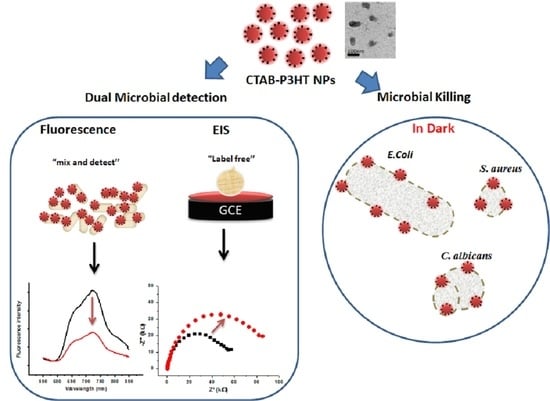
Antimicrobial Activity of Cationic Poly(3-hexylthiophene) Nanoparticles Coupled with Dual Fluorescent and Electrochemical Sensing: Theragnostic Prospect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
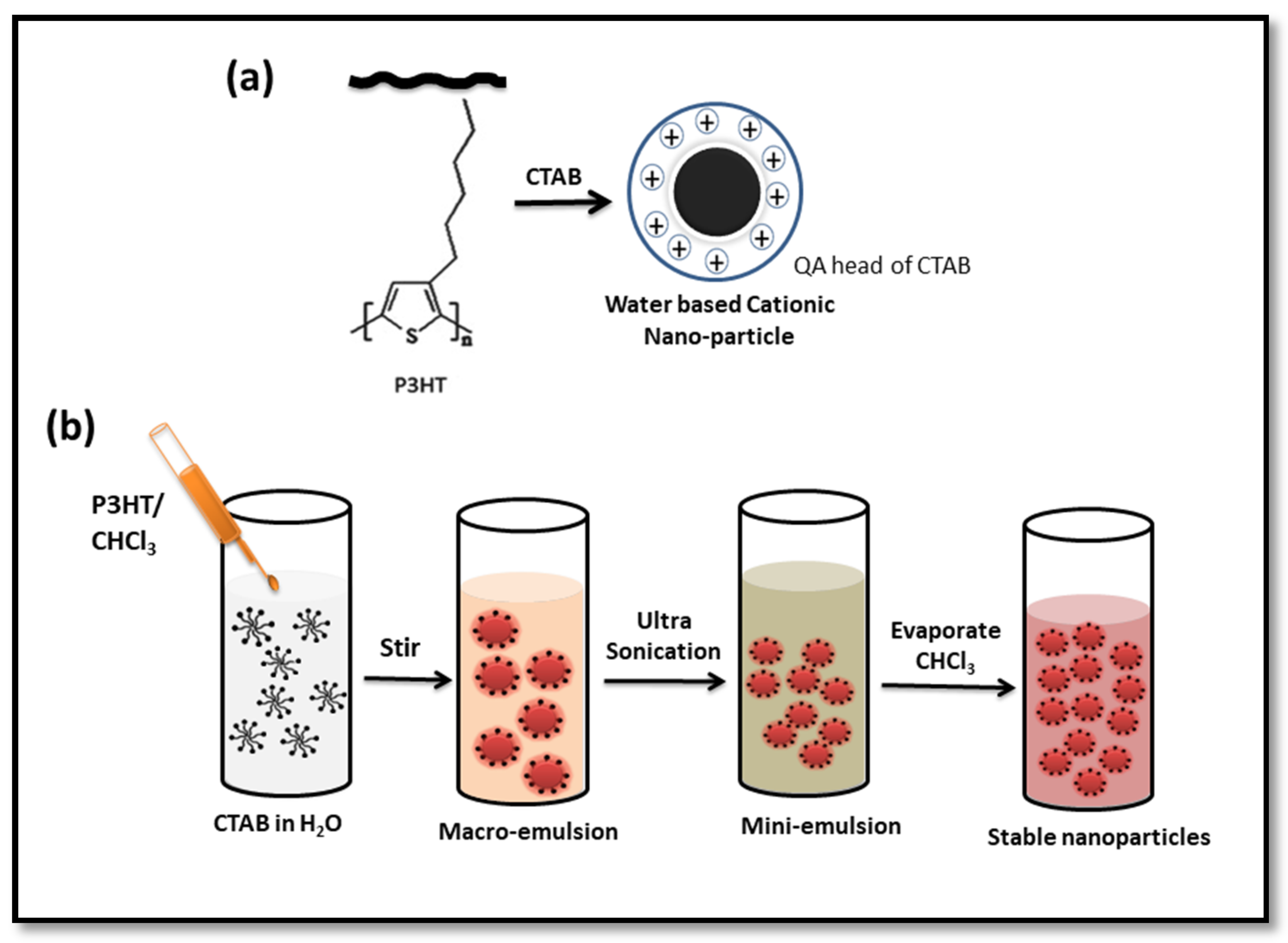
2.2. Preparation of P3HT-NPs
2.3. Bacterial Cell Biosensing Assay
2.3.1. Fluorescence Detection
2.3.2. Electrochemical Detection
2.4. Antimicrobial Activity of CTAB-P3HT NPs
2.4.1. Minimum Inhibitory Concentration (MIC)
2.4.2. MBC and MFC Determination
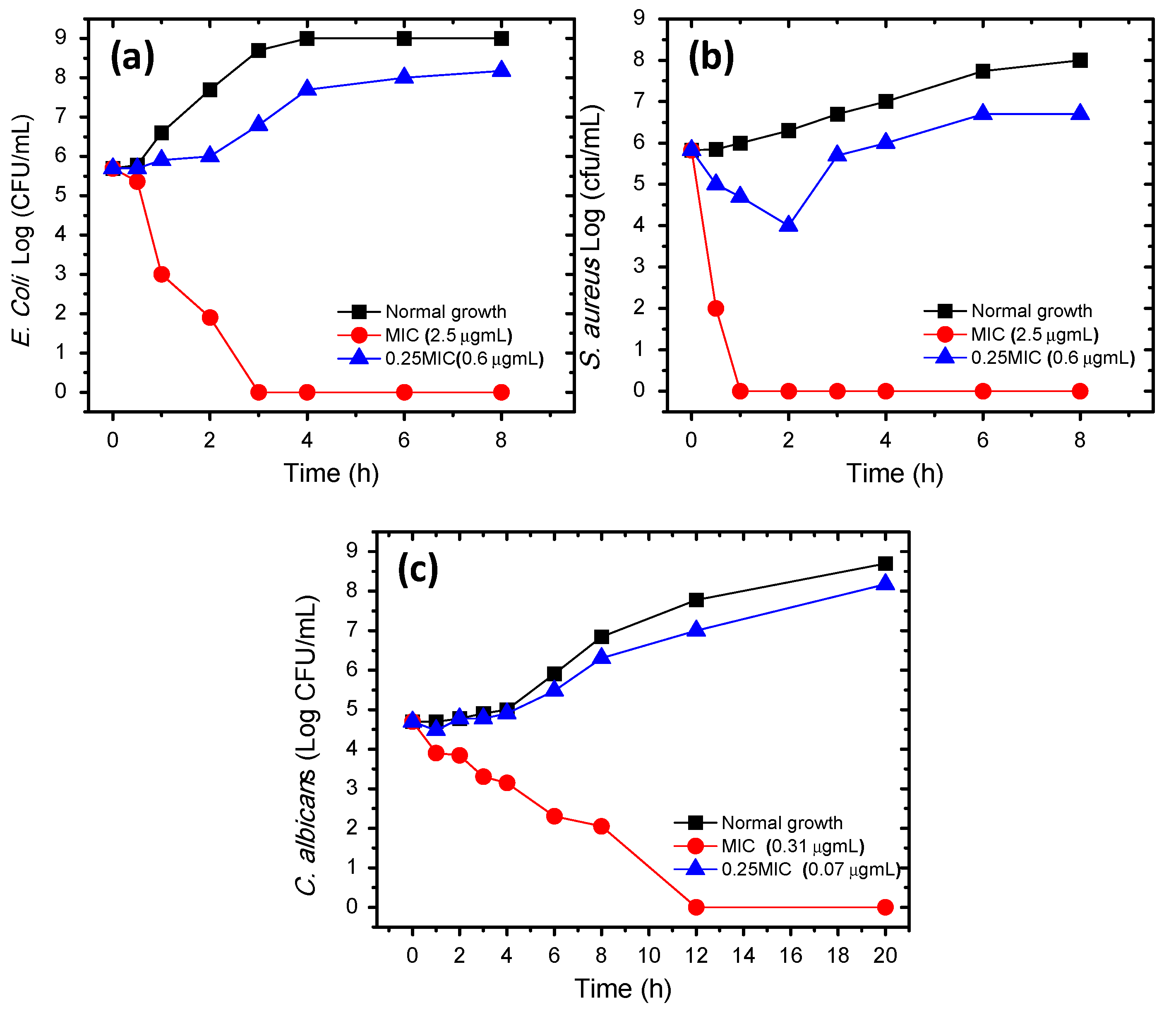
2.4.3. Time-Killing Assay
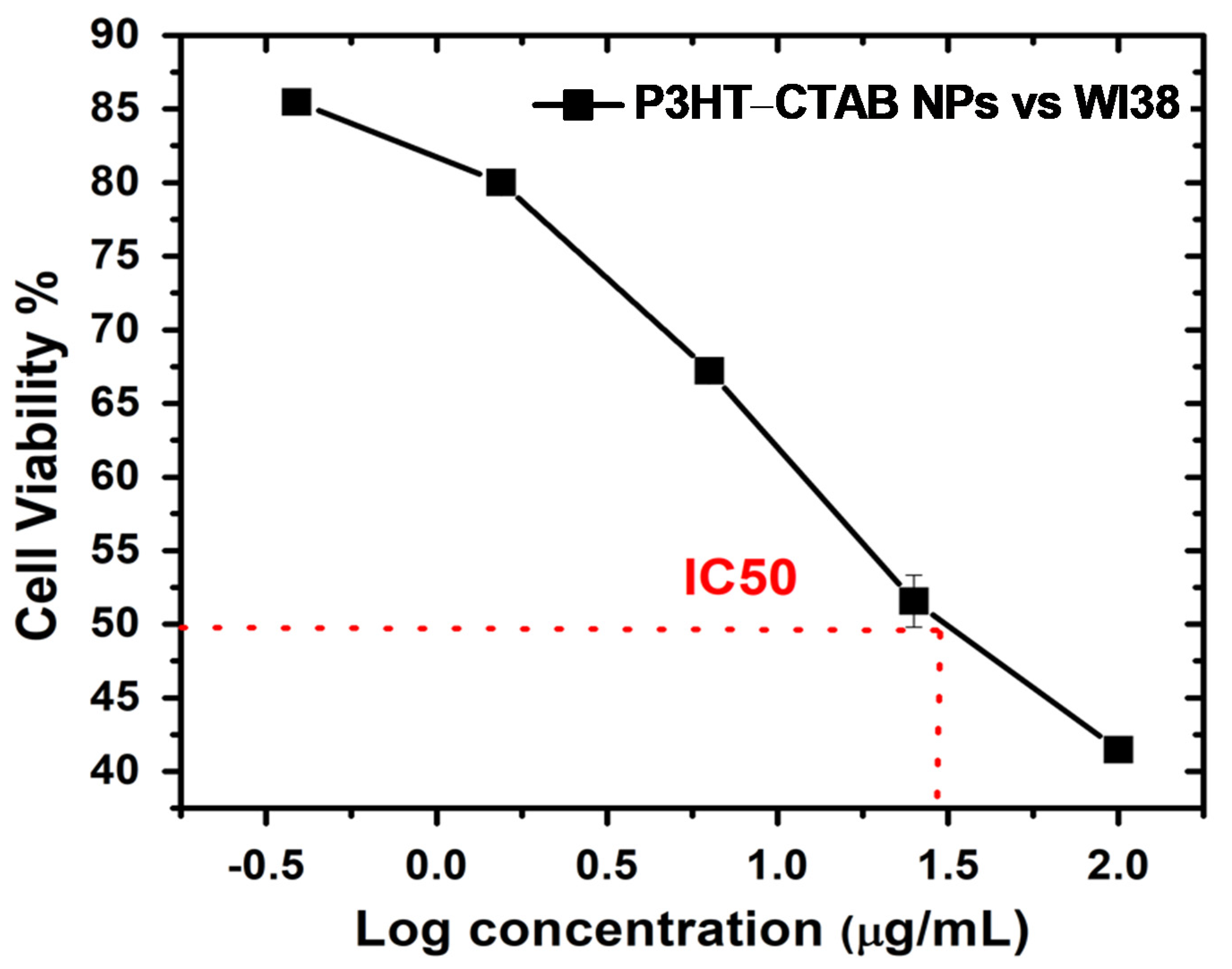
2.4.4. Cytotoxicity to Mammalian Cells
2.5. Instrumentation
3. Results and Discussion
3.1. Preparation of CTAB-P3HT NPs
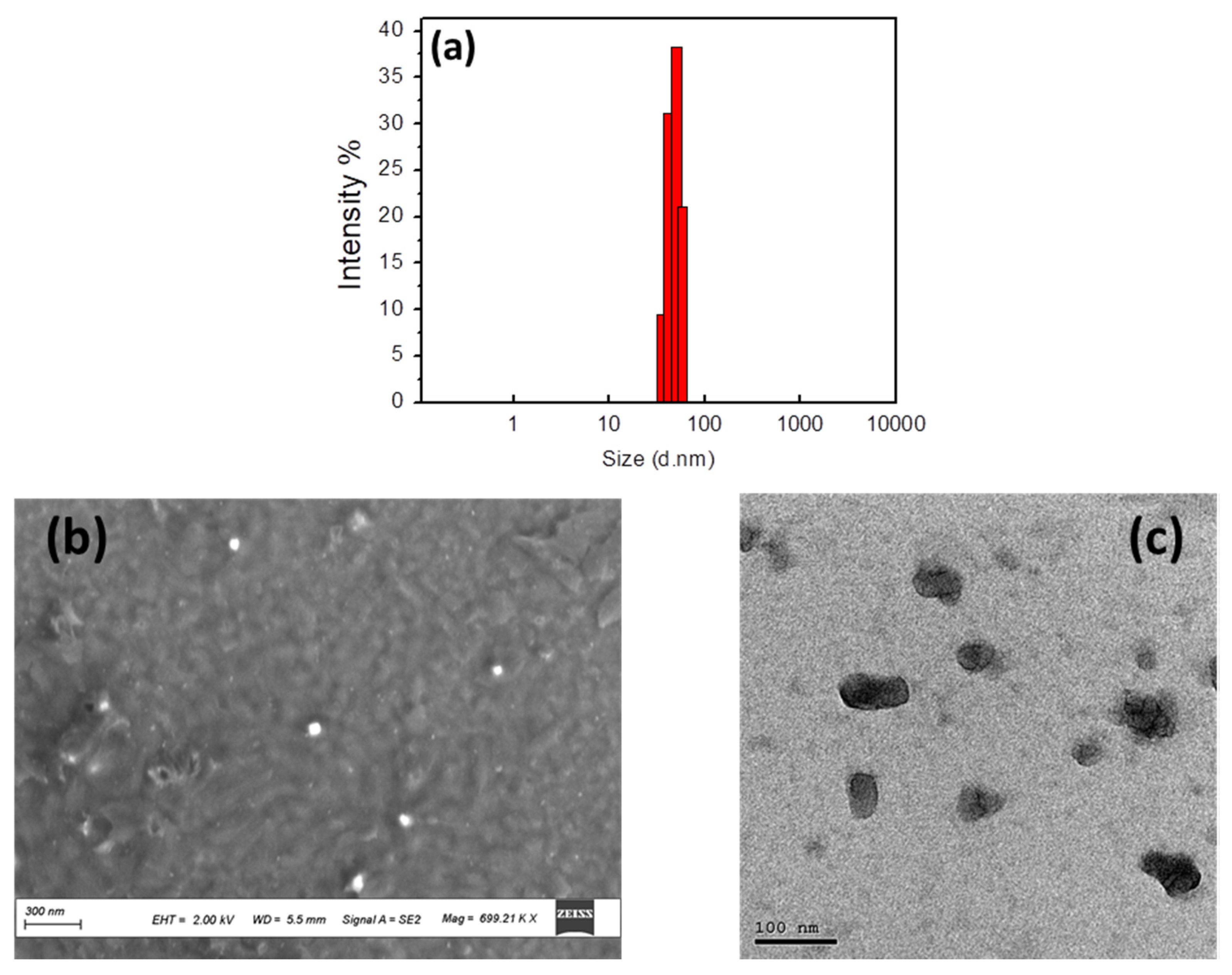
3.2. Characterization of CTAB-P3HT NPs
3.2.1. Morphological Characterization
3.2.2. Optical and Electrochemical Characterization
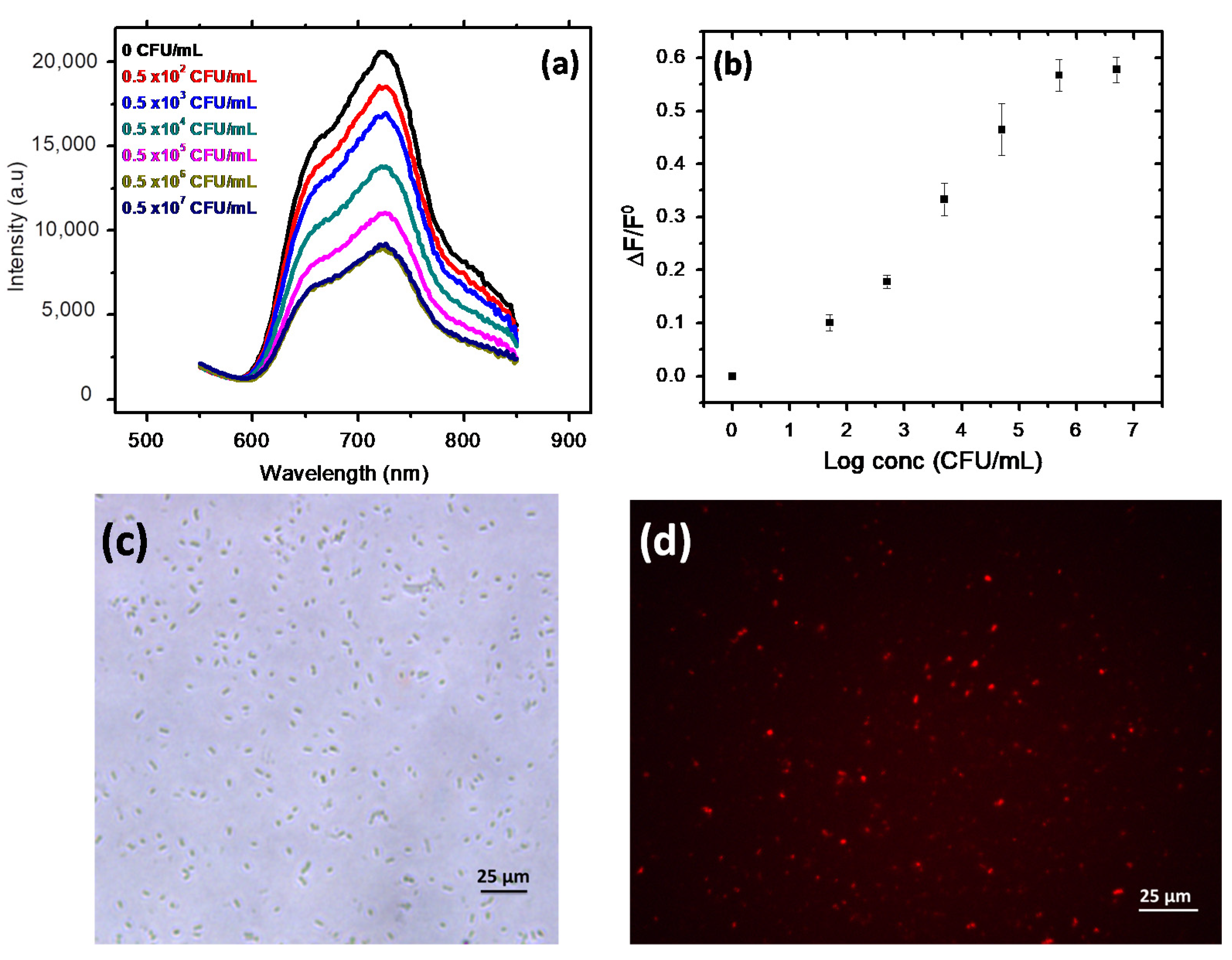
3.3. Microbial Cell Biosensing
3.3.1. Fluorescence Detection
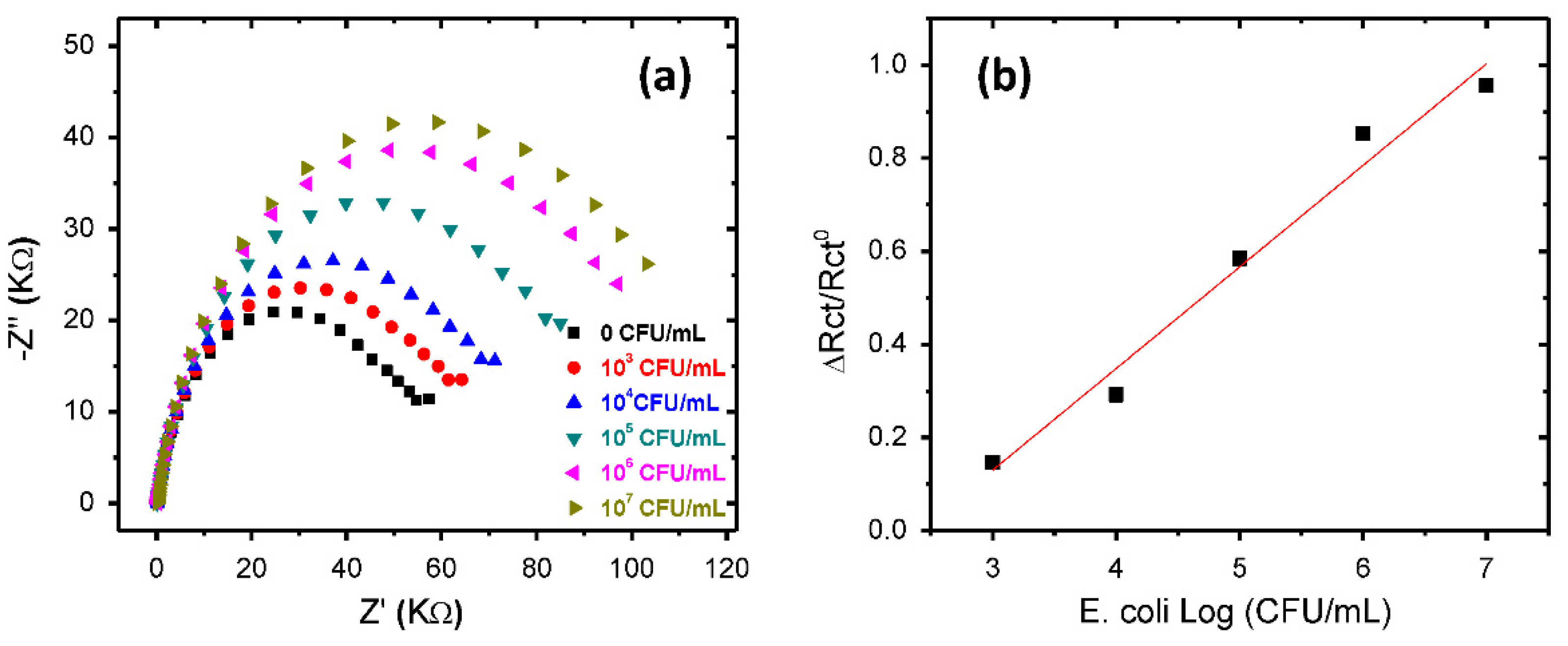
3.3.2. Electrochemical Detection
3.4. Antimicrobial Activity
3.5. Biocompatibility of CTAB-P3HT NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Lambert, G.; Liao, D.; Kim, H.; Robin, K.; Tung, C.K.; Pourmand, N.; Austin, R.H. Acceleration of emergence of bacterial antibiotic resistance in connected microenvironments. Science 2011, 333, 1764–1767. [Google Scholar] [CrossRef]
- Ma, B.C.; Ghasimi, S.; Landfester, K.; Zhang, K.A.I. Enhanced visible light promoted antibacterial efficiency of conjugated microporous polymer nanoparticles via molecular doping. J. Mater. Chem. B 2016, 4, 5112–5118. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Xie, S.; Manuguri, S.; Proietti, G.; Romson, J.; Fu, Y.; Inge, A.K.; Wu, B.; Zhang, Y.; Häll, D.; Ramström, O.; et al. Design and synthesis of theranostic antibiotic nanodrugs that display enhanced antibacterial activity and luminescence. Proc. Natl. Acad. Sci. USA 2017, 114, 8464–8469. [Google Scholar] [CrossRef]
- Ghosh, R.; Malhotra, M.; Sathe, R.R.M.; Jayakannan, M. Biodegradable polymer theranostic fluorescent nanoprobe for direct visualization and quantitative determination of antimicrobial activity. Biomacromolecules 2020, 21, 2896–2912. [Google Scholar] [CrossRef]
- Tagit, O.; Hildebrandt, N. Fluorescence sensing of circulating diagnostic biomarkers using molecular probes and nanoparticles. ACS Sens. 2017, 2, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, W.; Drzozga, K.; Baluta, S.; Cabaj, J.; Malecha, K. A fluorescent biosensors for detection vital body fluids’ agents. Sensors 2018, 18, 2357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yan, R.; Yi, X.; Li, J.; Rao, J.; Guo, Z.; Yang, Y.; Li, W.; Li, Y.Q.; Chen, C. Bacteria-activated theranostic nanoprobes against methicillin-resistant staphylococcus aureus infection. ACS Nano 2017, 11, 4428–4438. [Google Scholar] [CrossRef]
- Kim, T.; Zhang, Q.; Li, J.; Zhang, L.; Jokerst, J.V. A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano 2018, 12, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Jin, S.-M.; Han, E.H.; Ko, E.; Ahn, M.; Bang, W.-Y.; Bang, J.-K.; Lee, E. Structure-dependent antimicrobial theranostic functions of self-assembled short peptide nanoagents. Biomacromolecules 2017, 18, 3600–3610. [Google Scholar] [CrossRef] [PubMed]
