Synthesis of Cu2O-Modified Reduced Graphene Oxide for NO2 Sensors
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Polypyrrole-Coated Cu2O Nanowires
2.3. Preparation of rGO/PPy/Cu2O Nanocomposites
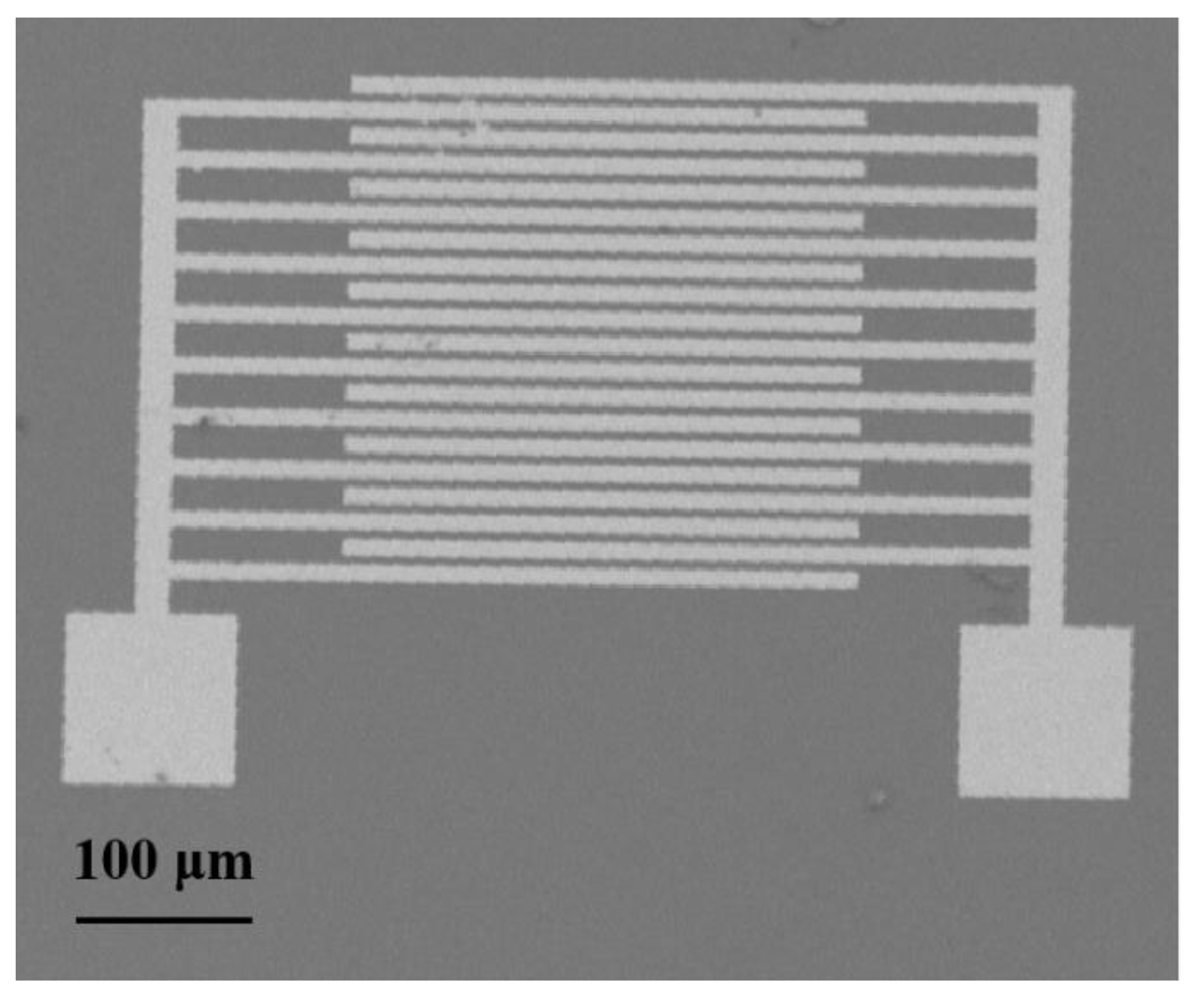
2.4. Fabrication of rGO/PPy/Cu2O-Based Gas Sensor
2.5. Gas Sensor Sensitivity
3. Results and Discussion
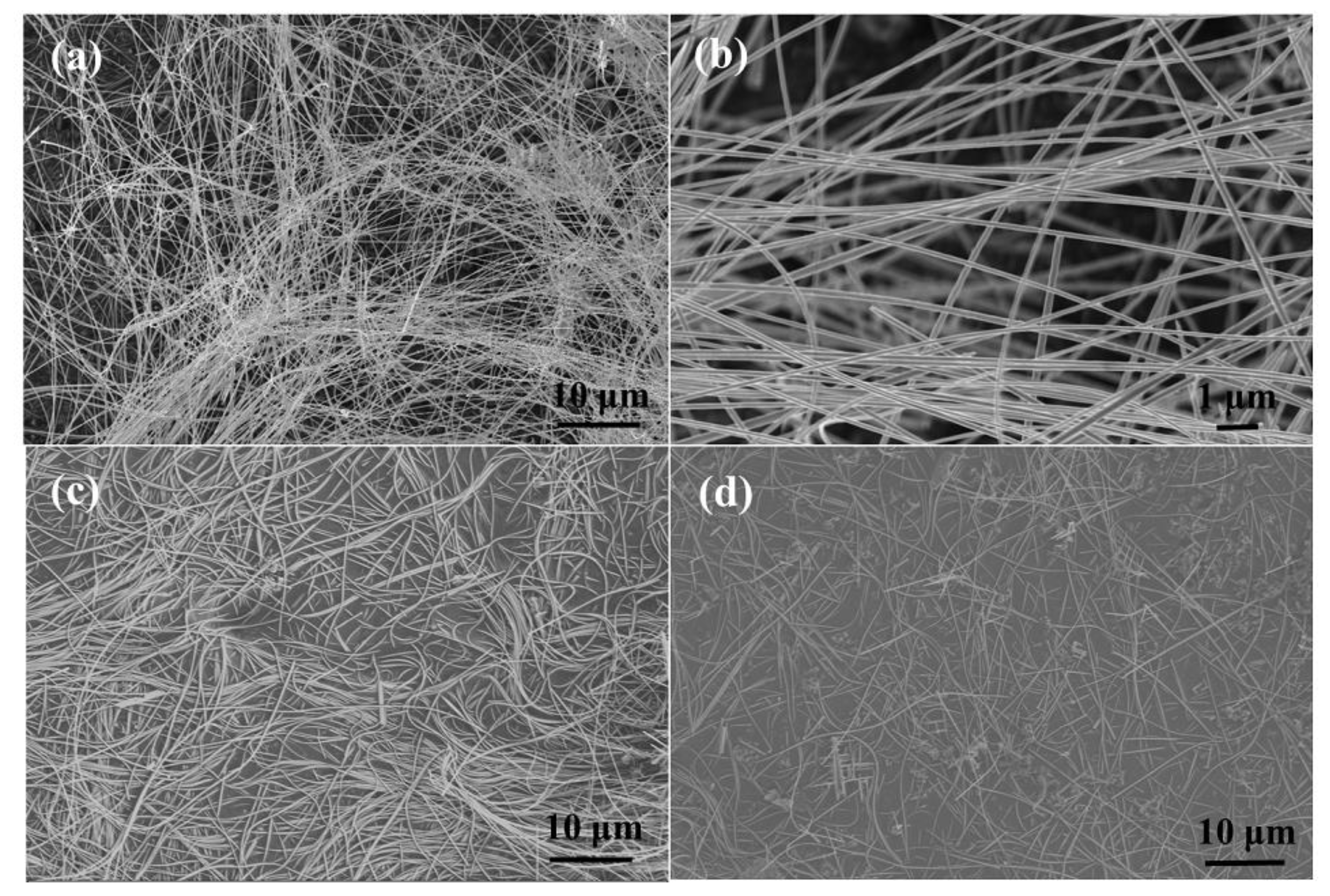
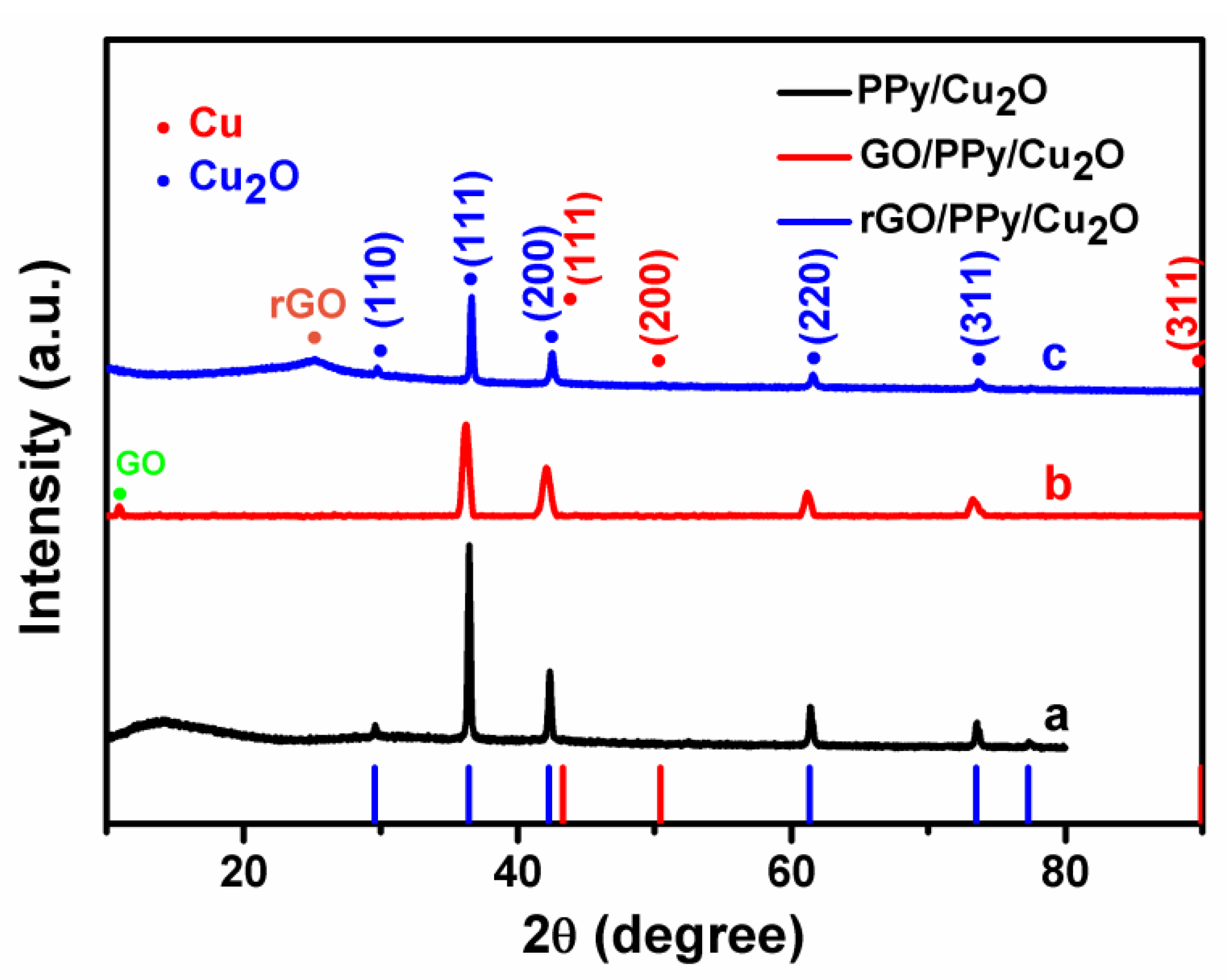
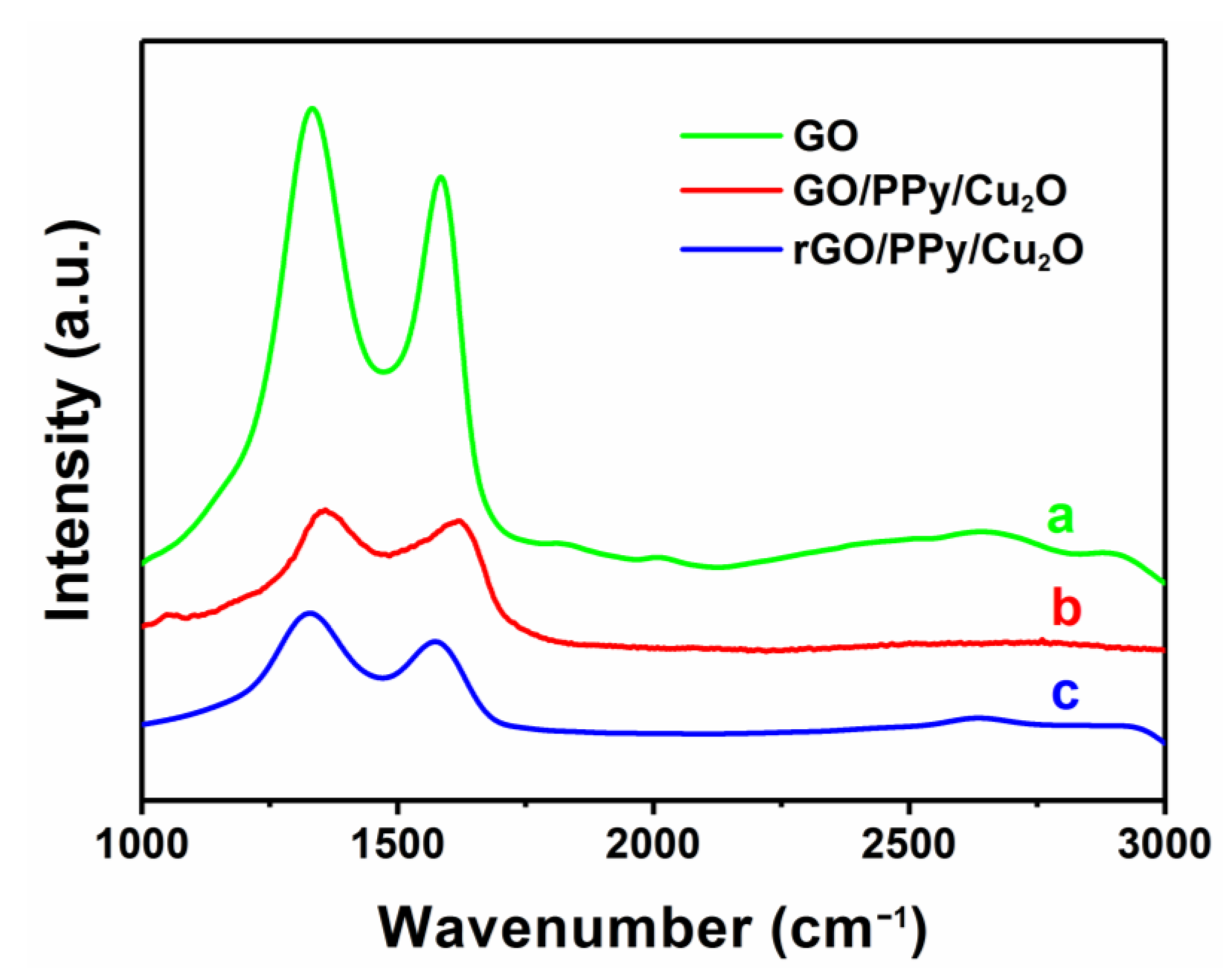
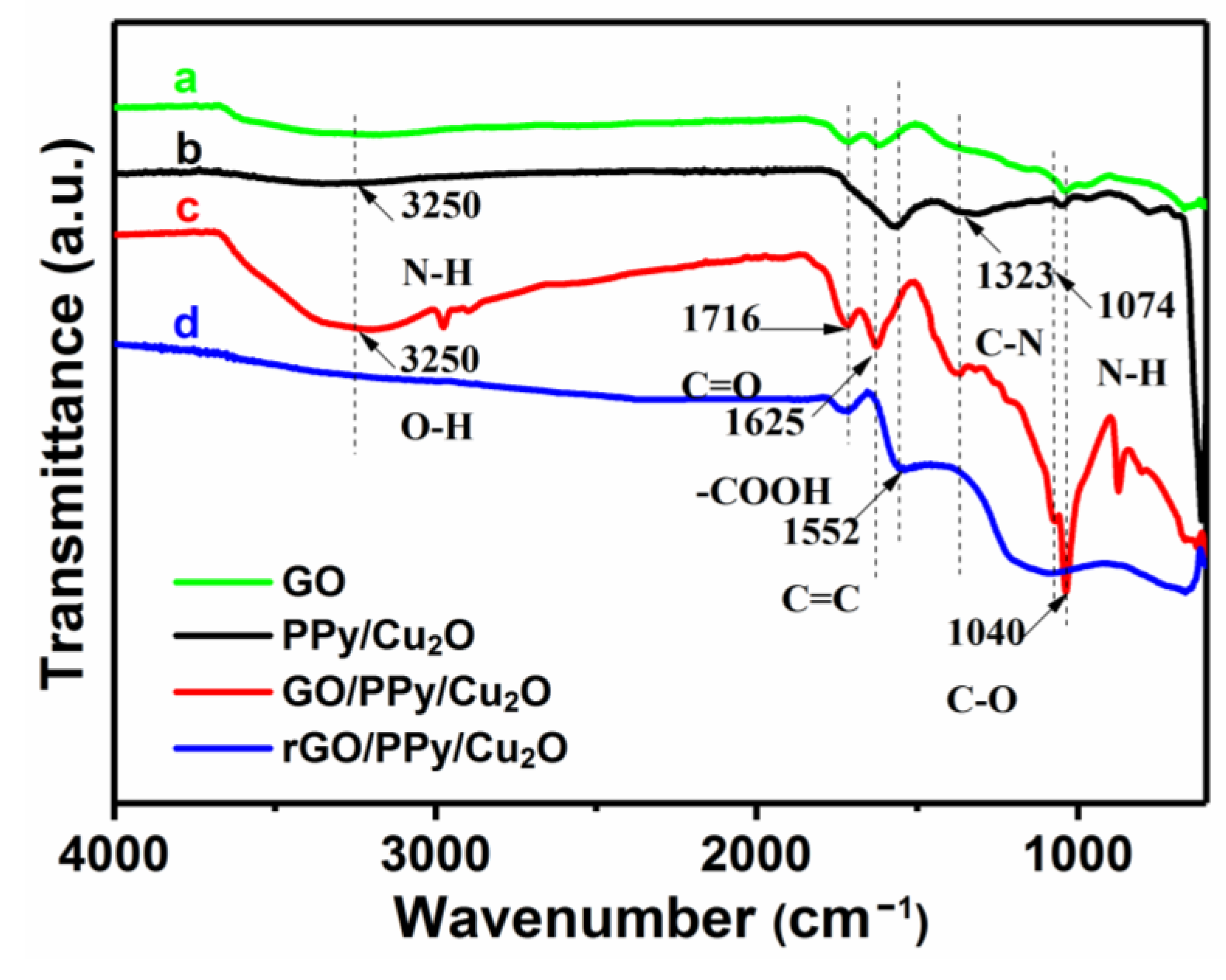
3.1. Characterization of as-Prepared Nanocomposites
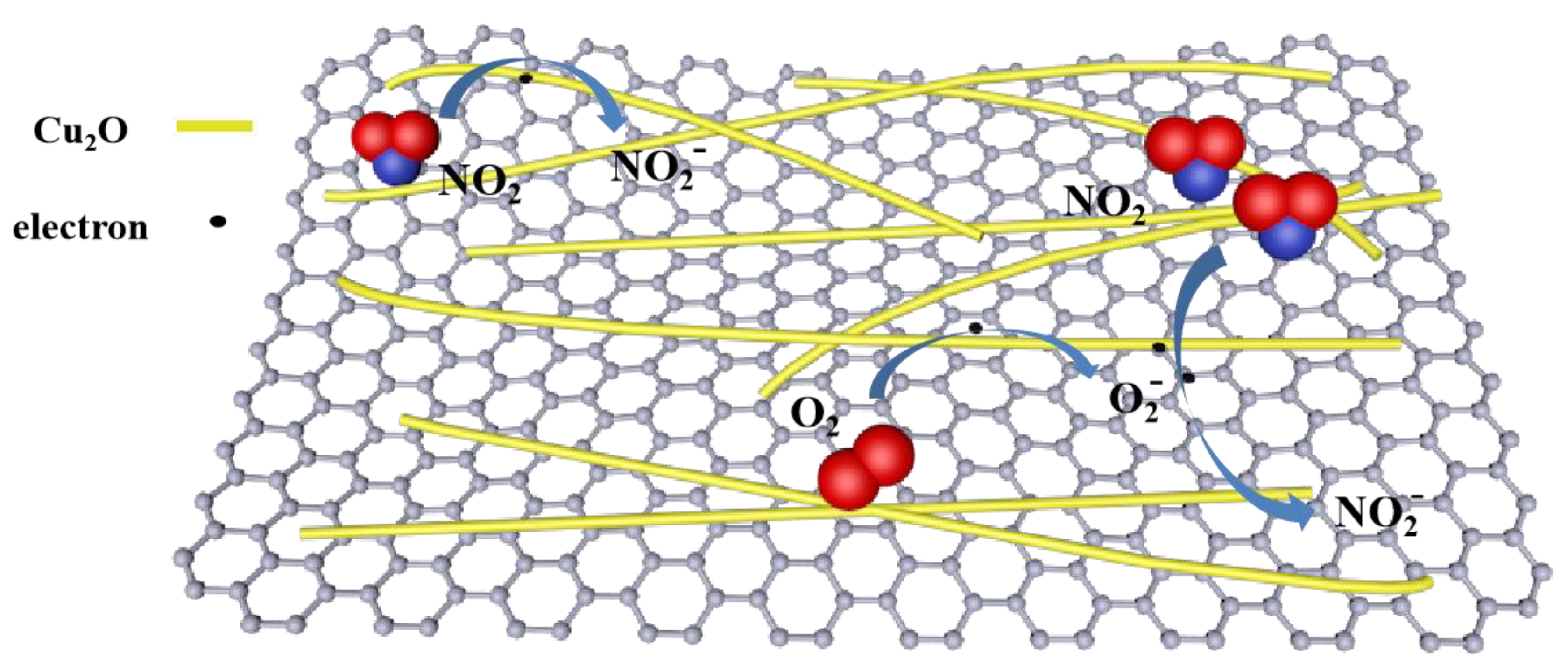
3.2. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Liu, G.; Zhu, X.; Guo, Y. Cu2O quantum dots modified by RGO nanosheets for ultrasensitive and selective NO2 gas detection. Ceram. Int. 2017, 43, 8372–8377. [Google Scholar] [CrossRef]
- Giancaterini, L.; Cantalini, C.; Cittadini, M.; Sturaro, M.; Guglielmi, M.; Martucci, A.; Resmini, A.; Anselmi-Tamburini, U. Au and Pt Nanoparticles Effects on the Optical and Electrical Gas Sensing Properties of Sol–Gel-Based ZnO Thin-Film Sensors. IEEE Sens. J. 2015, 15, 1068–1076. [Google Scholar] [CrossRef]
- Impeng, S.; Junkaew, A.; Maitarad, P.; Kungwan, N.; Zhang, D.; Shi, L.; Namuangruk, S. A MnN4 moiety embedded graphene as a magnetic gas sensor for CO detection: A first principle study. Appl. Surf. Sci. 2019, 473, 820–827. [Google Scholar] [CrossRef]
- Su, Y.; Xie, G.; Tai, H.; Li, S.; Yang, B.; Wang, S.; Zhang, Q.; Du, H.; Zhang, H.; Du, X.; et al. Self-powered room temperature NO2 detection driven by triboelectric nanogenerator under UV illumination. Nano Energy 2018, 47, 316–324. [Google Scholar] [CrossRef]
- Kim, D.-W.; Ha, S.; Ko, Y.-I.; Wee, J.-H.; Kim, H.J.; Jeong, S.Y.; Tojo, T.; Yang, C.-M.; Kim, Y.A. Rapid, repetitive and selective NO2 gas sensor based on boron-doped activated carbon fibers. Appl. Surf. Sci. 2020, 531, 147395. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Z.; Fang, R.; Li, H.; He, W.; Du, C. UV-assisted room temperature NO2 sensor using monolayer graphene dec-orated with SnO2 nanoparticles. Ceram. Int. 2020, 46, 2255–2260. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, C.; Wang, J.; Sun, L.; Shi, K.; Zhou, W.; Fu, H. Facile synthesis of novel 3D nanoflower-like CuxO/multilayer graphene composites for room temperature NOx gas sensor application. Nanoscale 2014, 6, 7369–7378. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, J.; Song, P.; Yang, Z.; Wang, Q. Synthesis of reduced graphene oxide/SnO2 nanosheets/Au nanoparticles ternary composites with enhanced formaldehyde sensing performance. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 118, 113953. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Lu, L.; Xue, J. Ultra-small Fe3O4 nanoparticle decorated graphene nanosheets with superior cyclic perfor-mance and rate capability. Nanoscale 2013, 5, 6797–6803. [Google Scholar] [CrossRef]
- You, X.; Yang, J.; Wang, M.; Wang, H.; Gao, L.; Dong, S. Interconnected graphene scaffolds for functional gas sensors with tunable sensitivity. J. Mater. Sci. Technol. 2020, 58, 16–23. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C.; et al. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Saleh, T.A.; Fadillah, G. Recent trends in the design of chemical sensors based on graphene–metal oxide nanocomposites for the analysis of toxic species and biomolecules. TrAC Trends Anal. Chem. 2019, 120, 115660. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Chen, R.M.; Lu, L.; Xue, J. A study of the superior electrochemical performance of 3 nm SnO2 nanoparticles supported by graphene. J. Mater. Chem. A 2014, 2, 5688–5695. [Google Scholar] [CrossRef]
- Yan, K.; Fu, L.; Peng, H.; Liu, Z. Designed CVD Growth of Graphene via Process Engineering. Accounts Chem. Res. 2012, 46, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, F.; Wang, H.; Liu, J.; Zheng, Y.; Hou, S. Chemical vapor deposition graphene combined with Pt nanoparticles applied in non-enzymatic sensing of ultralow concentrations of hydrogen peroxide. RSC Adv. 2017, 7, 30542–30547. [Google Scholar] [CrossRef]
