Comparison of Dual Beam Dispersive and FTNIR Spectroscopy for Lactate Detection
Abstract
1. Introduction
- Multiplexing: Interferometers, which are an integral part of the FTIR/FTNIR spectrometers, use a moving mirror which can scan the entire range of wavelengths at once for spectra collection. This makes it faster than the dual beam spectrophotometer, which considers each wavelength, individually, in the spectral range for collection. Each of the scans in FTIR/FTNIR can be summated for a better accuracy and signal to noise ratio (SNR) (squareroot of the total spectra) for each spectrum, known as Fellgett’s advantage [11]
- Throughput: A FTIR/FTNIR spectrometer has a higher throughput compared to the dual beam spectrophotometer, owing to the avoidance of the slits or optical apertures and the use of fewer mirrors in the instrument, which increases the total energy of the incident light on a sample and reduces reflection losses. This increases SNR, and is known as Jacquinot’s advantage [12].
2. Reagents and Materials
3. NIR Spectrometry
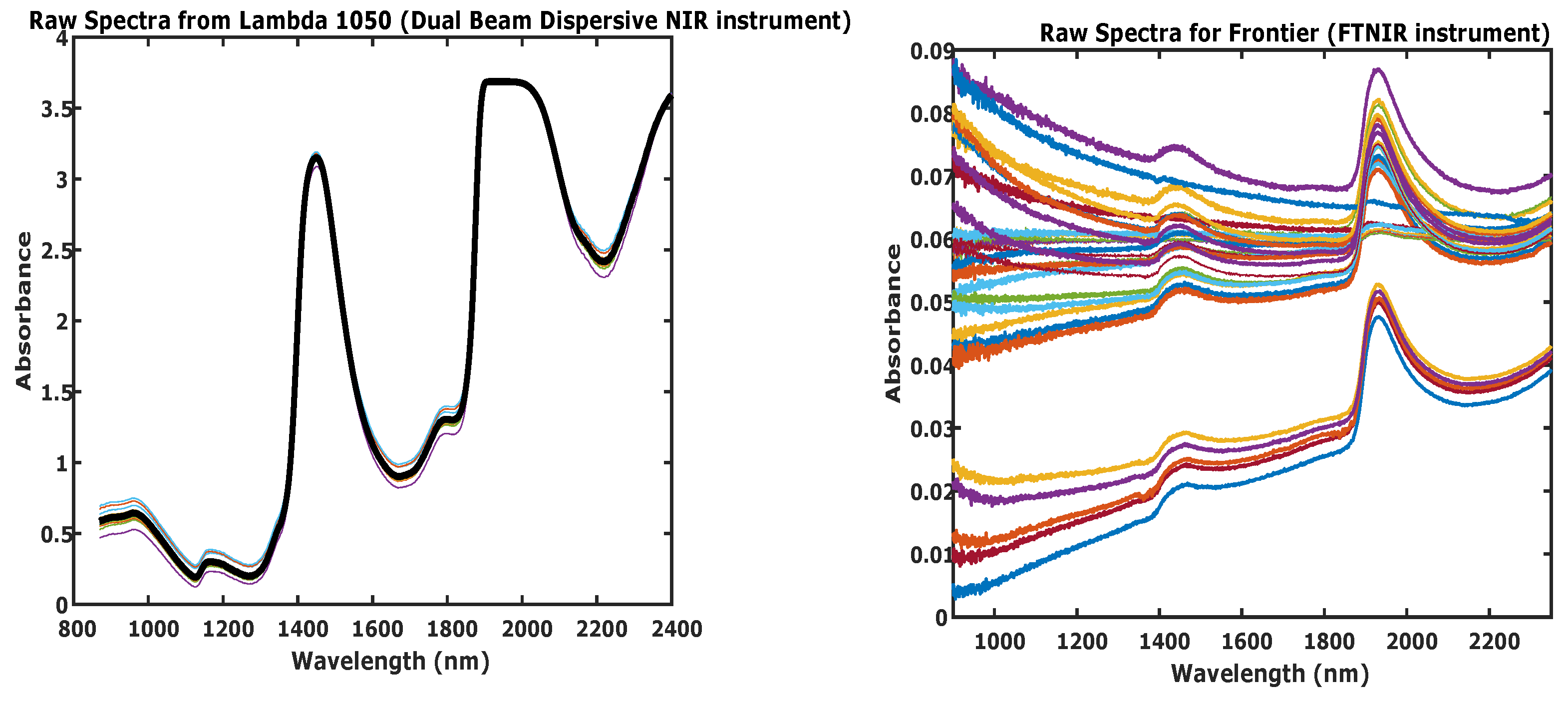
3.1. Dual Beam Dispersive NIR
3.2. FTNIR
3.3. Spectral Analysis
- UVWin Lab for LAMBDA 1050, from Perkin Elmer (Waltham, MA, USA) and
- Spectrum 10 for Frontier FTIR/NIR, from Perkin Elmer (Waltham, MA, USA).
