Design and Analysis of a Continuous and Non-Invasive Multi-Wavelength Optical Sensor for Measurement of Dermal Water Content †
Abstract
1. Introduction
2. Materials and Methods
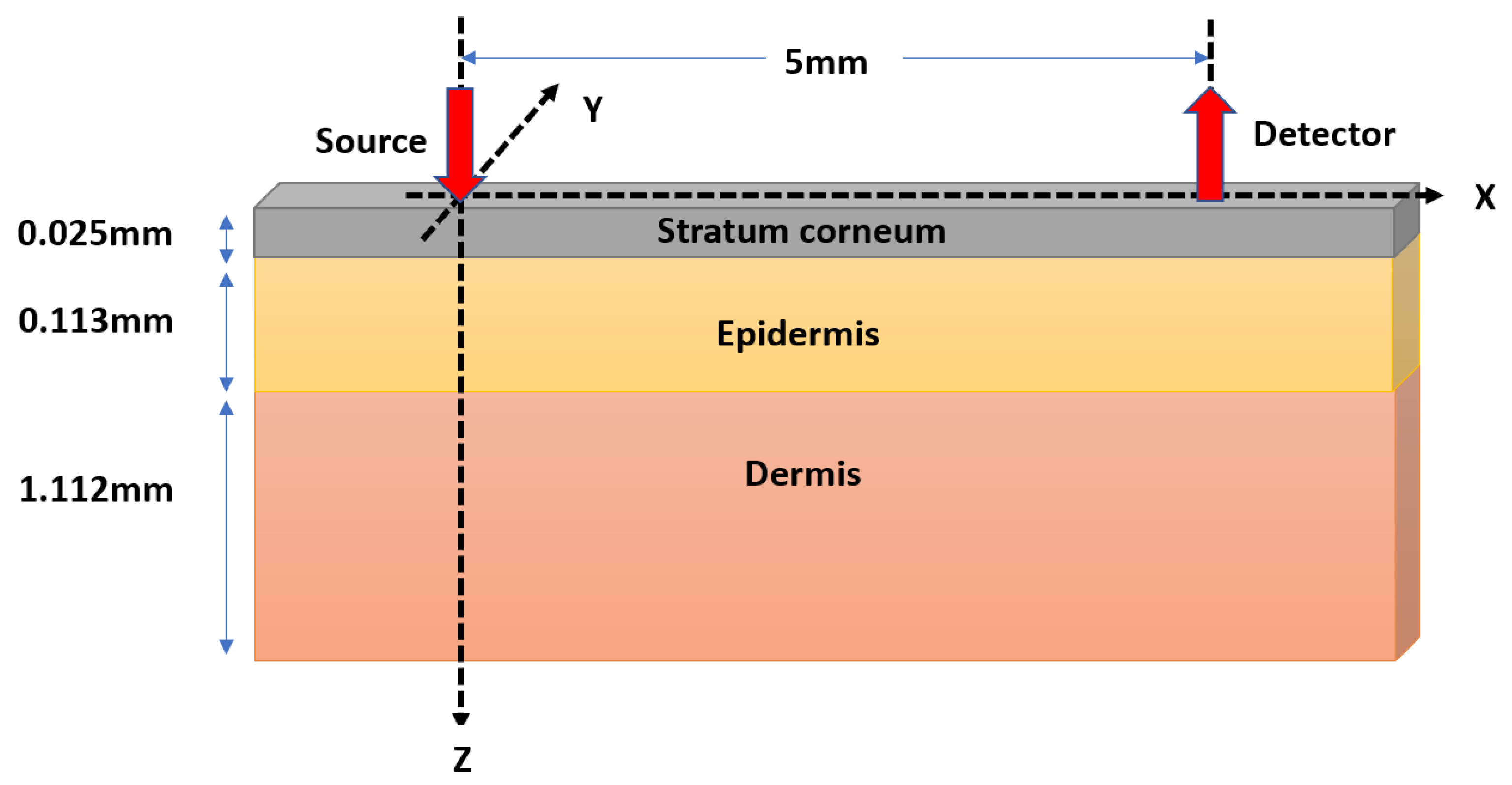
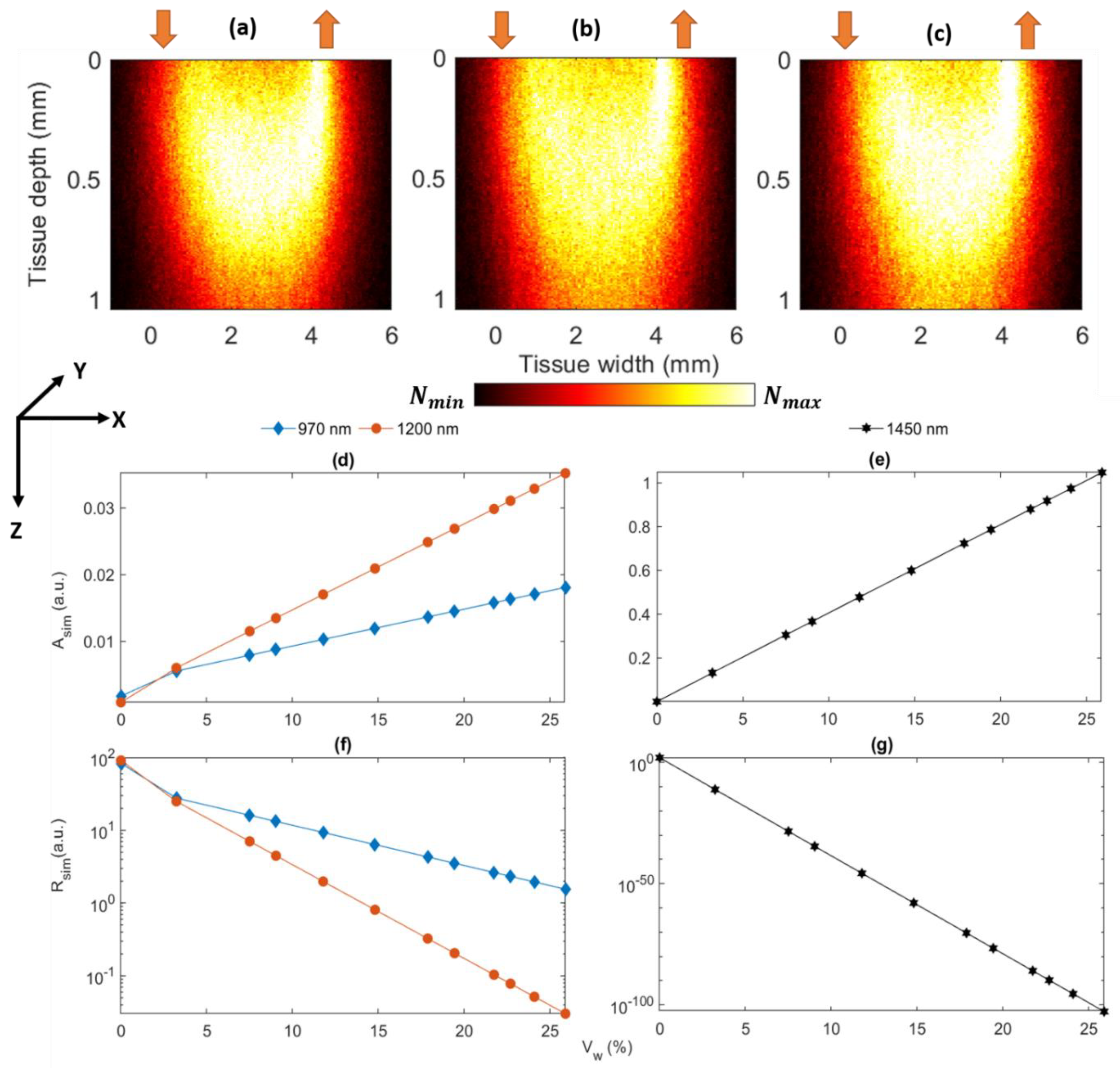
2.1. Monte Carlo Modeling of Optical Interactions with Porcine Skin
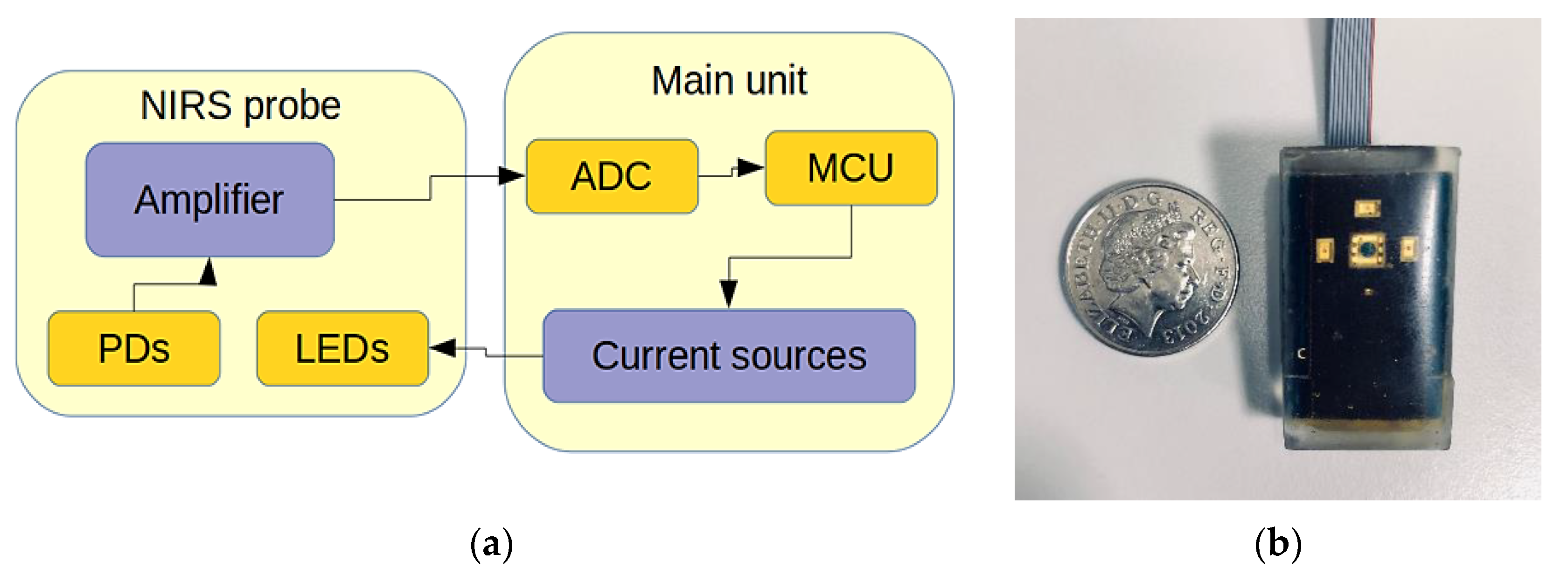
2.2. Design and Development of a Hydration Sensor
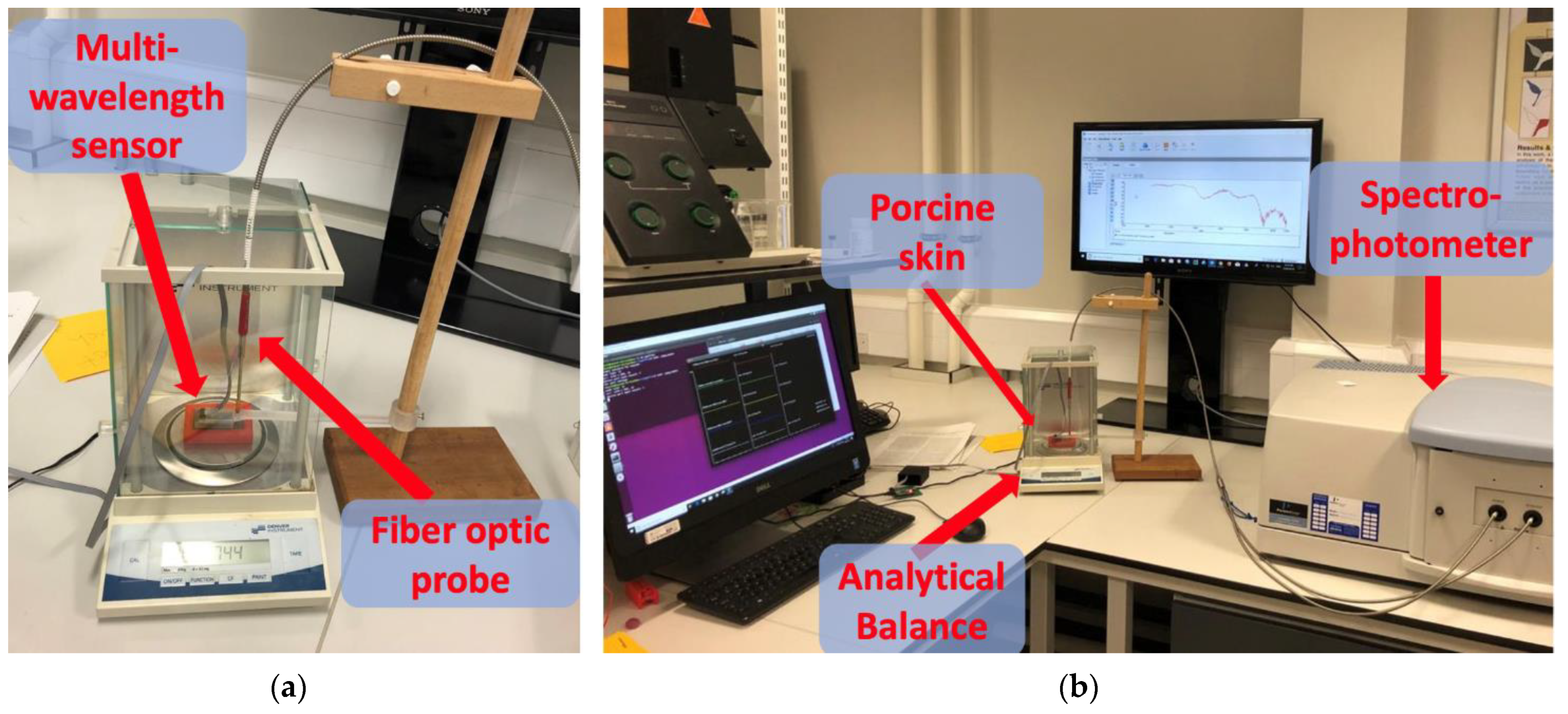
2.3. In Vitro Experiment
2.4. Instrument Settings and Data Analysis
- Intermittent weight measurements were recorded every 20 min, enabling the calculation of dermal water content.
- DC optical reflectance readings were recorded using the novel hydration sensor at every 30 s throughout the 3-h desorption process.
- A total of 40 reflectance spectra in the range of 872–2100 nm were collected in 3 h (every 4.5 min). Spectral acquisition was performed using the following instrument settings; InGaAs detection between 872 and 1800 nm, and PbS between 1800 and 2100 nm. The gain and response time for the InGaAs and PbS detectors were set to 3/0.2 s and 1/0.2 s, respectively. The attenuator settings were set to 1% for the reference beam and 100% for the sample compartment. This was done in order to reduce noise for high absorption values. Furthermore, an initial baseline correction of 100% transmittance and 0% absorption was also added.
- Benchmarking optical measurements of water content with full-range spectra: The full-range optical spectra obtained via Lambda 1050 were preprocessed and subsequently fit to the interpolated weight measurements as a proxy to skin water content.
- Investigations into the consistency of output readings from the novel skin hydration sensor and the benchtop spectrophotometer.
- Development of a multivariate model to predict dermal water content using the skin hydration sensor, the results of which are compared to the Lambda 1050.