- Tromp, R.M.; Hannon, J.B. Thermodynamics and kinetics of graphene growth on SiC(0001). Phys. Rev. Lett. 2009, 102, 106104. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Haridas, V.; Sukhananazerin, A.; Sneha, J.M.; Pullithadathil, B.; Narayanan, B. α-Fe2O3 loaded less-defective graphene sheets as chemiresistive gas sensor for selective sensing of NH3. Appl. Surf. Sci. 2020, 517, 146158. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; Cui, P.; Guo, S.; Liu, W.; Wang, X. Sensitivity investigation for the dependence of monolayer and stacking graphene NH3 gas sensor. Diam. Relat. Mater. 2017, 73, 56–61. [Google Scholar] [CrossRef]
- Li, G.; Shen, Y.; Zhou, P.; Hao, F.; Fang, P.; Wei, D.; Meng, D.; San, X. Design and application of highly responsive and selective rGO-SnO2 nanocomposites for NO2 monitoring. Mater. Charact. 2020, 163, 110284. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Heish, Y.-C.; Lee, C.-T. High sensitivity detection of nitrogen oxide gas at room temperature using zinc ox-ide-reduced graphene oxide sensing membrane. J. Alloy. Compd. 2019, 773, 950–954. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Wang, Q.; Geng, B. Microwave chemical route to self-assembled quasi-spherical Cu2O microarchitectures and their gas-sensing properties. Sens. Actuators B Chem. 2009, 143, 253–260. [Google Scholar] [CrossRef]
- Jayawardena, S.; Siriwardena, H.D.; Rajapakse, R.; Kubono, A.; Shimomura, M. Fabrication of a quartz crystal microbalance sensor based on graphene oxide/TiO2 composite for the detection of chemical vapors at room temperature. Appl. Surf. Sci. 2019, 493, 250–260. [Google Scholar] [CrossRef]
- Shafiei, M.; Bradford, J.; Khan, H.; Piloto, C.; Wlodarski, W.; Li, Y.; Motta, N. Low-operating temperature NO2 gas sensors based on hybrid two-dimensional SnS2-reduced graphene oxide. Appl. Surf. Sci. 2018, 462, 330–336. [Google Scholar] [CrossRef]