- Irwansyah, I.; Li, Y.Q.; Shi, W.; Qi, D.; Leow, W.R.; Tang, M.B.Y.; Li, S.; Chen, X. Gram-positive antimicrobial activity of amino acid-based hydrogels. Adv. Mater. 2015, 27, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Wang, L. Dual-mode antibacterial conjugated polymer nanoparticles for photothermal and photodynamic therapy. Macromol. Biosci. 2020, 2, 1900301. [Google Scholar] [CrossRef]
- Peng, F.; Qiu, L.; Chai, R.; Meng, F.; Yan, C.; Chen, Y.; Xing, C. Conjugated polymer-based nanoparticles for cancer cell-targeted and image-guided photodynamic therapy. Macromol. Chem. Phys. 2018, 4, 1700440. [Google Scholar] [CrossRef]
- Pan, H.M.; Gonuguntla, S.; Li, S.; Trau, D. 3.33 Conjugated polymers for biosensor devices. Compr. Biomater. 2017, 3, 716–754. [Google Scholar]
- Li, G.; Yang, T.; Zhao, W.; Liu, S.; Huang, W.; Zhao, Q. Luminescent conjugated polymer dots for biomedical applications. In Nanophotonics in Biomedical Engineering; Springer: Singapore, 2021. [Google Scholar]
- Wu, X.; Chiu, D.T. Conjugated polymer nanoparticles and semiconducting polymer dots for molecular sensing and in vivo and cellular imaging. Conjug. Polym. Biol. Biomed. Appl. 2018, 59–85. [Google Scholar] [CrossRef]
- Repenko, T.; Rix, A.; Ludwanowski, S.; Go, D.; Kiessling, F.; Lederle, W.; Kuehne, A.J.C. Bio-degradable highly fluorescent conjugated polymer nanoparticles for bio-medical imaging applications. Nat. Commun. 2017, 8, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pecher, J.; Mecking, S. Nanoparticles of conjugated polymers. Chem. Rev. 2010, 110, 6260–6279. [Google Scholar] [CrossRef]
- Kuehne, A.J.C. Conjugated polymer nanoparticles toward in vivo theranostics—Focus on targeting, imaging, therapy, and the importance of clearance. Adv. Biosyst. 2017, 1. [Google Scholar] [CrossRef]
