Smartphone-Based Prediction Model for Postoperative Cardiac Surgery Outcomes Using Preoperative Gait and Posture Measures
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doering, L.V.; McGuire, A.W.; Rourke, D. Recovering from cardiac surgery: What patients want you to know. Am. J. Crit. Care 2002, 11, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Zingone, B.; Gatti, G.; Rauber, E.; Tiziani, P.; Dreas, L.; Pappalardo, A.; Benussi, B.; Spina, A. Early and late outcomes of cardiac surgery in octogenarians. Ann. Thorac. Surg. 2009, 87, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gatti, G.; Cardu, G.; Lusa, A.M.; Pugliese, P. Predictors of postoperative complications in high-risk octogenarians undergoing cardiac operations. Ann. Thorac. Surg. 2002, 74, 671–677. [Google Scholar] [CrossRef]
- Graham, M.M. Survival After Coronary Revascularization in the Elderly. Circulation 2002, 105, 2378–2384. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef]
- Purser, J.L.; Kuchibhatla, M.N.; Fillenbaum, G.G.; Harding, T.; Peterson, E.D.; Alexander, K.P. Identifying frailty in hospitalized older adults with significant coronary artery disease. J. Am. Geriatr. Soc. 2006, 54, 1674–1681. [Google Scholar] [CrossRef]
- Dumurgier, J.; Elbaz, A.; Ducimetiere, P.; Tavernier, B.; Alperovitch, A.; Tzourio, C. Slow walking speed and cardiovascular death in well functioning older adults: Prospective cohort study. BMJ 2009, 339, b4460. [Google Scholar] [CrossRef]
- Afilalo, J.; Eisenberg, M.J.; Morin, J.F.; Bergman, H.; Monette, J.; Noiseux, N.; Perrault, L.P.; Alexander, K.P.; Langlois, Y.; Dendukuri, N.; et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 2010, 56, 1668–1676. [Google Scholar] [CrossRef]
- Soangra, R.; Lockhart, T.E. Agreement in gait speed from smartphone and stopwatch for five meter walk in laboratory and clinical environments. Biomed. Sci. Instrum. 2014, 50, 254–264. [Google Scholar]
- Soangra, R.; Lockhart, T.E. Inertial Sensor-Based Variables Are Indicators of Frailty and Adverse Post-Operative Outcomes in Cardiovascular Disease Patients. Sensors 2018, 18, 1792. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Sekine, M.; Tamura, T.; Yoshida, M.; Suda, Y.; Kimura, Y.; Miyoshi, H.; Kijima, Y.; Higashi, Y.; Fujimoto, T. A gait abnormality measure based on root mean square of trunk acceleration. J. Neuroeng. Rehabil. 2013, 10, 118. [Google Scholar] [CrossRef]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Harbourne, R.T.; Stergiou, N. Nonlinear analysis of the development of sitting postural control. Dev. Psychobiol. 