- Spectral Difference: The spectrum of the first sample (4.2 mM concentration of lactate) was subtracted from the rest of the spectra
- Linear Robust Multiplicative Scatter Correction (MSC) [26] was used for both the data-sets
4. Results and Discussion
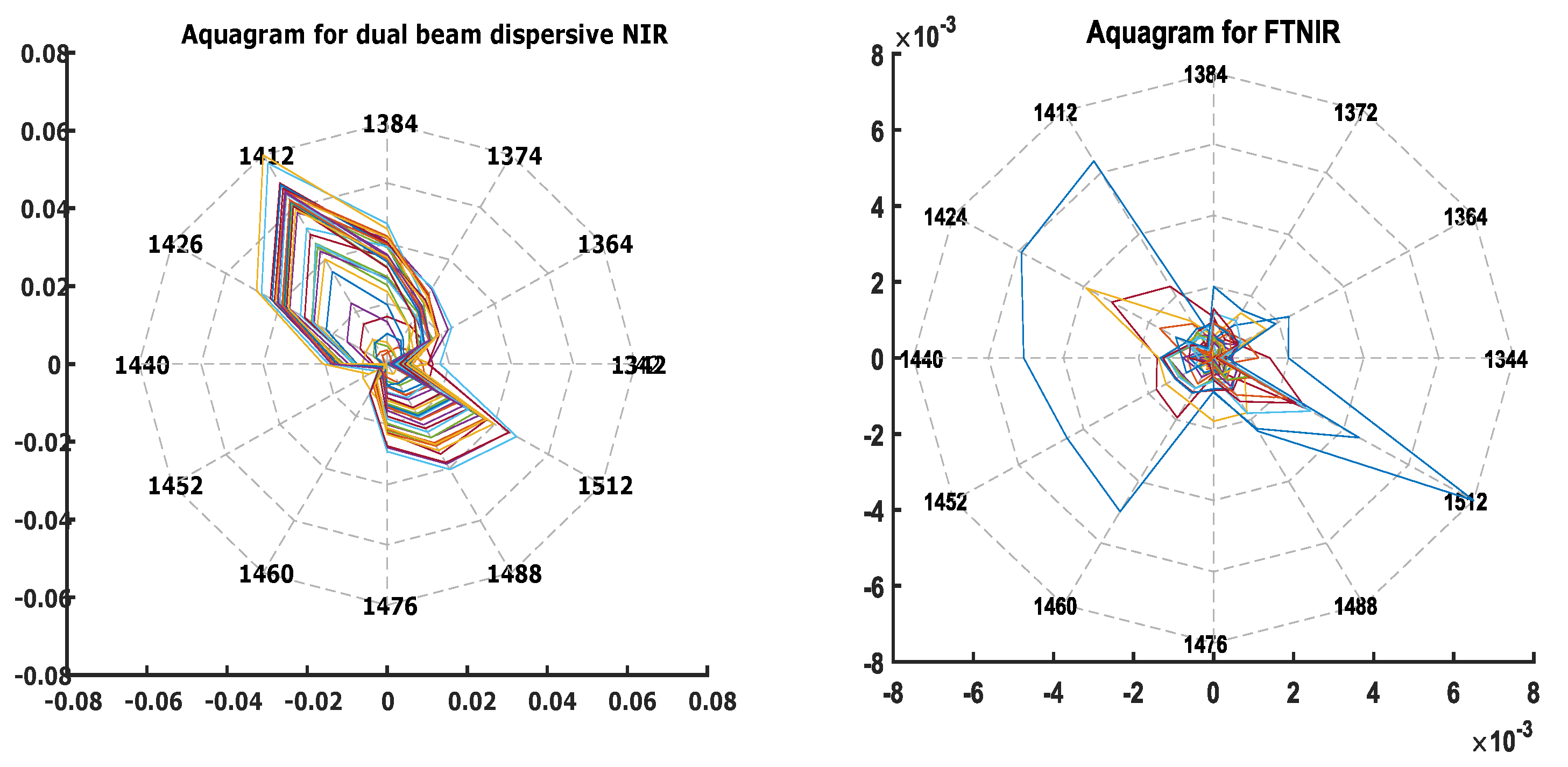
4.1. Spectral Regions with O-H Absorption Interference
4.2. Signature Wavelengths of Lactate
4.3. Full Spectral Regions for Predictive Modelling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Zhong, F.; Pan, B.; Li, Z.; Huang, C.; Deng, Z. A Brief Review of OPT101 Sensor Application in Near-Infrared Spectroscopy Instrumentation for Intensive Care Unit Clinics. Sensors 2017, 17, 1701. [Google Scholar] [CrossRef]
- Green, M.S.; Sehgal, S.; Tariq, R. Near-Infrared Spectroscopy: The New Must Have Tool in the Intensive Care Unit? Semin. Cardiothorac. Vasc. Anesth. 2016, 20, 213–224. [Google Scholar] [CrossRef]
- Jobsis, F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef]
- Ozaki, Y.; Matsunaga, T.; Miura, T. Nondestructive and Noninvasive Monitoring of Deoxyhemoglobin in the Vein by Use of a Near-Infrared Reflectance Spectrometer with a Fiber-Optic Probe. Appl. Spectrosc. 1992, 46, 180–182. [Google Scholar] [CrossRef]
- Uwadaira, Y.; Ikehata, A.; Momose, A.; Miura, M. Identification of informative bands in the short-wavelength NIR region for non-invasive blood glucose measurement. J. Jpn. Soc. Food Sci. Technol. 2016, 7, 2729. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liao, N.; Cheng, H.; Li, Y.; Bai, X.; Deng, C. Determination of NIR informative wavebands for transmission non-invasive blood glucose measurement using a Fourier transform spectrometer. Am. Inst. Phys. Adv. 2018, 8, 035216. [Google Scholar] [CrossRef]
- Lafrance, D.; Lands, L.C.; Burns, D.H. Measurement of lactate in whole human blood with near-infrared transmission spectroscopy. Talanta 2003, 60, 635–641. [Google Scholar] [CrossRef]
- Anonymous. The Infracord® Double Beam Spectrophotometer. Chem. Eng. News 1957, 35, 74. [Google Scholar] [CrossRef][Green Version]
- Griffiths, P.R. The Early Days of Commercial FT-IR Spectrometry: A Personal Perspective. Appl. Spectrosc. 2017, 71, 329–340. [Google Scholar] [CrossRef]
- Coates, J. Designing the ideal process analyser or at least making the attempt (continued). NIR News 1994, 5, 7–9. [Google Scholar] [CrossRef]