3. Results
3.1. Monte Carlo Characterization of the Developed Sensor
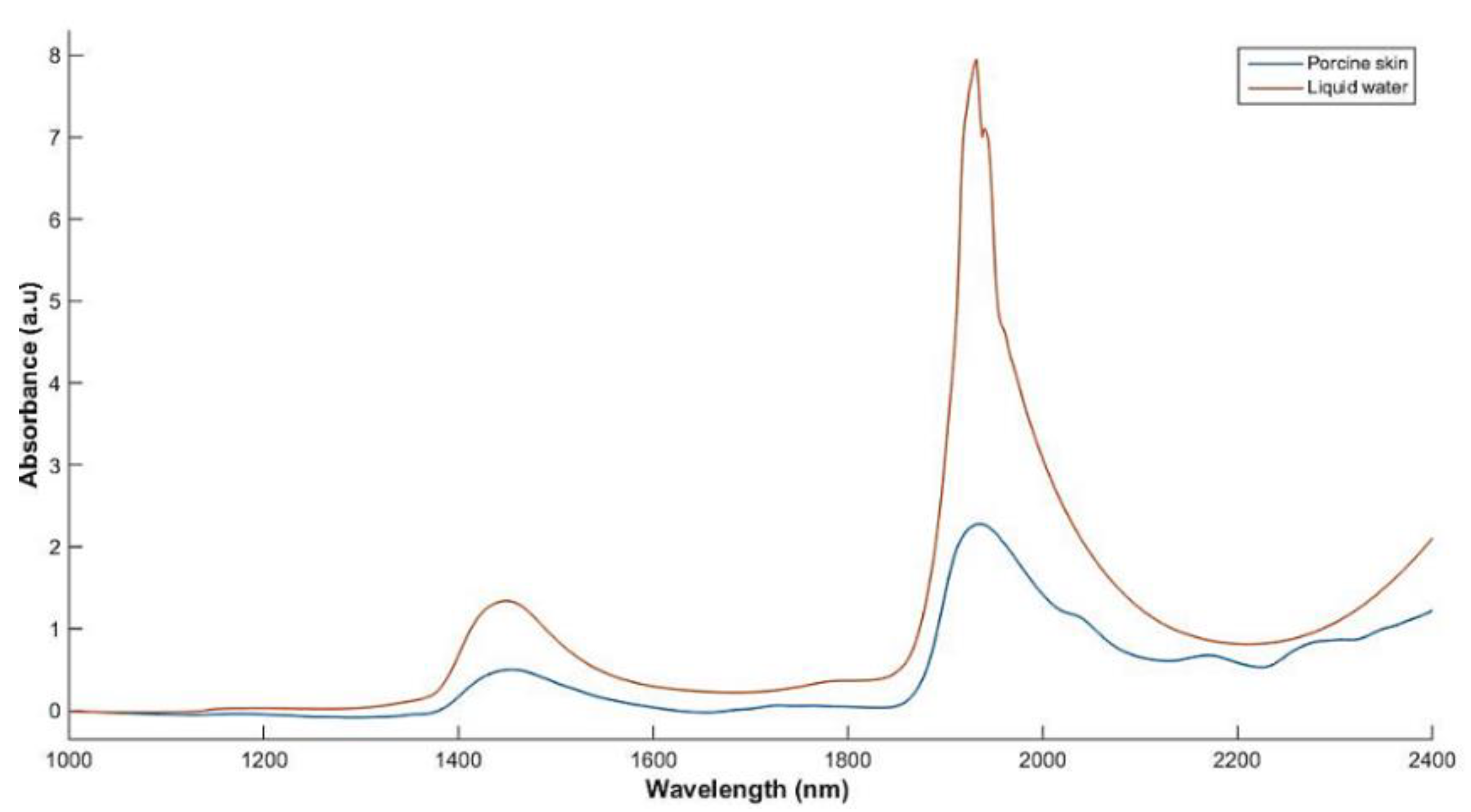
3.2. Benchmarking Optical Measurements of Weight with Full-Range Spectra
3.3. The Assessment of Agreement between the Developed Sensor and Lambda 1050 Spectrophotometer
3.4. Estimation of Water Content and Comparison with Lambda 1050
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madison, K.C. Barrier function of the skin: “La Raison d’Être” of the epidermis. J. Investig. Derm. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Derm. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, H. Use of Cutometer to assess epidermal hydration. Ski. Res. Technol. 2000, 6, 239–244. [Google Scholar] [CrossRef]
- Wiechers, J.W.; Barlow, T. Skin moisturisation and elasticity originate from at least two different mechanisms. Int. J. Cosmet. Sci. 1999, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (II): Protein, glycosaminoglycan, water, and lipid content and structure. Ski. Res. Technol. 2006, 2, 145–154. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Happillon, T.; Feru, J.; Brassart-Passco, S.; Angiboust, J.-F.; Manfait, M.; Piot, O. Raman comparison of skin dermis of different ages: Focus on spectral markers of collagen hydration. J. Raman Spectrosc. 2013, 44, 1230–1237. [Google Scholar] [CrossRef]
- del Rosso, J.Q.; Levin, J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J. Clin. Aesthetic Dermatol. 2011, 4, 22–42. [Google Scholar]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Ski. Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Farahmand, S.; Tien, L.; Hui, X.; Maibach, H.I. Measuring transepidermal water loss: A comparative in vivo study of condenser-chamber, unventilated-chamber and open-chamber systems. Ski. Res. Technol. 2009, 15, 392–398. [Google Scholar] [CrossRef]
- Netzlaff, F.; Kostka, K.H.; Lehr, C.M.; Schaefer, U.F. TEWL measurements as a routine method for evaluating the integrity of epidermis sheets in static Franz type diffusion cells in vitro. Limitations shown by transport data testing. Eur. J. Pharm. Biopharm. 2006, 63, 44–50. [Google Scholar] [CrossRef]
- Qassem, M.; Kyriacou, P.A. Review of modern techniques for the assessment of skin hydration. Cosmetics 2019, 6, 19. [Google Scholar] [CrossRef]
- Darvin, M.E.; Choe, C.S.; Schleusener, J.; Lademann, J. In vivo non-invasive determination of the water concentration and water bonding properties in the human stratum corneum using confocal Raman microspectroscopy (mini-review). Quantum Electron. 2021, 51, 28. [Google Scholar] [CrossRef]
- Sdobnov, A.Y.; Tuchin, V.V.; Lademann, J.; Darvin, M.E. Confocal Raman microscopy supported by optical clearing treatment of the skin—influence on collagen hydration. J. Phys. D Appl. Phys. 2017, 50, 28. [Google Scholar] [CrossRef]