- Wu, C.F.; Jin, Y.H.; Schneider, T.; Burnham, D.R.; Smithand, P.B.; Chiu, D.T. Ultrabright and bioorthogonal labeling of cellular targets using semiconducting polymer dots and click chemistry. Angew. Chem. 2010, 49, 9436–9440. [Google Scholar] [CrossRef]
- He, P.; Lv, F.; Liu, L.; Wang, S. Cationic conjugated polymers for detection and inactivation of pathogens. Sci. China Chem. 2017, 60, 1567–1574. [Google Scholar] [CrossRef]
- Zehra, N.; Dutta, D.; Malik, A.H. Fluorescence resonance energy transfer based wash-free bacterial imaging and antibacterial application using cationic conjugated polyelectrolyte. ACS Appl. Mater. Interfaces 2018, 10, 27603–27611. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, Z.; Zhao, Y.; Tang, Y. Efficient antibacterial performance and effect of structure on property based on cationic conjugated polymers. Macromolecules 2018, 51, 7239–7247. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Liu, L.; Li, L. Flexible antibacterial film based on conjugated polyelectrolyte/silver nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 9051–9058. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Zhu, J.; Tang, Y.; Canady, T.D.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Dark antimicrobial mechanisms of cationic phenylene ethynylene polymers and oligomers against Escherichia coli. Polymers 2011, 3, 1199–1214. [Google Scholar] [CrossRef]
- Wang, Y.; Corbitt, T.S.; Jett, S.D.; Tang, Y.; Schanze, K.S.; Chi, E.Y.; Whitten, D.G. Direct visualization of bactericidal action of cationic conjugated polyelectrolytes and oligomers. Langmuir 2012, 28, 65–70. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Ji, T.; Anderson, G.; Nie, G.; Zhao, Y. Biodegradable cationic ε-poly-L-lysine-conjugated polymeric nanoparticles as a new effective antibacterial agent. Sci. Bull. 2015, 60, 216–226. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, Z.; Liu, L.; Lv, F.; Wang, Y.; Wang, S. Cationic conjugated polymers for discrimination of microbial pathogens. Adv. Mater. 2014, 26, 4333–4338. [Google Scholar] [CrossRef]
