Review of Wearable Sensor-Based Health Monitoring Glove Devices for Rheumatoid Arthritis
Abstract
:1. Introduction
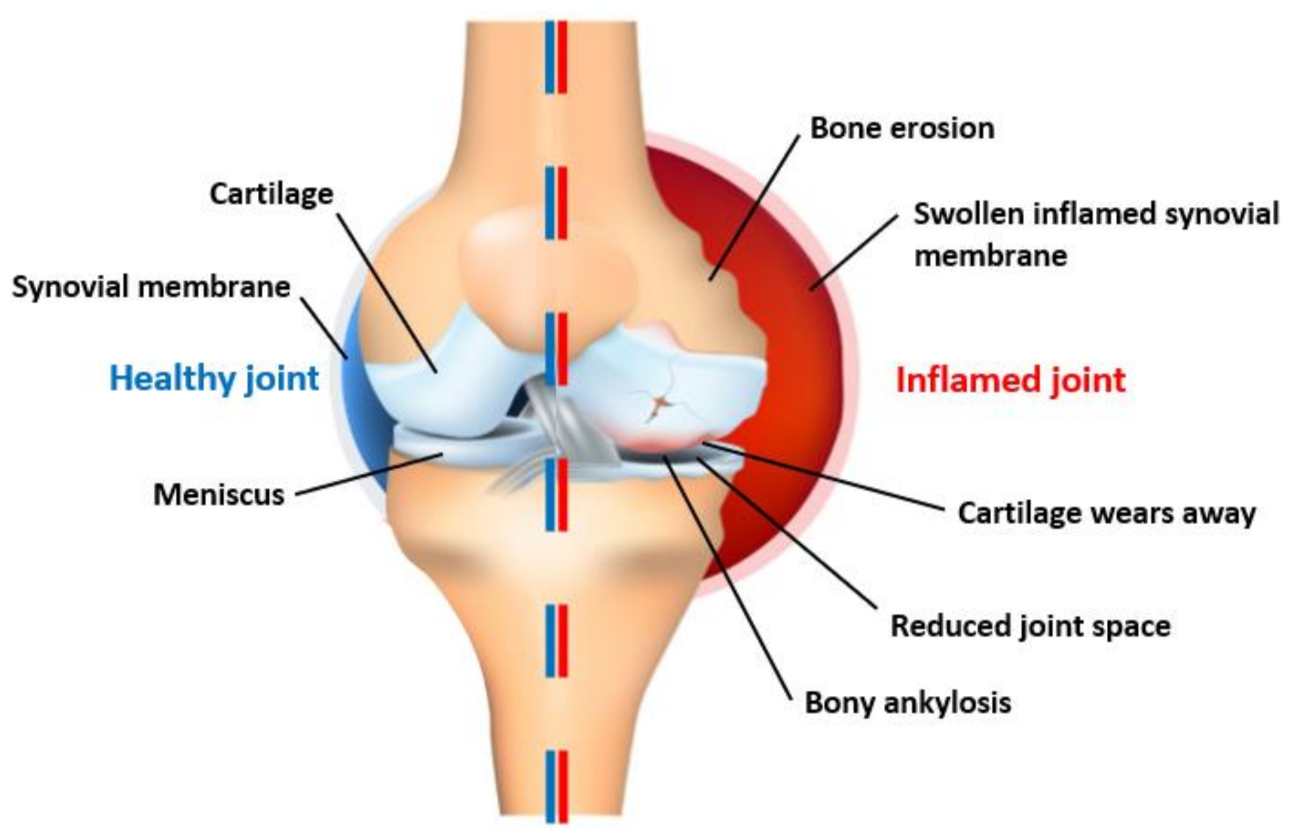
1.1. Problem Background
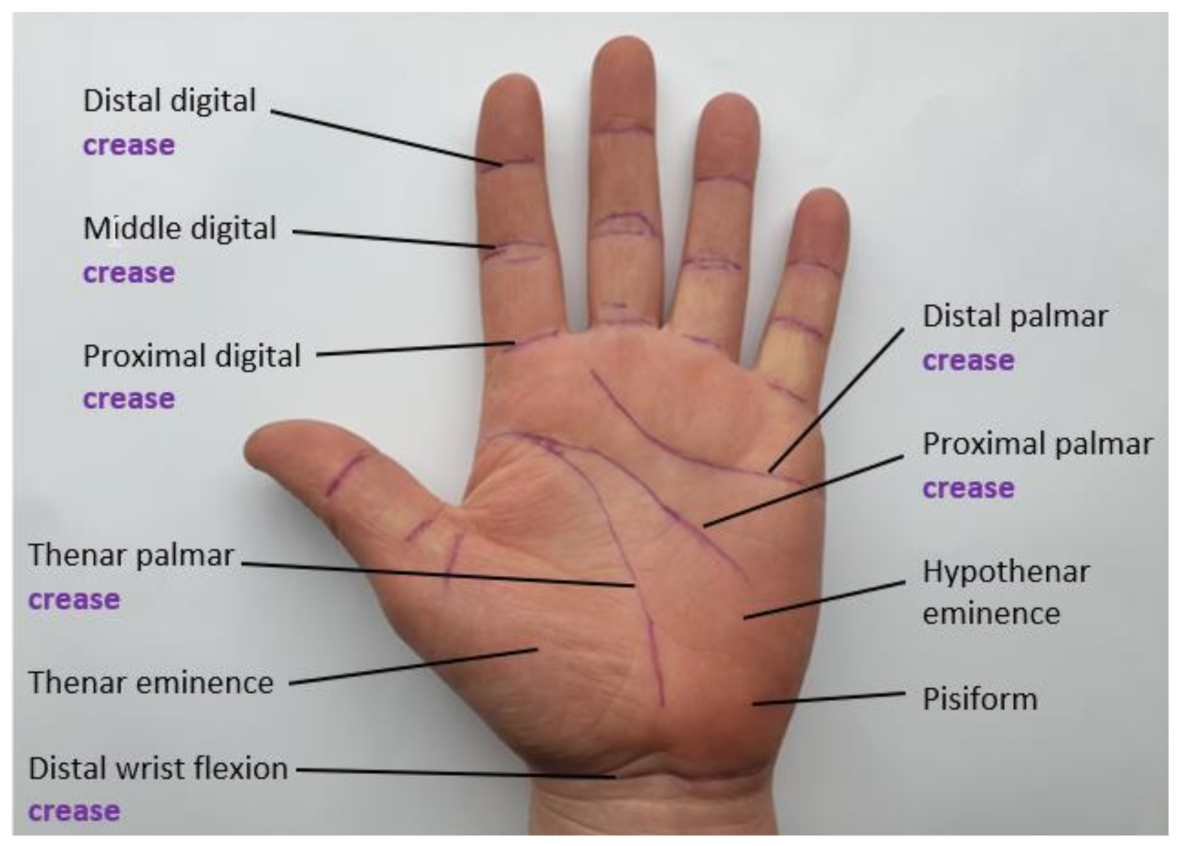
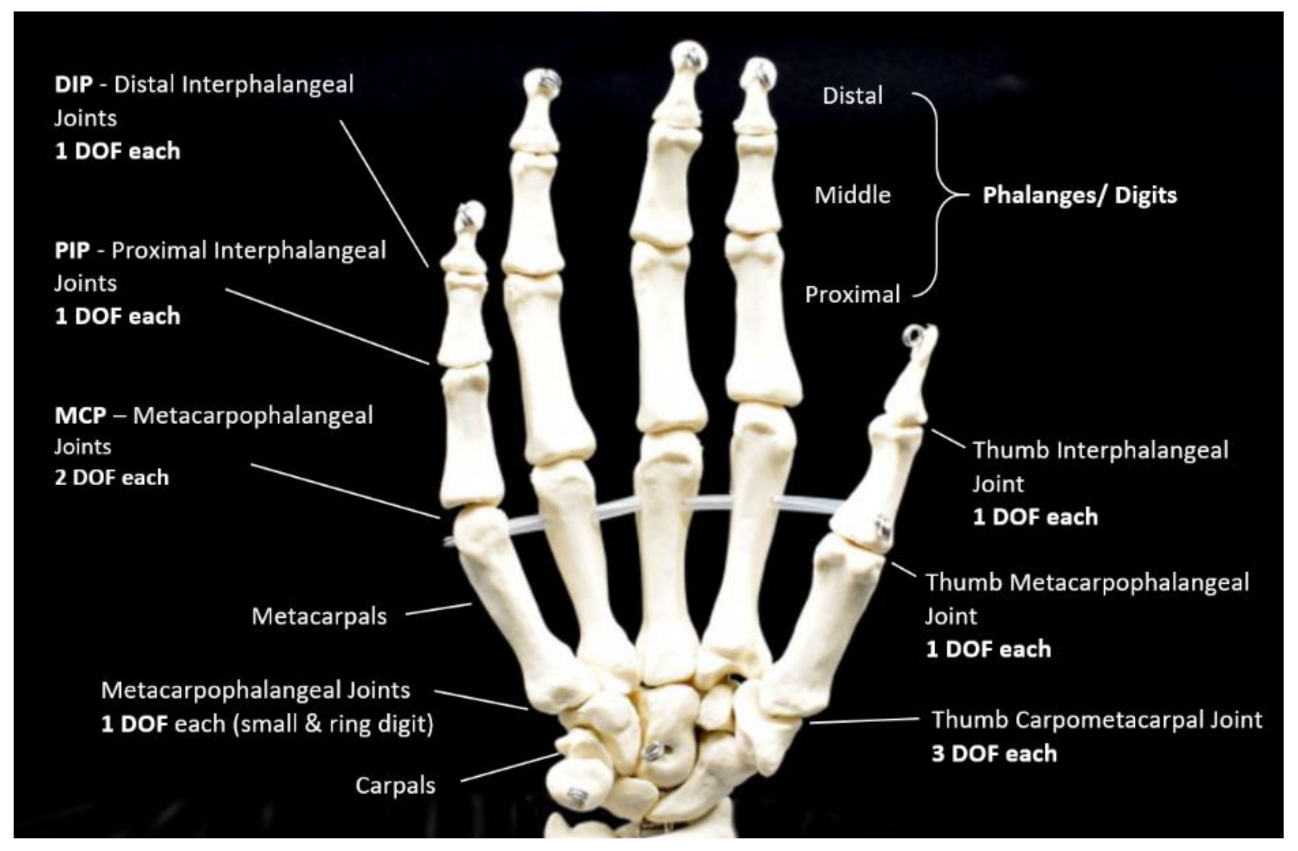
1.2. Hand-Based Functional Assessment
2. Sensors Characteristics & Signal Processing & Output
2.1. Sensor Topologies
2.1.1. Sensors Used to Monitor Finger ROM
2.1.2. Sensors Used to Monitor Finger ROM and Hand Orientation
2.2. Microcontroller/Processing Unit
2.3. Output Display Monitor
3. Commercial and Non-Commercial Glove-Based System
3.1. Glove Materials
3.2. Sensor and Device Calibration
4. Discussion
5. Conclusions
6. Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement.
Informed Consent Statement.
Data Availability Statement.
Acknowledgments
Conflicts of Interest
References
- Rashid, A.; Hasan, O. Wearable technologies for hand joints monitoring for rehabilitation: A survey. Microelectron. J. 2019, 88, 173–183. [Google Scholar] [CrossRef]
- Institute for Quality and Efficiency in Health Care. How do hands work? In NCBI; 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279362/ (accessed on 1 September 2020).