- Sdobnov, A.Y.; Darvin, M.E.; Schleusener, J.; Lademann, J.; Tuchin, V.V. Hydrogen bound water profiles in the skin influenced by optical clearing molecular agents—Quantitative analysis using confocal Raman microscopy. J. Biophotonics 2018, 12, e201800283. [Google Scholar] [CrossRef]
- Qassem, M.; Kyriacou, P.A. Reflectance near-infrared measurements for determining changes in skin barrier function and scattering in relation to moisturizer application. J. Biomed. Opt. 2015, 20. [Google Scholar] [CrossRef] [PubMed]
- Walling, P.L.; Dabney, J.M. Moisture in skin by near-infrared reflectance spectroscopy. J. Soc. Cosmet. Chem. 1989, 40, 151–171. [Google Scholar]
- Martin, K. In vivo measurements of water in skin by near-infrared reflectance. Appl. Spectrosc. 1998, 52, 1001–1007. [Google Scholar] [CrossRef]
- Martin, K. Direct measurement of moisture in skin by NIR spectroscopy. J. Soc. Cosmet. Chem. 1993, 44, 249–261. [Google Scholar]
- Bodén, I.; Nilsson, D.; Naredi, P.; Lindholm-Sethson, B. Characterization of healthy skin using near infrared spectroscopy and skin impedance. Med Biol. Eng. Comput. 2008, 46, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Qassem, M.; Kyriacou, P. Use of reflectance near-infrared spectroscopy to investigate the effects of daily moisturizer application on skin optical response and barrier function. J. Biomed. Opt. 2014, 19, 087007. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M. In vivo simultaneous measurement of urea and water in the human stratum corneum by diffuse-reflectance near-infrared spectroscopy. Ski. Res. Technol. 2009, 15, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Egawa, M.; Hirao, T.; Takahashi, M. In vivo estimation of stratum corneum thickness from water concentration profiles obtained with raman spectroscopy. Acta Derm.-Venereol. 2007, 87, 4–8. [Google Scholar] [CrossRef]
- Egawa, M.; Arimoto, H.; Hirao, T.; Takahashi, M.; Ozaki, Y. Regional difference of water content in human skin studied by diffuse-reflectance near-infrared spectroscopy: Consideration of measurement depth. Appl. Spectrosc. 2006, 60, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.A.; Ahn, J.W.; Chun, I.K.; Kim, H.J. Development of a method for the determination of human skin moisture using a portable near-infrared system. Anal. Chem. 2001, 73, 4964–4971. [Google Scholar] [CrossRef]
- Mohamad, M.; Msabbri, A.R.; Matjafri, M.Z. Conceptual design of near infrared spectroscopy instrumentation for skin moisture measurement. In Proceedings of the 2011 IEEE Colloquium on Humanities, Science and Engineering, CHUSER 2011, Penang, Malaysia, 5–6 December 2011; pp. 801–804. [Google Scholar] [CrossRef]
- Qassem, M.; Kyriacou, P.A. Comparing the rates of absorption and weight loss during a desorption test using near infrared spectroscopy. Ski. Res. Technol. 2013, 19, 137–144. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A.; Chatterjee, S. Investigating the origin of photoplethysmography using a multiwavelength monte carlo model. Physiol. Meas. 2020, 41, 084001. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Zamora-Rojas, E.; Aernouts, B.; Garrido-Varo, A.; Saeys, W.; Pérez-Marín, D.; Guerrero-Ginel, J.E. Optical properties of pig skin epidermis and dermis estimated with double integrating spheres measurements. Innov. Food Sci. Emerg. Technol. 2013, 20, 343–349. [Google Scholar] [CrossRef]