2003, 42, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.; Stergiou, N. Detecting altered postural control after cerebral concussion in athletes with normal postural stability. Br. J. Sports Med. 2005, 39, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M.; Goldberger, A.L. Physiological time-series analysis: What does regularity quantify? Am. J. Physiol. 1994, 266, H1643–H1656. [Google Scholar] [CrossRef]
- Pincus, S. Approximate entropy (ApEn) as a complexity measure. Chaos 1995, 5, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.T.; Guskiewicz, K.M.; Giuliani, C.; Marshall, S.; Mercer, V.S.; Stergiou, N. Recovery of postural control after cerebral concussion: New insights using approximate entropy. J. Athl. Train 2006, 41, 305–313. [Google Scholar] [PubMed]
- Vaillancourt, D.E.; Newell, K.M. The dynamics of resting and postural tremor in Parkinson’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2000, 111, 2046–2056. [Google Scholar] [CrossRef]
- Anderson, R.P. First publications from the Society of Thoracic Surgeons National Database. Ann. Thorac. Surg. 1994, 57, 6–7. [Google Scholar] [CrossRef]
- Soangra, R.; Lockhart, T.E.; Frames, C.W.; Zhang, J.; Moon, S.H.; Park, J. Potential for using Smartphone Accelerometers in Non-laboratory Environments. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2014, 58, 1672–1675. [Google Scholar] [CrossRef]
- Polanczyk, C.A.; Marcantonio, E.; Goldman, L.; Rohde, L.E.; Orav, J.; Mangione, C.M.; Lee, T.H. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann. Intern. Med. 2001, 134, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.M.; Darer, J.; Boult, C.; Fried, L.P.; Boult, L.; Wu, A.W. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA J. Am. Med. Assoc. 2005, 294, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Woods, N.F.; LaCroix, A.Z.; Gray, S.L.; Aragaki, A.; Cochrane, B.B.; Brunner, R.L.; Masaki, K.; Murray, A.; Newman, A.B.; Women’s Health, I. Frailty: Emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J. Am. Geriatr. Soc. 2005, 53, 1321–1330. [Google Scholar] [CrossRef]
- Fried, L.P.; Kronmal, R.A.; Newman, A.B.; Bild, D.E.; Mittelmark, M.B.; Polak, J.F.; Robbins, J.A.; Gardin, J.M. Risk factors for 5-year mortality in older adults: The Cardiovascular Health Study. JAMA J. Am. Med. Assoc. 1998, 279, 585–592. [Google Scholar] [CrossRef]