- Fellgett, P. I.—les principes généraux des méthodes nouvelles en spectroscopie interférentielle—A propos de la théorie du spectromètre interférentiel multiplex. J. Phys. Colloq. 1958, 19, 187–191. [Google Scholar] [CrossRef]
- Connes, P. Pierre Jacquinot and the beginnings of Fourier transform spectrometry. J. Phys. II EDP Sci. 1992, 2, 565–571. [Google Scholar] [CrossRef]
- Peirs, A.; Scheerlinck, N.; Touchant, K.; Nicolaï, B.M. PH—Postharvest technology: Comparison of fourier transform and dispersive near-infrared reflectance spectroscopy for apple quality measurements. Biosyst. Eng. 2002, 81, 305–311. [Google Scholar] [CrossRef]
- Armstrong, P.; Maghirang, E.; Xie, F.; Dowell, F. Comparison of dispersive and Fourier-transform NIR instruments for measuring grain and flour attributes. Appl. Eng. Agric. 2006, 22, 453–457. [Google Scholar] [CrossRef]
- Pandey, P.; Srivastava, S.; Mishra, H.N. Comparison of FT-NIR and NIR for evaluation of phyisco-chemical properties of stored wheat grains. Food Qual. Saf. 2018, 2, 165–172. [Google Scholar] [CrossRef]
- Jiang, H.; Zhuang, H.; Sohn, M.; Wang, W. Measurement of soy contents in ground beef using near-infrared spectroscopy. Appl. Sci. 2017, 7, 97. [Google Scholar] [CrossRef]
- Gergely, S.; Izsó, E.; Salgó, A. Comparison of different types of near infrared (NIR) instruments in ability to measure alkaloids in capsule of poppy (P. somniferum). Period. Polytech. Chem. Eng. 2015, 59, 277–282. [Google Scholar] [CrossRef]
- Steuer, B.; Schulz, H. Near-infrared analysis of fennel (Foeniculum vulgare Miller) on different spectrometers—Basic considerations for a reliable network. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2003, 14, 285–289. [Google Scholar] [CrossRef]
- Sinnaeve, G.; Dardenne, P.; Agneessens, R.; Lateur, M.; Hallet, A. Quantitative analysis of raw apple juices using near infrared, Fourier-transform near infrared and Fourier-transform infrared instruments: A comparison of their analytical performances. J. Infrared Spectrosc. 1997, 5, 1–17. [Google Scholar] [CrossRef]