- Salawu, F.K.; Danburam, A.; Olokoba, A.B. Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Niger. J. Med. 2010, 19, 126–131. [Google Scholar] [CrossRef]
- Baldominos, A.; Saez, Y.; del Pozo, C.G. An Approach to Physical Rehabilitation Using State-of-the-art Virtual Reality and Motion Tracking Technologies. Procedia Comput. Sci. 2015, 64, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Davarzani, S.; Pajouh, M.A.A. Design and Fabrication of Sensing System for Rehabilitation of Finger. In Proceedings of the 2020 28th Iranian Conference on Electrical Engineering (ICEE), Tabriz, Iran, 4–6 August 2020. [Google Scholar]
- Brigante, C.M.N.; Abbate, N.; Basile, A.; Faulisi, A.C.; Sessa, S. Towards miniaturization of a MEMS-based wearable motion capture system. IEEE Trans. Ind. Electron. 2011, 58, 3234–3241. [Google Scholar] [CrossRef]
- Orbai, A.M.; Smith, K.C.; Bartlett, S.J.; de Leon, E.; Bingham, C.O. ‘Stiffness Has Different Meanings, I Think, to Everyone’: Examining Stiffness from the Perspective of People Living With Rheumatoid Arthritis. Arthritis Care Res. 2014, 66, 1662–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajak, R.; Zaman, M.; Jones, T.; Sheikh, F.; Sharif, M. Thu0617 Wrist Ultrasound (Us) Pathology in Early Rheumatoid Arthritis (Ra); Observations from an Early Inflammatory Arthritis (Eia) Diagnostic Service. Ann. Rheum. Dis. 2019, 78, 601. [Google Scholar]
- Abbas, S.; Condell, J.; Gardiner, P.; McCann, M.; Todd, S.; Connolly, J. Can multiple wearable sensors be used to detect the early onset of Parkinson’s Disease? In In Proceedings of the 2020 31st Irish Signals and Systems Conference (ISSC), Letterkenny, Ireland, 11–12 June 2020. [Google Scholar]
- Majithia, V.; Geraci, S.A. Rheumatoid Arthritis: Diagnosis and Management. Am. J. Med. 2007, 120, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J. Symmetric vs. asymmetric arthritis: What to know. Medical News Today. 2019. Available online: https://www.medicalnewstoday.com/articles/326840 (accessed on 1 September 2020).
- Bukhari, M.; Kent, A. How rheumatologists assess disability in the current era needs an overhaul: Focus on the Health Assessment Questionnaire. Rheumatology (Oxford) 2020, 59, 267–268. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.C.; Nixon, M.F.; Dias, J.J.; Graham, T.; Cook, S. How accurately does a simulation glove reflect function compared to rheumatoid arthritis sufferers? Ann. R. Coll. Surg. Engl. 2010, 92, 605–609. [Google Scholar] [CrossRef]
- Rat, A.C.; Boissier, M.C. Rheumatoid arthritis: Direct and indirect costs. Jt. Bone Spine 2004, 71, 518–524. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; Van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.W.; Deriche, M.A.; Hafeedh, A.B.; Almasawa, H.; Jofan, K.B.; Alsakkaf, H.; Bahumran, A.; Salem, M. An IOT based wearable smart glove for remote monitoring of rheumatoid arthritis patients. BIOSIGNALS 2019, 2019, 224–228. [Google Scholar]
- Debes, C.; Merentitis, A.; Sukhanov, S.; Niessen, M.; Frangiadakis, N.; Bauer, A. Monitoring activities of daily living in smart homes: Understanding human behavior. IEEE Signal Process. Mag. 2016, 33, 81–94. [Google Scholar] [CrossRef]
- Nasir, S.H.; Troynikov, O.; Westropp, N.M. Therapy gloves for patients with rheumatoid arthritis: A review. Ther. Adv. Musculoskelet. Dis. 2014, 6, 226–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkinen, H.; Kautiainen, H.; Hannonen, P.; Sokka, T. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann. Rheum. Dis. 2005, 64, 1410–1413. [Google Scholar] [CrossRef] [Green Version]
- NRAS. The DAS28 score. National Rheumatoid Arthritis Society. 2017. Available online: https://nras.org.uk/resource/the-das28-score/ (accessed on 1 August 2020).