- Dasgupta, M.; Rolfson, D.B.; Stolee, P.; Borrie, M.J.; Speechley, M. Frailty is associated with postoperative complications in older adults with medical problems. Arch. Gerontol. Geriatr. 2009, 48, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Kim, S.; O’Brien, S.; Brennan, J.M.; Edwards, F.H.; Mack, M.J.; McClurken, J.B.; Cleveland, J.C.; Smith, P.K.; Shahian, D.M.; et al. Gait Speed and Operative Mortality in Older Adults Following Cardiac Surgery. JAMA Cardiol. 2016, 1, 314. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Alexander, K.P.; Mack, M.J.; Maurer, M.S.; Green, P.; Allen, L.A.; Popma, J.J.; Ferrucci, L.; Forman, D.E. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 2014, 63, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Afilalo, J.; Sharma, A.; Zhang, S.; Brennan, J.M.; Edwards, F.H.; Mack, M.J.; McClurken, J.B.; Cleveland, J.C.; Smith, P.K.; Shahian, D.M.; et al. Gait Speed and 1-Year Mortality Following Cardiac Surgery: A Landmark Analysis From the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Arozullah, A.M.; Daley, J.; Henderson, W.G.; Khuri, S.F. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann. Surg. 2000, 232, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Arozullah, A.M.; Khuri, S.F.; Henderson, W.G.; Daley, J. Participants in the National Veterans Affairs Surgical Quality Improvement, P. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann. Intern. Med. 2001, 135, 847–857. [Google Scholar] [CrossRef]
- Marcantonio, E.R.; Goldman, L.; Mangione, C.M.; Ludwig, L.E.; Muraca, B.; Haslauer, C.M.; Donaldson, M.C.; Whittemore, A.D.; Sugarbaker, D.J.; Poss, R.; et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA J. Am. Med. Assoc. 1994, 271, 134–139. [Google Scholar] [CrossRef]
- Alibhai, S.M.; Leach, M.; Tomlinson, G.; Krahn, M.D.; Fleshner, N.; Holowaty, E.; Naglie, G. 30-day mortality and major complications after radical prostatectomy: Influence of age and comorbidity. J. Natl. Cancer Inst. 2005, 97, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.F. Frailty and Its Dangerous Effects—Might Be Preventable. Ann. Intern. Med. 2004, 141, 489–492. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Graham, J.E.; Mogilner, A.J.; Rockwood, K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002, 2, 1. [Google Scholar] [CrossRef]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef]
- Haapanen, M.J.; Perälä, M.M.; Salonen, M.K.; Kajantie, E.; Simonen, M.; Pohjolainen, P.; Pesonen, A.K.; Räikkönen, K.; Eriksson, J.G.; von Bonsdorff, M.B. Early life stress and frailty in old age: The Helsinki birth cohort study. BMC Geriatr. 2018, 18. [Google Scholar] [CrossRef]
