated with silver particles. Mater. Sci. Eng. B 2021, 263. [Google Scholar] [CrossRef]
- Liua, Q.; Zhangb, T.; Yuc, L.; Nengqin, J.D.P.Y. 3D Nanoporous Ag@BSA Composite Microspheres As Hydrogen Peroxide Sensor. R. Soc. Chem. 2013, 138, 5559–5562. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, X.; Wang, W.; Liu, C.; Li, Z.; Zhang, Z. Fabrication of TiN nanostructure as a hydrogen peroxide sensor by oblique angle deposition. Nanoscale Res. Lett. 2014, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Sophia, J.; Muralidharan, G. Amperometric sensing of hydrogen peroxide using glassy carbon electrode modified with copper nanoparticles. Mater. Res. Bull. 2015, 70, 315–320. [Google Scholar] [CrossRef]
- Li, S.J. A Novel Enzyme-Free Hydrogen Peroxide Sensor Based on Electrode Modified with Gold Nanoparticles-Overoxidized Polydopamine Composites. Int. J. Electrochem. Sci. 2016, 11, 2887–2896. [Google Scholar] [CrossRef]
- Cernat, A.; Petica, A.; Anastasoaie, V.; Lazar, O.; Szabolcs, J.; Irimes, M.B.; Stefan, G.; Tertis, M.; Enachescu, M.; Anic, L.; et al. Detection of hydrogen peroxide involving bismuth nanowires via template-free electrochemical synthesis using deep eutectic solvents. Electrochem. Commun. 2020, 121, 5. [Google Scholar] [CrossRef]
- Hira, A.S.; Annas, D.; Nagappan, S.; Anil, Y.; Song, S.; Kim, H.j.; Park, S.; Hyun, K. Electrochemical sensor based on nitrogen-enriched metal – organic framework for selective and sensitive detection of hydrazine and hydrogen peroxide. J. Environ. Chem. Eng. 2021, 9, 105182. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lan, W.J.; Chen, C.H. Redox preparation of mixed-valence cobalt manganese oxide nanostructured materials: Highly efficient noble metal-free electrocatalysts for sensing hydrogen peroxide. Nanoscale 2014, 6, 334–341. [Google Scholar] [CrossRef]
- Shu, X.; Chen, Y.; Yuan, H.; Gao, S.; Xiao, D. H2O2 Sensor Based on the Room-Temperature Phosphorescence of Nano TiO2/SiO2 Composite. Anal. Chem. 2007, 79, 3695–3702. [Google Scholar] [CrossRef]
- Sivalingam, D.; Gopalakrishnan, J.B.; Krishnan, U.M.; Madanagurusamy, S.; Rayappan, J.B.B. Nanostructured ZnO thin film for hydrogen peroxide sensing. Phys. Low Dimens. Syst. Nanostruc. 2011, 43, 1804–1808. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; Zhang, X.; Yang, L. Facile synthesis of flower like copper oxide and their application to hydrogen peroxide and nitrite sensing. Chem. Cent. J. 2011, 5, 75. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, Y.; Wang, X.; Zhao, G. Direct electrocatalytic oxidation of nitric oxide and reduction of hydrogen peroxide based on alpha-Fe2O3 nanoparticles-chitosan composite. Talanta 2010, 82, 196–201. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Ni, Y.; Li, J.; Liao, K.; Zhao, G. Porous cuprous oxide microcubes for non-enzymatic amperometric hydrogen peroxide and glucose sensing. Electrochem. Commun. 2009, 11, 812–815. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, B.; Xu, Y.; Wu, D. Hexagonal [small alpha]-Fe2O3 nanorods bound by high-index facets as high-performance electrochemical sensor. J. Mater. Chem. A 2013, 1, 3040–3046. [Google Scholar] [CrossRef]