- Huang, Y.; Pappas, H.C.; Zhang, L.; Wang, S.; Cai, R.; Tan, W.; Wang, S.; Whitten, D.G.; Schanze, K.S. Selective imaging and inactivation of bacteria over mammalian cells by imidazolium-substituted polythiophene. Chem. Mater. 2017, 29, 6389–6395. [Google Scholar] [CrossRef]
- Brown, D.M.; Yang, J.; Strach, E.W.; Khalil, M.I.; Whitten, D.G. Size and substitution effect on antimicrobial activity of polythiophene polyelectrolyte derivatives under photolysis and dark conditions. Photochem. Photobiol. 2018, 94, 1116–1123. [Google Scholar] [CrossRef]
- Scheberl, A.; Khalil, M.L.; Maghsoodi, F.; Strach, E.W.; Yang, J.; Chi, E.Y.; Schanze, K.S.; Reimhult, E.; Whitten, D.G. Quantitative determination of dark and light-activated antimicrobial activity of poly(phenylene ethynylene), polythiophene, and oligo(phenylene ethynylene) electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 21322–21329. [Google Scholar] [CrossRef] [PubMed]
- Bag, M.; Gehan, T.S.; Algaier, D.D.; Liu, F.; Nagarjuna, G.; Lahti, P.M.; Russell, T.P.; Venkataraman, D. Efficient charge transport in assemblies of surfactant-stabilized semiconducting nanoparticles. Adv. Mater. 2013, 25, 6411–6415. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Khalil, W.F.; El-Sayyad, G.S.; El Rouby, W.M.A.; Sadek, M.A.; Farghali, A.A.; El-Batal, A.I. Graphene oxide-based nanocomposites (GO-chitosan and GO-EDTA) for outstanding antimicrobial potential against some Candida species and pathogenic bacteria. Int. J. Biol. Macromol. 2020, 164, 1370–1383. [Google Scholar] [CrossRef]