- Broskey, N.T.; Zou, K.; Dohm, G.L.; Houmard, J.A. Plasma Lactate as a Marker for Metabolic Health. Exerc. Sport Sci. Rev. 2020, 48, 119–124. [Google Scholar] [CrossRef]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Jones, T.E.; Pories, W.J.; Houmard, J.A.; Tanner, C.J.; Zheng, D.; Zou, K.; Coen, P.M.; Goodpaster, B.H.; Kraus, W.E.; Dohm, G.L. Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 2019, 166, 861–866. [Google Scholar] [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2. [Google Scholar] [CrossRef]
- Baishya, N.; Mamouei, M.; Budidha, K.; Qassem, M.; Vadgama, P.; Kyriacou, P.A. Investigations into the Effects of pH on Quantitative Measurements of Lactate in Biological Media Using ATR-FTIR Spectroscopy. Molecules 2020, 25, 3695. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.L.M.; Lima, K.M.G.; Singh, M.; Martin, F.L. Tutorial: Multivariate classification for vibrational spectroscopy in biological samples. Nat. Protoc. 2020, 15, 2143–2162. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and Scatter-Correction for Near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Tsenkova, R.; Munćan, J.; Pollner, B.; Kovacs, Z. Essentials of Aquaphotomics and Its Chemometrics Approaches. Front. Chem. 2018, 6, 363. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Partial Least Squares Methods: Partial Least Squares Correlation and Partial Least Square Regression; Humana Press: Totowa, NJ, USA, 2013; pp. 549–579. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). WIREs Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Budidha, K.; Mamouei, M.; Baishya, N.; Qassem, M.; Vadgama, P.; Kyriacou, P. Identification and Quantitative Determination of Lactate Using Optical Spectroscopy—Towards a Noninvasive Tool for Early Recognition of Sepsis. Sensors 2020, 20, 5402. [Google Scholar] [CrossRef] [PubMed]
- Baishya, N.; Momouei, M.; Budidha, K.; Qassem, M.; Vadgama, P.; Kyriacou, P.A. Near infrared spectrometric investigation of lactate in a varying pH buffer. J. Near Infrared Spectrosc. 2020. [Google Scholar] [CrossRef]
- Baishya, N.; Mamouei, M.; Budidha, K.; Qassem, M.; Vadgama, P.; Kyriacou, P.A. Near Infrared and Aquaphotomic analysis of water absorption in lactate containing media. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar] [CrossRef]
- Tsenkova, R. Aquaphotomics: Dynamic Spectroscopy of Aqueous and Biological Systems Describes Peculiarities of Water. J. Infrared Spectrosc. 2009, 17, 303–313. [Google Scholar] [CrossRef]