- Boyd, C.M.; Xue, Q.L.; Simpson, C.F.; Guralnik, J.M.; Fried, L.P. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am. J. Med. 2005, 118, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Stadnyk, K.; MacKnight, C.; McDowell, I.; Hebert, R.; Hogan, D.B. A brief clinical instrument to classify frailty in elderly people. Lancet 1999, 353, 205–206. [Google Scholar] [CrossRef]
- Afilalo, J. Frailty in Patients with Cardiovascular Disease: Why, When, and How to Measure. Curr. Cardiovasc. Risk Rep. 2011, 5, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; Kostsuca, S.R.; Boura, J.A. Utilization of a 5-Meter Walk Test in Evaluating Self-selected Gait Speed during Preoperative Screening of Patients Scheduled for Cardiac Surgery. Cardiopulm. Phys. Ther. J. 2013, 24, 36–43. [Google Scholar] [CrossRef]
- Soangra, R.; Lockhart, T.E.; Lach, J.; Abdel-Rahman, E.M. Effects of hemodialysis therapy on sit-to-walk characteristics in end stage renal disease patients. Ann. Biomed. Eng. 2013, 41, 795–805. [Google Scholar] [CrossRef]
- Lockhart, T.E.; Barth, A.T.; Zhang, X.; Songra, R.; Abdel-Rahman, E.; Lach, J. Portable, Non-Invasive Fall Risk Assessment in End Stage Renal Disease Patients on Hemodialysis. ACM Trans. Comput. Hum. Interact. 2010, 84–93. [Google Scholar] [CrossRef]
- Handrigan, G.A.; Corbeil, P.; Simoneau, M.; Teasdale, N. Balance control is altered in obese individuals. J. Biomech. 2010, 43, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Hue, O.; Simoneau, M.; Marcotte, J.; Berrigan, F.; Dore, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Body weight is a strong predictor of postural stability. Gait Posture 2007, 26, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A.; Goldberger, A.L. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Manor, B.; Costa, M.D.; Hu, K.; Newton, E.; Starobinets, O.; Kang, H.G.; Peng, C.K.; Novak, V.; Lipsitz, L.A. Physiological complexity and system adaptability: Evidence from postural control dynamics of older adults. J. Appl. Physiol. 2010, 109, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, T.E.; Woldstad, J.C.; Smith, J.L. Effects of age-related gait changes on the biomechanics of slips and falls. Ergonomics 2003, 46, 1136–1160. [Google Scholar] [CrossRef]
- Zarrugh, M.Y.; Todd, F.N.; Ralston, H.J. Optimization of energy expenditure during level walking. Eur. J. Appl. Physiol. Occup. Physiol. 1974, 33, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Nagasaki, H. Temporal variability in the phase durations during treadmill walking. Hum. Mov. Sci. 1992, 11, 335–348. [Google Scholar] [CrossRef]