- Lemos, J.D.A.; Costa, C.R.; Araújo, C.R.D.; Silva, M.D.R.R. Susceptibility testing of candida albicans isolated from oropharyngeal mucosa of HIV+ patients to fluconazole, amphotericin b and caspofungin. killing kinetics of caspofungin and amphotericin b against fluconazole resistant and susceptible isolates. Brazilian J. Microbiol. 2009, 40, 163–169. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards; Barry, A.L. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999. [Google Scholar]
- Nagarjuna, G.; Baghgar, M.; Labastide, J.A.; Algaier, D.D.; Barnes, M.D.; Venkataraman, D. Tuning aggregation of poly(3-hexylthiophene) within nanoparticles. ACS Nano 2012, 12, 10750–10758. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, S.; Fortin, P.; Tiwari, V.; Srivastva, U.; Sharma, A.; Holdcroft, S. Photocathodic hydrogen evolution from catalysed nanoparticle films prepared from stable aqueous dispersions of P3HT and PCBM. Synth. Met. 2019, 247, 10–17. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Q.; Zhang, Z.; Lu, Z.; Zhao, Y.; Tang, Y. Fluorescent conjugated polymer/quarternary ammonium salt co-assembly nanoparticles: Applications in highly effective antibacteria and bioimaging. ACS Appl. Bio Mater. 2018, 1, 1478–1486. [Google Scholar] [CrossRef]
- Algaier, D.D. Impact of Fabrication Parameters on the Internal Structure of Poly(3-Hexylthiophene) Nanoparticles. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2015. [Google Scholar]
- Istif, E.; Kagkoura, A.; Hernandez-Ferrer, J.; Stergiou, A.; Skaltsas, T.; Arenal, R.; Tagmatarchis, N. Self-assembled core–shell cdte/poly (3-hexylthiophene) nanoensembles as novel donor–acceptor light-harvesting systems. ACS Appl. Mater. Interfaces 2017, 51, 44695–44703. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.P.; Bérubé, E.; Bissonnette, L.; Bergeron, M.G.; Leclerc, M. Polythiophene biosensor for rapid detection of microbial particles in water. ACS Appl. Mater. Interfaces 2013, 5, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Disney, M.D.; Zheng, J.; Swager, T.M.; Seeberger, P.H. Detection of bacteria with carbohydrate-functionalized fluorescent polymers. J. Am. Chem. Soc. 2004, 126, 13343–13346. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.R.; Singh, A.K.; Ramesh, A.; Chattopadhyay, A. Rapid estimation of bacteria by a fluorescent gold nanoparticle- polythiophene composite. Langmuir 2008, 24, 11995–12000. [Google Scholar] [CrossRef]
- Elgiddawy, N.; Ren, S.; Yassar, A.; Louis-Joseph, A.; Sauriat-Dorizon, H.; El Rouby, W.M.A.; El-Gendy, A.O.; Farghali, A.A.; Korri-Youssoufi, H. Dispersible conjugated polymer nanoparticles as biointerface materials for label-free bacteria detection. ACS Appl. Mater. Interfaces 2020, 12, 39979–39990. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yuan, H.; Li, R.; Fan, Y.; Zhan, Y.; Qi, J.; An, H.; Niu, R.; Li, G.; Xing, C. Conjugated polythiophene/porphyrin complex for rapid and simple detection of bacteria in drinking water. Macromol. Chem. Phys. 2015, 15, 1603–1608. [Google Scholar] [CrossRef]
- Maneedaeng, A.; Phoemboon, S.; Chanthasena, P.; Chudapongse, N. Synthesis, interfacial properties, and antimicrobial activity of a new cationic gemini surfactant. Korean J. Chem. Eng. 2018, 35, 2313–2320. [Google Scholar] [CrossRef]

| Name | Zeta Potential (mv) | SD |
|---|---|---|
| CTAB-P3HT NPs | +44.1 mv | ±5.07 mv |
| E. coli | −45.5 mv | ±5.59 mv |
| S. aureus | −39.3 mv | ±4.81 mv |
| C. albicans | −32.8 mv | ±4.54 mv |
| Element | Rs (Ω) | Rct (KΩ) | CPE (µF) | N | W1 | X2 |