- Li, L.; Du, Z.; Liu, S.; Hao, Q.; Wang, Y.; Li, Q.; Wang, T. A novel nonenzymatic hydrogen peroxide sensor based on MnO2/graphene oxide nanocomposite. Talanta 2010, 82, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- He, S.J.; Zhang, B.Y.; Liu, M.M.; Chen, W. Non-enzymatic hydrogen peroxide electrochemical sensor based on a three-dimensional MnO2 nanosheets/carbon foam composite. Rsc Adv. 2014, 4, 49315–49323. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. A novel δ-MnO2 with carbon nanotubes nanocomposite as an enzyme-free sensor for hydrogen peroxide electrosensing. RSC Adv. 2016, 6, 50572–50580. [Google Scholar] [CrossRef]
- Kong, L.; Ren, Z.; Zheng, N.; Du, S.; Wu, J.; Tang, J.; Fu, H. Interconnected 1D Co3O4 nanowires on reduced graphene oxide for enzymeless H2O2 detection. Nano Res. 2015, 8, 469–480. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Z.; Yin, L.; Zhu, Y.; Zhao, J.; Zhu, B.; Zheng, L.; Jin, Q.; Wang, L. A novel self-powered bioelectrochemical sensor based on CoMn2O4 nanoparticle modified cathode for sensitive and rapid detection of hydrogen peroxide. Sens. Actuators B Chem. 2018, 271, 247–255. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, L.; Zhang, J.; Dai, W.; Mo, G.; Ye, J. Bifunctional and highly sensitive electrochemical non-enzymatic glucose and hydrogen peroxide biosensor based on NiCo2O4 nano fl owers decorated 3D nitrogen doped holey graphene hydrogel. Mater. Sci. Eng. C 2019, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Loh, P.Y.; Sow, C.H.; Chin, W.S. CoOOH nanosheet electrodes: Simple fabrication for sensitive electrochemical sensing of hydrogen peroxide and hydrazine. Biosens. Bioelectron. 2013, 39, 255–260. [Google Scholar] [CrossRef]
- Mai, L.N.T.; Bui, Q.B.; Bach, L.G.; Nhac-vu, H. A novel nanohybrid of cobalt oxide-sulfide nanosheets deposited three-dimensional foam as efficient sensor for hydrogen peroxide detection. J. Electroanal. Chem. 2020, 857, 113757. [Google Scholar] [CrossRef]
- Khan, S.B.; Rahman, M.M.; Asiri, A.M.; Asif, S.A.B.; Al-Qarni, S.A.S.; Al-Sehemi, A.G.; Al-Sayari, S.A.; Al-Assiri, M.S. Fabrication of non-enzymatic sensor using Co doped ZnO nanoparticles as a marker of H2O2. Phys. E Low Dimens. Syst. Nanostruct. 2014, 62, 21–27. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.C.; Gucciardi, E.; Cavallaro, S. Lubricants: Properties and characteristics. In Biolubricants; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 24–73. [Google Scholar] [CrossRef]
- Drogman, M.; Drogman, D. 2D Nanoelectronics; Springer: Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. Electrochemical and bio-sensing platform based on a novel 3D Cu nano-flowers/layered MoS2 composite. Biosens. Bioelectron. 2016, 79, 685–692. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor based on ultrasmall MoS2 nanoparticles for electrochemical detection of H2O2 released by cells at the nanomolar level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Bohlooli, F.; Yamatogi, A.; Mori, S. Manganese oxides/carbon nanowall nanocomposite electrode as an efficient non-enzymatic electrochemical sensor for hydrogen peroxide. Sens. Bio-Sens. Res. 2021, 31, 100392. [Google Scholar] [CrossRef]
- Sankarasubramanian, K.; Babu, K.J.; Soundarrajan, P.; Logu, T.; Gnanakumar, G. A new catalyst Ti doped CdO thin film for non-enzymatic hydrogen peroxide sensor application. Sens. Actuators B Chem. 2019, 285, 164–172. [Google Scholar] [CrossRef]
- Chang, Y.J.; Periasamy, A.P.; Chen, S.M. Poly (toluidine blue) and zirconia nanoparticles electrochemically deposited at gelatin-multiwalled carbon nanotube film for amperometric H2O2 sensor. Int. J. Electrochem. Sci. 2011, 6, 4188–4203. [Google Scholar]