- Sekiya, N.; Nagasaki, H.; Ito, H.; Furuna, T. Optimal Walking in Terms of Variability in Step Length. J. Orthop. Sports Phys. Ther. 1997, 26, 266–272. [Google Scholar] [CrossRef]
- Kurosawa, K. Effects of various walking speeds on probe reaction time during treadmill walking. Percept. Motor Skills 1994, 78, 768–770. [Google Scholar] [CrossRef]
- Cali, C.M.; Kiel, D.P. An epidemiologic study of fall-related fractures among institutionalized older people. J. Am. Geriatr. Soc. 1995, 43, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.P.; Alessio, H.M.; Mills, E.M.; Tong, C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing 1997, 26, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Moe-Nilssen, R. A new method for evaluating motor control in gait under real-life environmental conditions. Part 2: Gait analysis. Clin. Biomech. 1998, 13, 328–335. [Google Scholar] [CrossRef]
- Moe-Nilssen, R. A new method for evaluating motor control in gait under real-life environmental conditions. Part 1: The instrument. Clin. Biomech. 1998, 13, 320–327. [Google Scholar] [CrossRef]
- Moe-Nilssen, R. Test-retest reliability of trunk accelerometry during standing and walking. Arch. Phys. Med. Rehabil. 1998, 79, 1377–1385. [Google Scholar] [CrossRef]
- Latt, M.D.; Menz, H.B.; Fung, V.S.; Lord, S.R. Walking speed, cadence and step length are selected to optimize the stability of head and pelvis accelerations. Exp. Brain Res. 2008, 184, 201–209. [Google Scholar] [CrossRef] [PubMed]
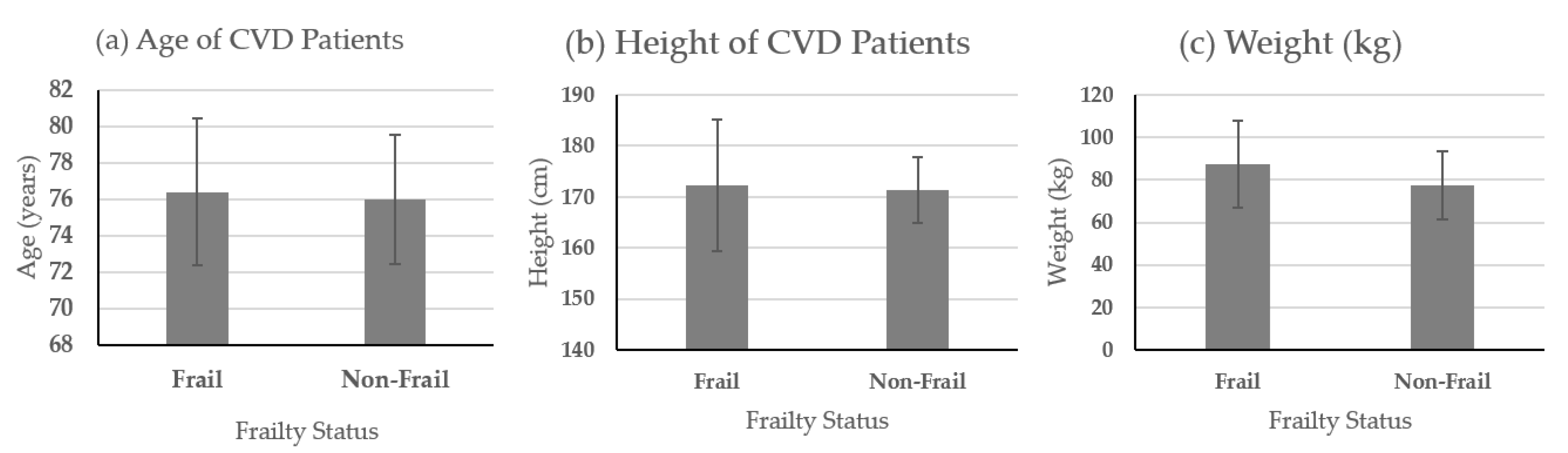
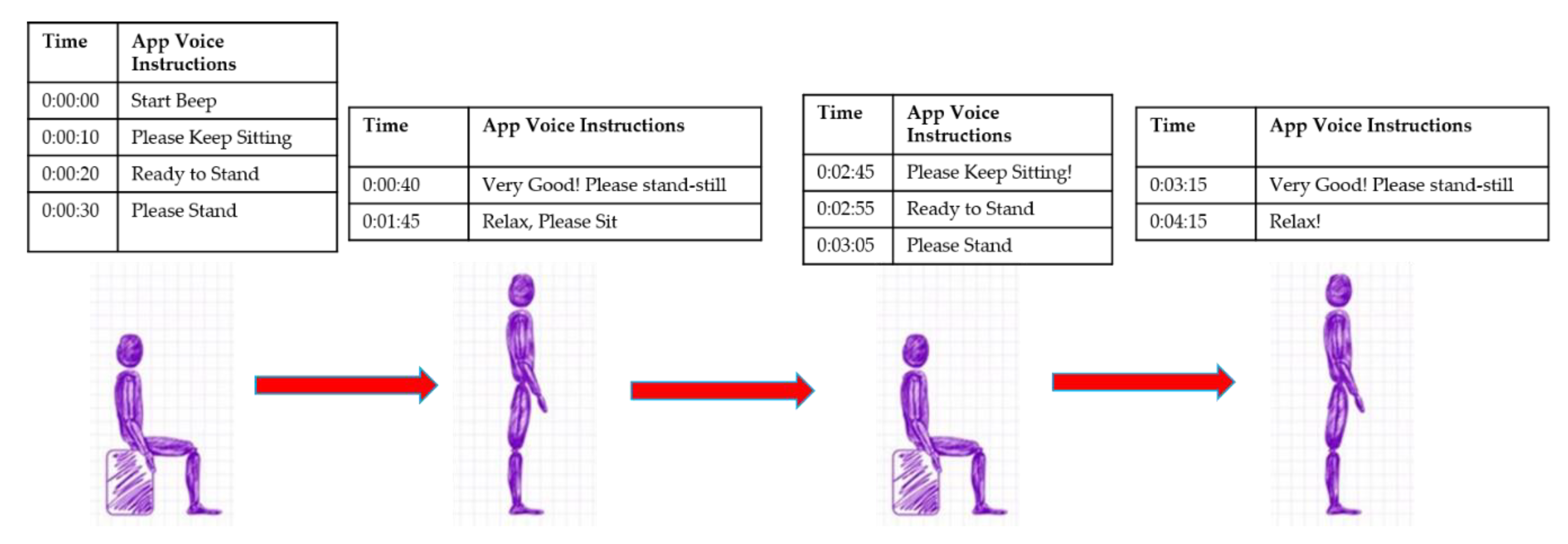

| ID | Surgical Procedure | STS Risk Score |
|---|---|---|
| ID04 | CABG × 4 | 0.012 |
| ID06 | AVR, MV repair, CABG × 3, Maze | NS |
| ID08 | AVR, CABG × 1 | 0.035 |
| ID09 | AVR, CABG × 2 | 0.013 |
| ID10 | AVR, CABG × 2 | 0.027 |
| ID11 | CABG × 2, Extensive Maze | 0.021 |
| ID13 | AVR, CABG × 3 | 0.013 |
| ID14 | AVR | 0.023 |
| ID17 | AVR | 0.019 |
| ID18 | AVR, CABG × 2 | 0.042 |
| ID19 | AVR (re-do sternotomy) | 0.044 |
| ID20 | AVR, Root Replacement, coronary reconstruction | NS |
| ID21 | MVR, CABG × 1 (re-do sternotomy) | 0.164 |
| ID22 | MV Repair | 0.020 |
| ID23 | AVR, CABG × 1, limited concomitant Maze | NS |
| ID24 | AVR, CABG × 3 | 0.014 |
| Variables | Health Status | |||||
|---|---|---|---|---|---|---|
| Frail | Non-Frail | |||||
| Mean | SD | CV | Mean | SD | CV | |
| RMS_AP * | 0.12 | 0.03 | 24.50 | 0.15 | 0.02 | 15.23 |
| RMS_V * | 0.11 | 0.01 | 11.82 | 0.17 | 0.02 | 10.40 |
| RMS_ML * | 0.11 | 0.02 | 19.63 | 0.15 | 0.03 | 22.48 |