- Yu, T.; Wen, X.; Tuchin, V.V.; Luo, Q.; Zhu, D. Quantitative analysis of dehydration in porcine skin for assessing mechanism of optical clearing. J. Biomed. Opt. 2011, 16, 095002. [Google Scholar] [CrossRef] [PubMed]
- Mamouei, M.; Qassem, M.; Razban, M.; Kyriacou, P.A. Measurement of dermal water content using a multi-wavelength optical sensor. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Montreal, QC, Canada, 20–24 July 2020; pp. 4353–4356. [Google Scholar] [CrossRef]

| Weight (g) | ||||
|---|---|---|---|---|
| 970 nm | 1200 nm | 1450 nm | ||
| 1.8244 | 0 | 0.0149 | 0.0074 | 0.0040 |
| 1.8567 | 0.0323 | 0.0289 | 0.0408 | 0.9276 |
| 1.8994 | 0.075 | 0.0475 | 0.0849 | 2.1487 |
| 1.9147 | 0.0903 | 0.0541 | 0.1006 | 2.5862 |
| 1.9422 | 0.1178 | 0.0661 | 0.1291 | 3.3726 |
| 1.9724 | 0.148 | 0.0793 | 0.1602 | 4.2362 |
| 2.0031 | 0.1787 | 0.0926 | 0.1920 | 5.114 |
| 2.0187 | 0.1943 | 0.0994 | 0.2081 | 5.5602 |
| 2.0417 | 0.2173 | 0.1094 | 0.2318 | 6.2179 |
| 2.0513 | 0.2269 | 0.1136 | 0.2417 | 6.4924 |
| 2.0652 | 0.2408 | 0.1196 | 0.2560 | 6.8899 |
| 2.0833 | 0.2589 | 0.1275 | 0.2747 | 7.4075 |
| Wavelength (nm) | ||
|---|---|---|
| 1450 | 1.30 (0.078) * | 0.993 |
| 1200 | 1.15 (0.313) ** | 0.91 |
| 970 | 0.13 (0.004) *** | 0.837 |
| Model | RMSECV (g) | |
|---|---|---|
| Skin Hydration Sensor | 0.0038 | 0.9975 |
| Lambda 1050 full spectrum (#LVs = 5) | 0.0149 | 0.9590 |
| Lambda 1050 4 wavelengths | 0.0183 | 0.9429 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamouei, M.; Chatterjee, S.; Razban, M.; Qassem, M.; Kyriacou, P.A. Design and Analysis of a Continuous and Non-Invasive Multi-Wavelength Optical Sensor for Measurement of Dermal Water Content. Sensors 2021, 21, 2162. https://doi.org/10.3390/s21062162
Mamouei M, Chatterjee S, Razban M, Qassem M, Kyriacou PA. Design and Analysis of a Continuous and Non-Invasive Multi-Wavelength Optical Sensor for Measurement of Dermal Water Content. Sensors. 2021; 21(6):2162. https://doi.org/10.3390/s21062162
Chicago/Turabian StyleMamouei, Mohammad, Subhasri Chatterjee, Meysam Razban, Meha Qassem, and Panayiotis A. Kyriacou. 2021. "Design and Analysis of a Continuous and Non-Invasive Multi-Wavelength Optical Sensor for Measurement of Dermal Water Content" Sensors 21, no. 6: 2162. https://doi.org/10.3390/s21062162
APA StyleMamouei, M., Chatterjee, S., Razban, M., Qassem, M., & Kyriacou, P. A. (2021). Design and Analysis of a Continuous and Non-Invasive Multi-Wavelength Optical Sensor for Measurement of Dermal Water Content. Sensors, 21(6), 2162. https://doi.org/10.3390/s21062162