- Huang, J.; Wang, K.; Wei, Z. Conducting polymernanowire arrays with enhanced electrochemical performance. J. Mater. Chem. 2010, 20, 1117–1121. [Google Scholar] [CrossRef]
- Guerrero-Bermea, C.; Rajukumar, L.P.; Dasgupta, A.; Lei, Y.; Hashimoto, Y.; Sepulveda-Guzman, S.; Cruz-Silva, R.; Endo, M.; Terrones, M. Two-dimensional and three-dimensional hybrid assemblies based on graphene oxide and other layered structures: A carbon science perspective. Carbon 2017, 125, 437–453. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020. [Google Scholar] [CrossRef]
- Lecouvet, B.; Sclavons, M.; Bourbigot, S.; Devaux, J.; Bailly, C. Water-assisted extrusion as a novel processing route to prepare polypropylene/halloysite nanotube nanocomposites: Structure and properties. Polymer 2011, 52, 4284–4295. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, X.; Liu, J.; Guo, H.; Liu, C. Highly sensitive H2O2 sensor based on Co3O4 hollow sphere prepared via a template-free method. Electrochim. Acta 2015, 182, 613–620. [Google Scholar] [CrossRef]
- Dimcheva, N. Nanostructures of noble metals as functional materials in biosensors. Curr. Opin. Electrochem. 2020, 19, 35–41. [Google Scholar] [CrossRef]
- Subramoney, S. Carbon Nanotubes. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–8. [Google Scholar]
- Pandey, P.; Dahiya, M. Carbon Nanotubes: Types, Methods of Preparation and Applications. Int. J. Pharm. Sci. Res. 2016, 1, 15–21. [Google Scholar] [CrossRef]
- Mintmire, J.W.; White, C.T. Electronic and structural properties of carbon nanotubes. Carbon 1995, 33, 893–902. [Google Scholar] [CrossRef]
- Gayathri, P.; Kumar, A.S. An iron impurity in multiwalled carbon nanotube complexes with chitosan that biomimics the heme-peroxidase function. Chem. A Eur. J. 2013, 19, 17103–17112. [Google Scholar] [CrossRef]
- Lin, K.C.; Tsai, T.H.; Chen, S.M. Performing enzyme-free H2O2 biosensor and simultaneous determination for AA, DA, and UA by MWCNT-PEDOT film. Biosens. Bioelectron. 2010, 26, 608–614. [Google Scholar] [CrossRef]
- Shan, C.; Wang, L.; Han, D.; Li, F.; Zhang, Q.; Zhang, X.; Niu, L. Polyethyleneimine-functionalized graphene and its layer-by-layer assembly with Prussian blue. Thin Solid Films 2013, 534, 572–576. [Google Scholar] [CrossRef]
- Zöpfl, A.; Sisakthi, M.; Eroms, J.; Matysik, F.M.; Strunk, C.; Hirsch, T. Signal enhancement in amperometric peroxide detection by using graphene materials with low number of defects. Microchim. Acta 2016, 183, 83–90. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.S.; Pessoa, C.A.; Mello, L.D.; Kubota, L.T. Direct electron transfer: An approach for electrochemical biosensors with higher selectivity and sensitivity. J. Braz. Chem. Soc. 2003, 14, 230–243. [Google Scholar] [CrossRef]
- Ruzgas, T.; Csöregi, E.; Emnéus, J.; Gorton, L.; Marko-Varga, G. Peroxidase-modified electrodes: Fundamentals and application. Anal. Chim. Acta 1996, 330, 123–138. [Google Scholar] [CrossRef]
- Prakash, P.A.; Yogeswaran, U.; Chen, S.M. A review on direct electrochemistry of catalase for electrochemical sensors. Sensors 2009, 9, 1821–1844. [Google Scholar] [CrossRef]
- Adediran, S.A.; Lambeir, A. Kinetics of the reaction of compound II of horseradish peroxidase with hydrogen peroxide to form compound III. Eur. J. Biochem. 1989, 186, 571–576. [Google Scholar] [CrossRef]