- Na, C.W.; Kim, J.-H.; Kim, H.-J.; Woo, H.-S.; Gupta, A.; Kim, H.-K.; Lee, J.-H. Highly selective and sensitive detection of NO2 using rGO-In2O3 structure on flexible substrate at low temperature. Sens. Actuators B Chem. 2018, 255, 1671–1679. [Google Scholar] [CrossRef]
- Bai, S.; Li, Q.; Han, N.; Zhang, K.; Tang, P.; Feng, Y.; Luo, R.; Li, D.; Chen, A. Synthesis of novel BiVO4/Cu2O heterojunctions for improving BiVO4 towards NO2 sensing properties. J. Colloid Interface Sci. 2020, 567, 37–44. [Google Scholar] [CrossRef]
- Li, Q.; Han, N.; Zhang, K.; Bai, S.; Guo, J.; Luo, R.; Li, D.; Chen, A. Novel p-n heterojunction of BiVO4/Cu2O decorated with rGO for low concentration of NO2 detection. Sens. Actuators B Chem. 2020, 320, 128284. [Google Scholar] [CrossRef]
- Lu, W.-C.; Kumar, S.S.; Chen, Y.-C.; Hsu, C.-M.; Lin, H.-N. Au/Cu2O/ZnO ternary nanocomposite for low concentration NO2 gas sensing at room temperature. Mater. Lett. 2019, 256, 126657. [Google Scholar] [CrossRef]
- Ha, N.H.; Thinh, D.D.; Huong, N.T.; Phuong, N.H.; Thach, P.D.; Hong, H.S. Fast response of carbon monoxide gas sensors using a highly porous network of ZnO nanoparticles decorated on 3D reduced graphene oxide. Appl. Surf. Sci. 2018, 434, 1048–1054. [Google Scholar] [CrossRef]
- Hong, H.S.; Phuong, N.H.; Huong, N.T.; Nam, N.H.; Hue, N.T. Highly sensitive and low detection limit of resistive NO2 gas sensor based on a MoS2/graphene two-dimensional heterostructures. Appl. Surf. Sci. 2019, 492, 449–454. [Google Scholar] [CrossRef]
- Su, Y.; Xie, G.; Chen, J.; Du, H.; Zhang, H.; Yuan, Z.; Ye, Z.; Du, X.; Tai, H.; Jiang, Y. Reduced graphene oxide–polyethylene oxide hybrid films for toluene sensing at room temperature. RSC Adv. 2016, 6, 97840–97847. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, X.; Wang, Y.; Liu, G.; Zhu, X.; Huang, Y.; Guo, Y.; Gao, C.; Zhou, M. Study on gas sensing of reduced graphene oxide/ZnO thin film at room temperature. Sens. Actuators B Chem. 2017, 240, 870–880. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, A.X.; Li, Y. Nearly Monodisperse Cu2O and CuO Nanospheres: Preparation and Applications for Sensitive Gas Sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yang, W.; Ding, K.; Feng, L.; Guan, Y. Cu2O nanorods modified by reduced graphene oxide for NH3 sensing at room temperature. J. Mater. Chem. A 2015, 3, 1174–1181. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, R.; Zhou, T.; Lou, Z.; Deng, J.; Zhang, T. Concave Cu2O octahedral nanoparticles as an advanced sensing material for benzene (C6H6) and nitrogen dioxide (NO2) detection. Sens. Actuators B Chem. 2016, 223, 311–317. [Google Scholar] [CrossRef]
- Shen, Y.; Tian, F.H.; Chen, S.; Ma, Z.; Zhao, L.; Jia, X. Density functional theory study on the mechanism of CO sensing on Cu2O (111) surface: Influence of the pre-adsorbed oxygen atom. Appl. Surf. Sci. 2014, 288, 452–457. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.; Liu, Y.; Zhang, J.; Liu, J.; Shu, S.; Kang, X.; Song, Q.; Liu, D.; Huang, F.; et al. NiO-rGO composite for supercapacitor electrode. Surf. Interfaces 2020, 18, 100420. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Guo, Y. Cuprous oxide nanowires/nanoparticles decorated on reduced graphene oxide nanosheets: Sensitive and selective H2S detection at low temperature. Mater. Lett. 2019, 254, 336–339. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K.; Motokawa, M. Magneto-electropolymerization of conducting polypyrrole. Phys. B Condens. Matter 1998, 246-247, 412–415. [Google Scholar] [CrossRef]
- Bora, C.; Dolui, S. Fabrication of polypyrrole/graphene oxide nanocomposites by liquid/liquid interfacial polymerization and evaluation of their optical, electrical and electrochemical properties. Polymer 2012, 53, 923–932. [Google Scholar] [CrossRef]