- Muncan, J.; Tsenkova, R. Aquaphotomics—From Innovative Knowledge to Integrative Platform in Science and Technology. Molecules 2019, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Chatham, J.C.; Forder, J.R. Lactic acid and protein interactions: Implications for the NMR visibility of lactate in biological systems. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1426, 177–184. [Google Scholar] [CrossRef]
- Guesnon, P.; Poyart, C.; Bursaux, E.; Bohn, B. The binding of lactate and chloride ions to human adult hemoglobin. Respir. Physiol. 1979, 38, 115–129. [Google Scholar] [CrossRef]
- Pérez-Hernández, N.; Pérez, C.; Rodríguez, M.L.; Foces-Foces, C.; Tolstoy, P.M.; Limbach, H.H.; Morales, E.Q.; Pérez, R.; Martín, J.D. Water molecules in hydroxy/acid networks as a competition between dynamics and bonding. Synthesis of a wet hydrophobic pore. Bioorg. Med. Chem. 2004, 12, 1305–1314. [Google Scholar] [CrossRef]
- Baishya, N.; Mamouei, M.; Budidha, K.; Qassem, M.; Vadgama, P.; Kyriacou, P.A. In-vitro spectrometric analysis of hyperlactatemia and lactic acidosis in buffer relating to sepsis. J. Near Infrared Spectrosc. 2020. accepted for publication. [Google Scholar] [CrossRef]
- Kossowski, T.; Kossowska, K.; Stasiński, R. Non-invasive measurement of chemical compounds levels in human body. Meas. Autom. Monit. 2017, 63, 200–202. [Google Scholar]
- Lafrance, D.; Lands, L.C.; Burns, D.H. In vivo lactate measurement in human tissue by near-infrared diffuse reflectance spectroscopy. Vib. Spectrosc. 2004, 36, 195–202. [Google Scholar] [CrossRef]
- Lafrance, D.; Lands, L.C.; Hornby, L.; Burns, D.H. Near-infrared spectroscopic measurement of lactate in human plasma. Appl. Spectrosc. 2000, 54, 300–304. [Google Scholar] [CrossRef]
- Crocombe, R.A. Portable Spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.J.; Ettenberg, M.H.; Lange, M.J.; Olsen, G.H. Commercial and industrial applications of indium gallium arsenide near-infrared focal plane arrays. In Proceedings of the Conference on Infrared Technology and Applications XXV, Orlando, FL, USA, 5–9 April 1999. [Google Scholar] [CrossRef]

| Data-Set | Polynomial Order (PO) | Derivative (D) | Window Length (WL) |
|---|---|---|---|
| Dual Beam | |||
| Dispersive NIR | 2 | 1 | 7 |
| FTNIR | 2 | 1 | 31 |
| Wavelength (nm)/Data-Set | Dual Beam Dispersive NIR | FTNIR |
|---|---|---|
| 970 | 1.51 × (9.15 × ) | −2.00 × (1.43 × ) |
| 1450 | −1.11 × (4.17 × ) 1 | −1.72 × (6.8 × ) |