- Huang, R.; Hu, N. Direct electrochemistry and electrocatalysis with horseradish peroxidase in Eastman AQ films. Bioelectrochemistry 2001, 54, 75–81. [Google Scholar] [CrossRef]
- Yan, R.; Zhao, F.; Li, J.; Xiao, F.; Fan, S.; Zeng, B. Direct electrochemistry of horseradish peroxidase in gelatin-hydrophobic ionic liquid gel films. Electrochim. Acta 2007, 52, 7425–7431. [Google Scholar] [CrossRef]
- Yao, Y.; Wen, Y.; Zhang, L.; Xu, J.; Wang, Z.; Duan, X. A stable sandwich-type hydrogen peroxide sensor based on immobilizing horseradish peroxidase to a silver nanoparticle monolayer supported by PEDOT:PSS-nafion composite electrode. Int. J. Electrochem. Sci. 2013, 8, 9348–9359. [Google Scholar]
- Yu, C.; Wang, Y.; Wang, L.; Zhu, Z.; Bao, N.; Gu, H. Nanostructured biosensors built with layer-by-layer electrostatic assembly of hemoglobin and Fe3O4@Pt nanoparticles. Colloids Surfaces Biointerfaces 2013, 103, 231–237. [Google Scholar] [CrossRef]
- Nandini, S.; Nalini, S.; Niranjana, P.; Melo, J.S.; Suresh, G.S. Metal-ion co-ordination assembly based multilayer of one-dimensional, gold nanostructures and catalase as electrochemical sensor for the analysis of hydrogen peroxide. Sens. Actuators B Chem. 2017, 245, 726–740. [Google Scholar] [CrossRef]
- Bollella, P.; Medici, L.; Tessema, M.; Poloznikov, A.A.; Hushpulian, D.M.; Tishkov, V.I.; Andreu, R.; Leech, D.; Megersa, N.; Marcaccio, M. Highly sensitive, stable and selective hydrogen peroxide amperometric biosensors based on peroxidases from different sources wired by Os-polymer: A comparative study. Solid State Ionics 2018, 314, 178–186. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.H.; Seo, S.E.; il Kim, M.; Park, S.J.; Kwon, O.S. Cytochrome C-decorated graphene field-effect transistor for highly sensitive hydrogen peroxide detection. J. Ind. Eng. Chem. 2020, 83, 29–34. [Google Scholar] [CrossRef]
- Nieto, C.H.D.; Granero, A.M.; Lopez, J.C.; Pierini, G.D.; Levin, G.J.; Fernandez, H.; Zon, M.A. Development of a third generation biosensor to determine hydrogen peroxide based on a composite of soybean peroxidase/chemically reduced graphene oxide deposited on glassy carbon electrodes. Sens. Actuators B Chem. 2018. [Google Scholar] [CrossRef]
- Murphy, M.; Theyagarajan, K.; Prabusankar, G.; Senthilkumar, S. Electrochemical biosensor for the detection of hydrogen peroxide using cytochrome c covalently immobilized on carboxyl functionalized ionic liquid/multiwalled carbon nanotube hybrid. Appl. Surf. Sci. 2019, 492, 718–725. [Google Scholar] [CrossRef]
- Fatima, B.; Hussain, D.; Bashir, S.; Aslam, R.; Naeem, M.; Najam-ul haq, M. Catalase immobilized antimonene quantum dots used as an electrochemical biosensor for quantitative determination of H2O2 from CA-125 diagnosed ovarian cancer samples. Mater. Sci. Eng. C 2020, 117. [Google Scholar] [CrossRef] [PubMed]







| Nanomaterial | Transd. Princp. | Sensitivity [A·mM·cm] | LR [mM] | LOD [M] | Ref |
|---|---|---|---|---|---|
| PB@PtNPs/GF | Amp | 40.9 × 10 | – | 1.2 × 10 | [25] |
| PB NPs | Amp | 0.762 × 10 | 0–4.5 | 0.2 | [29] |
| PB-MWCNTs | Amp | 0.436 × 10 | 5–1645 × 10 | 0.35 | [32] |
| PB | Amp | 0.35 × 10 | up to 2.5 | 50 | [39] |
| PB | CV | 0.3 × 10 | – | 0.5 | [15] |
| GC-R/PB | Amp | 0.25 × 10 | 50 × 10–10 | 0.1 | [21] |
| PB@Au NPs | Amp | 39.72 | 2 × 10–8.56 | 0.1 | [28] |
| PB-PPy NWs | Amp | 10 | 0.2 × 10–7.2 | – | [20] |
| NIHCF-GS | Photocurrent | 3.53 | 2.0 × 10–2.3 | 1.0 | [35] |