- Kim, D.-H.; Richardson-Burns, S.M.; Hendricks, J.L.; Sequera, C.; Martin, D.C. Effect of Immobilized Nerve Growth Factor on Conductive Polymers: Electrical Properties and Cellular Response. Adv. Funct. Mater. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Pernaut, J.-M.; Reynolds, J.R. Use of Conducting Electroactive Polymers for Drug Delivery and Sensing of Bioactive Molecules. A Redox Chemistry Approach. J. Phys. Chem. B 2000, 104, 4080–4090. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Li, J.; Kou, L.; Huang, J.; Feng, Y.; Chen, S. Cu/Cu2O@Ppy nanowires as a long-life and high-capacity anode for lithium ion battery. Chem. Eng. J. 2020, 391, 123597. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, Y.; Liu, A.; Zhang, Y.; Sui, Y.; Hu, S.; Li, J.; Kang, H.; Wang, S.; Zhao, S.; et al. One-step hydrothermal synthesis of three-dimensional structures of MoS2/Cu2S hybrids via a copper foam-assisted method. Mater. Lett. 2020, 273, 127928. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Yan, X.-Y.; Tong, X.-L.; Zhang, Y.-F.; Han, X.-D.; Wang, Y.-Y.; Jin, G.-Q.; Qin, Y.; Guo, X.-Y. Cuprous oxide nanoparticles dis-persed on reduced graphene oxide as an efficient electrocatalyst for oxygen reduction reaction. Chem. Commun. 2012, 48, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, T.; Hu, L.; Wang, Y. A facile one-pot synthesis of Cu2O/RGO nanocomposite for removal of organic pollutant. J. Phys. Chem. Solids 2013, 74, 635–640. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Dong, L.; Xu, Z.; Liu, Y.; Hu, N.; Kong, E.S.-W.; Zhao, J.; Peng, C. Gas Sensors Based on Chemically Reduced Holey Graphene Oxide Thin Films. Nanoscale Res. Lett. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Highly conductive chemically converted graphene prepared from mildly oxidized graphene oxide. J. Mater. Chem. 2011, 21, 7376–7380. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal Reduction of Chemically Exfoliated Graphene Sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef]