|---|---|---|---|---|---|---|
| P3HT | 172 | 79.2 | 4.2 | 0.85 | ---- | 0.019 |
| CTAB-P3HT NPs | 123 | 51.3 | 2.64 | 0.86 | 0.00014 | 0.012 |
| Pathogen | CTAB-P3HT NPs | |
|---|---|---|
| MIC (µg/mL) | MBC/MFC(µg/mL) | |
| E. coli | 2.5 | 2.5 |
| S. aureus | 2.5 | 2.5 |
| C. albicans | 0.312 | 0.312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgiddawy, N.; Ren, S.; Ghattas, W.; Rouby, W.M.A.E.; El-Gendy, A.O.; Farghali, A.A.; Yassar, A.; Korri-Youssoufi, H. Antimicrobial Activity of Cationic Poly(3-hexylthiophene) Nanoparticles Coupled with Dual Fluorescent and Electrochemical Sensing: Theragnostic Prospect. Sensors 2021, 21, 1715. https://doi.org/10.3390/s21051715
Elgiddawy N, Ren S, Ghattas W, Rouby WMAE, El-Gendy AO, Farghali AA, Yassar A, Korri-Youssoufi H. Antimicrobial Activity of Cationic Poly(3-hexylthiophene) Nanoparticles Coupled with Dual Fluorescent and Electrochemical Sensing: Theragnostic Prospect. Sensors. 2021; 21(5):1715. https://doi.org/10.3390/s21051715
Chicago/Turabian StyleElgiddawy, Nada, Shiwei Ren, Wadih Ghattas, Waleed M. A. El Rouby, Ahmed O. El-Gendy, Ahmed A. Farghali, Abderrahim Yassar, and Hafsa Korri-Youssoufi. 2021. "Antimicrobial Activity of Cationic Poly(3-hexylthiophene) Nanoparticles Coupled with Dual Fluorescent and Electrochemical Sensing: Theragnostic Prospect" Sensors 21, no. 5: 1715. https://doi.org/10.3390/s21051715
APA StyleElgiddawy, N., Ren, S., Ghattas, W., Rouby, W. M. A. E., El-Gendy, A. O., Farghali, A. A., Yassar, A., & Korri-Youssoufi, H. (2021). Antimicrobial Activity of Cationic Poly(3-hexylthiophene) Nanoparticles Coupled with Dual Fluorescent and Electrochemical Sensing: Theragnostic Prospect. Sensors, 21(5), 1715. https://doi.org/10.3390/s21051715