| CuHCF | 2.34 | up to 10 | 250 | [39] | |
| PB-PANI-HNTs | Amp | 0.98 | 4–1064 × 10 | 0.226 | [14] |
| ENM | Amp | 0.237 | up to 100 × 10 | 6.1 | [40] |
| PB-RGO | Amp | 0.1617 | 0.5 × 10–0.7 | – | [27] |
| CPE/CFe*-RP | Amp | – | up to 0.8 | 33 × 10 | [26] |
| CoHCNFPs-GR | Amp | – | 0.6–379.5 × 10 | 0.1 | [37] |
| PB | Amp | – | 1 × 10–10 | 1 | [16] |
| NiHCF | CV | – | 0.2 × 10–1.5 | 1.2 | [34] |
| CrHCF-SWNTs | Amp | – | 0.5 × 10–10 | – | [36] |
| Nanomaterial | Transd. Princp. | Sensitivity [A·mM·cm] | LR [mM] | LOD [M] | Ref |
|---|---|---|---|---|---|
| Ag NWs | Amp | 4.705 × 10 | 50 × 10–10 | 10 | [9] |
| nano Pd | Amp | 1.42 × 10 | 1–14 × 10 | 1 | [50] |
| Ag NWs | Amp | 749 | 0.2 to 1.5 | 46 | [54] |
| PtRu | Amp | 539.01 | – | 1.7 | [53] |
| PtAu | 415.46 | – | 2.0 | [53] | |
| PtIr | 404.52 | – | 0.8 | [53] | |
| C@Ag-Ps | Amp | 128 | – | 100 | [55] |
| Au NPs | Amp | 52.94 | 10 × 10–8 | 0.5 | [59] |
| Pt NPs | Amp | 9.15 | 0.5 × 10–4 | 500 | [49] |
| TiN nanofilm | Amp | 3.99 | 2 × 10–3 | – | [57] |
| Pt NPs/SWCNT | Amp | 3.57 | 25 × 10–10 × 10 | 25 | [46] |
| Au/Pt NPs | Amp | 2.92 | 10–80 × 10 | 10 | [47] |
| Pt/TiO | Amp | 0.85 | 4 × 10–1.25 | 4 | [48] |
| Ag NPs/ATP | Amp | – | 10 × 10–21.53 | 2.4 | [51] |
| Ag NPs/GO | Amp | – | 0.1–20 | 1.9 | [52] |
| Cu NPs/Chi/CNT | Amp | – | 0.05–12 | 20 | [42] |
| Cu NPs | Amp | – | 8–70 × 10 | 3.4 | [58] |
| TiN NRs | CV | – | 0.5 × 10–2 | 0.5 | [45] |
| Nanomaterial | Transd. Princp. | Sensitivity A·mM·cm | LR [mM] | LOD M | Ref |
|---|---|---|---|---|---|
| NHGH/NiCoO | Amp | 2072 | 1–510 × 10 | 0.136 | [74] |
| CoO NWs | Amp | 1.14 × 10 | 0.015–0.675 | 2.4 | [72] |
| MnO/CNW | Amp | 698.6 | 40 × 10–10 | 0.55 | [83] |
| -MnO/CNTs | Amp | 243.9 | 0.05–22 | 1 | [71] |
| CoOOH nanosheets | Amp | 99 | up to 1.6 | 40 | [75] |
| Co doped ZnO NPs | Amp | 92.4 | – | 14.3 | [77] |
| ZrO NPs | Amp | 82.13 | 0.05–0.25 | – | [85] |
| PTBO/GCNT | |||||
| CuO | Amp | 50.6 | up to 1.5 | 1.5 | [67] |
| MnO | Amp | 38.2 | 5–600 × 10 | 0.8 | [69] |
| FeO NPs | Amp | 21.62 | 1.0–44.0 × 10 | 0.4 | [66] |
| CoMnO@GE | Amp | 13.2 | 1–1000 | 40.2 | [73] |
| TiO/SiO | Phosphorescence | – | 7.0 × 10–70 | – | [63] |
| CuO | Amp | – | 5.0–180.0 × 10 | 1.6 | [65] |
| -FeO NRs | CV | – | 40 × 10–4.66 | – | [68] |
| MoS NPs | Amp | – | 10 × LOD | 2.5 × 10 | [82] |
| cobalt manganese oxide | Amp | – | 0.1 to 25 | 15 | [62] |
| MnO | Amp | – | 2.5 × 10–2.05 | 12 | [70] |
| CuNFs/MoS | Amp | – | 0.04–1.88 × 10 | 0.021 | [81] |
| CoO-CoS/NF | Amp | 0.059 | 2–254 × 10 | 0.89 | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo, R.M.; Barraza, D.E.; Zamora, M.L.; Cattani-Scholz, A.; Madrid, R.E. Nanostructures in Hydrogen Peroxide Sensing. Sensors 2021, 21, 2204. https://doi.org/10.3390/s21062204
Trujillo RM, Barraza DE, Zamora ML, Cattani-Scholz A, Madrid RE. Nanostructures in Hydrogen Peroxide Sensing. Sensors. 2021; 21(6):2204. https://doi.org/10.3390/s21062204
Chicago/Turabian StyleTrujillo, Ricardo Matias, Daniela Estefanía Barraza, Martin Lucas Zamora, Anna Cattani-Scholz, and Rossana Elena Madrid. 2021. "Nanostructures in Hydrogen Peroxide Sensing" Sensors 21, no. 6: 2204. https://doi.org/10.3390/s21062204
APA StyleTrujillo, R. M., Barraza, D. E., Zamora, M. L., Cattani-Scholz, A., & Madrid, R. E. (2021). Nanostructures in Hydrogen Peroxide Sensing. Sensors, 21(6), 2204. https://doi.org/10.3390/s21062204