- Miankushki, H.N.; Sedghi, A.; Baghshahi, S. Facile and scalable fabrication of graphene/polypyrrole/MnOx/Cu(OH)2 composite for high-performance supercapacitors. J. Solid State Electrochem. 2018, 22, 3317–3329. [Google Scholar] [CrossRef]
- Chakravarty, A.; Bhowmik, K.; Mukherjee, A.; De, G. Cu2O Nanoparticles Anchored on Amine-Functionalized Graphite Nanosheet: A Potential Reusable Catalyst. Langmuir 2015, 31, 5210–5219. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.-Q.; Zu, S.-Z.; Peng, K.; Zhou, D.; Han, B.-H.; Zhang, Z. The Effect of Interlayer Adhesion on the Mechanical Behaviors of Macroscopic Graphene Oxide Papers. ACS Nano 2011, 5, 2134–2141. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, N.; Zhou, Z.; Xu, D.; Wang, Z.; Yang, Z.; Wei, H.; Kong, E.S.-W.; Zhang, Y. Single-walled carbon nanotube/cobalt phthalocyanine derivative hybrid material: Preparation, characterization and its gas sensing properties. J. Mater. Chem. 2011, 21, 3779–3787. [Google Scholar] [CrossRef]
- Sun, J.; Sun, L.; Han, N.; Chu, H.; Bai, S.; Shu, X.; Luo, R.; Chen, A. rGO decorated CdS/CdO composite for detection of low concentration NO2. Sens. Actuators B Chem. 2019, 299, 126832. [Google Scholar] [CrossRef]
- Mane, A.; Navale, S.; Sen, S.; Aswal, D.; Gupta, S.K.; Patil, V. Nitrogen dioxide (NO2) sensing performance of p-polypyrrole/n-tungsten oxide hybrid nanocomposites at room temperature. Org. Electron. 2015, 16, 195–204. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Rehman, A.U.; Gong, L.; Kan, K.; Li, L.; Shi, K. Synthesis of NiO@CuO nanocomposite as high-performance gas sensing material for NO2 at room temperature. Appl. Surf. Sci. 2017, 412, 230–237. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Wang, Y.; Tai, H.; Guo, Y. UV Illumination-Enhanced Molecular Ammonia Detection Based on a Ternary-Reduced Graphene Oxide–Titanium Dioxide–Au Composite Film at Room Temperature. Anal. Chem. 2018, 91, 3311–3318. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, C.; Guo, Y. UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet-ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 2018, 6, 10286–10296. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wu, X.-H.; Zhou, Z.-L. A new type of semiconductor gas sensor based on the n+n combined structure. Sens. Actuators B Chem. 2001, 73, 216–220. [Google Scholar] [CrossRef]
- Lu, G.; Ocola, L.E.; Chen, J. Gas detection using low-temperature reduced graphene oxide sheets. Appl. Phys. Lett. 2009, 94, 083111. [Google Scholar] [CrossRef]







| Material | Response | Concentration | Working Temperature | Reference |
|---|---|---|---|---|
| SnO2/graphene | 0.25 (∆R/Ra) | 10 ppm | Room temperature | [6] |
| rGO-SnO2 | 53.57 (Rg/Ra) | 3 ppm | 125 °C | [22] |
| SnS2-rGO | 9.8% (∆R/Ra) | 0.6 ppm | 80 °C | [27] |
| rGO/In2O3 | 22.3 (Rg/Ra) | 500 ppb | 150 °C | [28] |
| BiVO4/Cu2O | 4.2 (Rg/Ra) | 4 ppm | 60 °C | [29] |
| BiVO4/Cu2O/rGO | 8.2 (Rg/Ra) | 1 ppm | 60 °C | [30] |
| Au/Cu2O/ZnO | 26% (∆R/Ra) | 5 ppb | Room temperature | [31] |
| MoS2/graphene | 69% (∆R/Ra) | 10 ppm | 200 °C | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Wang, Y.; Ying, S.; Wu, Z.; Liu, W.; Chen, D.; Peng, C. Synthesis of Cu2O-Modified Reduced Graphene Oxide for NO2 Sensors. Sensors 2021, 21, 1958. https://doi.org/10.3390/s21061958
Huang M, Wang Y, Ying S, Wu Z, Liu W, Chen D, Peng C. Synthesis of Cu2O-Modified Reduced Graphene Oxide for NO2 Sensors. Sensors. 2021; 21(6):1958. https://doi.org/10.3390/s21061958
Chicago/Turabian StyleHuang, Manman, Yanyan Wang, Shuyang Ying, Zhekun Wu, Weixiao Liu, Da Chen, and Changsi Peng. 2021. "Synthesis of Cu2O-Modified Reduced Graphene Oxide for NO2 Sensors" Sensors 21, no. 6: 1958. https://doi.org/10.3390/s21061958
APA StyleHuang, M., Wang, Y., Ying, S., Wu, Z., Liu, W., Chen, D., & Peng, C. (2021). Synthesis of Cu2O-Modified Reduced Graphene Oxide for NO2 Sensors. Sensors, 21(6), 1958. https://doi.org/10.3390/s21061958