| (a) | No Morbidity | Morbidity | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | |
| Stopwatch Velocity * [m/s] | 0.865 | 0.217 | 0.701 | 0.097 | 0.0078 |
| SmartphoneVelocity * [m/s] | 0.793 | 0.222 | 0.637 | 0.064 | 0.009 |
| RMSAP * [g] | 0.128 | 0.026 | 0.113 | 0.033 | 0.029 |
| RMSV * [g] | 0.136 | 0.034 | 0.114 | 0.016 | 0.013 |
| ApEnMLCOP | 1.077 | 0.068 | 1.101 | 0.018 | 0.335 |
| ApEnRCOP | 1.141 | 0.033 | 1.113 | 0.021 | 0.088 |
| StepTime * [s] | 0.573 | 0.081 | 0.654 | 0.150 | 0.034 |
| (b) Adverse Outcome Score | Score = 44.13 − 26.96 × RMSAP − 28.29 × RMSV + 13.33 × ApEnMLCOP − 46.85 × ApEnRCOP + 5.35 × StepTime | ||||
| Prediction Model | |||||
| (c) Scoring Criteria (Cp = 2.77, R-square = 0.52) | Death = 7; Prolonged Ventilation = 6; Prolonged length of stay = 5; Discharge to Skilled Nursing Facility = 4; Stroke/Renal Failure = 3; Reoperation = 2; Deep Sternal Wound Infection = 1 | ||||
| ID | Surgical Procedure | STS Risk Score |
|---|---|---|
| ID02 | AVR, Root Replacement, coronary reconstruction | NS |
| ID05 | AVR, Root Replacement, reimplantation of coronaries | NS |
| ID07 | AVR, MV repair, CABG × 3, Extensive Maze | NS |
| ID12 | MVR | 0.062 |
| ID25 | AVR | 0.018 |
| ID | RMS_AP | RMS_V | APEN_ML_COP | APEN_R_COP | STEP TIME | MODEL SCORE |
|---|---|---|---|---|---|---|
| ID02 | 0.093 | 0.118 | 0.991 | 1.105 | 0.550 | 2.613 |
| ID05 | 0.105 | 0.107 | 0.126 | 1.174 | 0.650 | 0.648 |
| D07 | 0.121 | 0.111 | 0.087 | 1.148 | 0.550 | 1.346 |
| ID12 | 0.039 | 0.039 | 1.118 | 0.141 | 0.980 | 8.633 |
| D25 | 0.111 | 0.137 | 1.097 | 1.076 | 0.540 | 4.340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soangra, R.; Lockhart, T. Smartphone-Based Prediction Model for Postoperative Cardiac Surgery Outcomes Using Preoperative Gait and Posture Measures. Sensors 2021, 21, 1704. https://doi.org/10.3390/s21051704
Soangra R, Lockhart T. Smartphone-Based Prediction Model for Postoperative Cardiac Surgery Outcomes Using Preoperative Gait and Posture Measures. Sensors. 2021; 21(5):1704. https://doi.org/10.3390/s21051704
Chicago/Turabian StyleSoangra, Rahul, and Thurmon Lockhart. 2021. "Smartphone-Based Prediction Model for Postoperative Cardiac Surgery Outcomes Using Preoperative Gait and Posture Measures" Sensors 21, no. 5: 1704. https://doi.org/10.3390/s21051704
APA StyleSoangra, R., & Lockhart, T. (2021). Smartphone-Based Prediction Model for Postoperative Cardiac Surgery Outcomes Using Preoperative Gait and Posture Measures. Sensors, 21(5), 1704. https://doi.org/10.3390/s21051704