| 1490 | −1.16 × (3.56 × ) | −1.28 × (5.42 × ) 1 |
| 1540 | 2.28 × (1.17 × ) | −4.94 × (5.26 × ) |
| 1820 | 3.73 × (1.46 × ) 1 | 1.33 × (3.57 × ) |
| 1960 | 4.25 × (4.42 × ) | −5.97 × (3.03 × ) |
| 2070 | 2.77 × (3.79 × ) | −1.72 × (9.26 × ) |
| 2100 | 7.51 × (3.41 × ) 1 | −1.62 × (6.59 × ) 1 |
| Wavelength (nm)/Data-Set | Dual Beam Dispersive NIR | FTNIR |
|---|---|---|
| 1142 | 1.08 × (1.27 × ) 3 | −6.71 × (1.01 × ) |
| 1232 | −5.26 × (9.28 × ) 3 | −7.30 × (6.72 × ) |
| 1280 | 1.08 × (4.85 × ) 1 | −5.72 × (6.86 × ) |
| 1330 | 4.29 × (1.69 × ) 1 | −5.84 × (7.01 × ) |
| 1710 | −5.34 × (1.51 × ) 2 | 3.64 × (4.88 × ) |
| 1750 | 3.03 × (1.09 × ) 2 | 7.04 × (4.62 × ) |
| 1882 | −2.18 × (0.09 × ) | 1.46 × (7.50 × ) |
| 2204 | 2.19 × (2.38 × ) | 3.12 × (3.15 × ) |
| 2320 | −1.26 × (3.26 × ) 3 | 8.18 × (8.18 × ) |
| 2340 | 1.3833 × (6.07 × ) 1 | 2.41 × (9.93 × ) |
| Wavelength (nm)/Data-Set | Dual Beam Dispersive NIR | FTNIR |
|---|---|---|
| 922 | 1.354 × (9.59 × ) | −3.42 × (1.73 × ) |
| 1046 | 5.57 × (1.52 × ) | −1.67 × (1.30 × ) |
| 1674 | −1.63 × (2.84 × ) | 1.47 × (5.47 × ) |
| 1690 | 3.72 × (9.10 × ) | −1.05 × (3.65 × ) |
| 1730 | 4.29 × (1.10 × ) | −1.59 × (5.44 × ) |
| 2166 | −6.09 × (2.66 × ) | −2.52 × (2.96 × ) |
| 2254 | 3.72 × (3.04 × ) | 5.85 × (7.17 × ) |
| 2292 | −3.36 × (4.69 × ) | 1.77 × (5.60 × ) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baishya, N.; Mamouei, M.; Budidha, K.; Qassem, M.; Vadgama, P.; Kyriacou, P.A. Comparison of Dual Beam Dispersive and FTNIR Spectroscopy for Lactate Detection. Sensors 2021, 21, 1891. https://doi.org/10.3390/s21051891
Baishya N, Mamouei M, Budidha K, Qassem M, Vadgama P, Kyriacou PA. Comparison of Dual Beam Dispersive and FTNIR Spectroscopy for Lactate Detection. Sensors. 2021; 21(5):1891. https://doi.org/10.3390/s21051891
Chicago/Turabian StyleBaishya, Nystha, Mohammad Mamouei, Karthik Budidha, Meha Qassem, Pankaj Vadgama, and Panayiotis A. Kyriacou. 2021. "Comparison of Dual Beam Dispersive and FTNIR Spectroscopy for Lactate Detection" Sensors 21, no. 5: 1891. https://doi.org/10.3390/s21051891
APA StyleBaishya, N., Mamouei, M., Budidha, K., Qassem, M., Vadgama, P., & Kyriacou, P. A. (2021). Comparison of Dual Beam Dispersive and FTNIR Spectroscopy for Lactate Detection. Sensors, 21(5), 1891. https://doi.org/10.3390/s21051891