- Van Riel, P.L. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28). Clin. Exp. Rheumatol. 2014, 32, S65–S74. [Google Scholar]
- Connolly, J. Wearable Rehabilitative Technology for the Movement Measurement of Patients with Arthritis. Ulster University: Northern Ireland, UK, 2015. [Google Scholar]
- Milanese, S.; Gordon, S.; Buettner, P.; Flavell, C.; Ruston, S.; Coe, D.; O’Sullivan, W.; McCormack, S. Reliability and concurrent validity of knee angle measurement: Smart phone app versus universal goniometer used by experienced and novice clinicians. Man. Ther. 2014, 19, 569–574. [Google Scholar] [CrossRef] [Green Version]
- North Coast Medical. Hand Goniometer. North Coast Medical. 2020. Available online: https://www.ncmedical.com/item_3728.html#!prettyPhoto[pp_gal]/0/ (accessed on 10 December 2020).
- Ghosh, S. Capturing Human Hand Kinematics for Object Grasping and Manipulation; Texas A&M University: College Station, TX, USA, 2013. [Google Scholar]
- Lorig, K.R.; Mazonson, P.D.; Holman, H.R. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis Rheum. 1993, 36, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.P.; Gossec, L.; Mak, A.; March, L. Fc literature review. Semin. Arthritis Rheum. 2014, 43, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.W.; Cox, A.; Anderson, S.; Liew, B.; Olsen, A.; Schram, B.; Furness, J. Reliability and validity of clinically accessible smartphone applications to measure joint range of motion: A systematic review. PLoS ONE 2019, 14, e0215806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, B.S.; Lee, I.J.; Yang, S.Y.; Lo, Y.C.; Lee, J.; Chen, J.L. Design of an Inertial-Sensor-Based Data Glove for Hand Function Evaluation. Sensors 2018, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Salter, N. Methods of measurement of muscle and joint function. J. Bone Joint Surg. Br. 1955, 37, 474–491. [Google Scholar] [CrossRef]
- Burr, N. Inter-rater and Intra-rater Reliability when Measuring Interphalangeal Joints. Physiotherapy 2003, 89, 641–652. [Google Scholar] [CrossRef]
- Ellis, B.; Bruton, A. A study to compare the reliability of composite finger flexion with goniometry for measurement of range of motion in the hand. Clin. Rehabil. 2002, 16, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Jung, M.C. Ergonomic evaluation of biomechanical hand function. Saf. Health Work 2015, 6, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerschan-Schindl, K.; MacHold, K. Rehabilitation von Patienten mit rheumatoider Arthritis. Phys. Medizin Rehabil. Kurortmed. 2011, 21, 297–310. [Google Scholar] [CrossRef]
- Carteron, N. What are the symptoms of rheumatoid arthritis? Healthline. 2020. Available online: https://www.medicalnewstoday.com/articles/323374#complications (accessed on 1 November 2020).
- Arthritus Foundation, How Rheumatoid Arthritis Affects More Than Joints. 2020. Available online: https://www.arthritis.org/diseases/more-about/how-rheumatoid-arthritis-affects-more-than-joints (accessed on 1 November 2020).
- Bakir, E.; Samancioglu, S.; Gursoy, S. Complementary Therapies in Clinical Practice the effects of re fl exology on pain and sleep deprivation in patients with rheumatoid arthritis: A randomized controlled trial. Complement. Ther. Clin. Pract. 2018, 31, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Metsios, G.S.; Kitas, G.D. Best Practice & Research Clinical Rheumatology Physical activity, exercise and rheumatoid arthritis: Effectiveness, mechanisms and implementation. Best Pract. Res. Clin. Rheumatol. 2019, 32, 669–682. [Google Scholar]
- Rapoliene, J.; Krisciunas, A. The effectiveness of occupational therapy in restoring the functional state of hands in rheumatoid arthritis patients. Medicina (Kaunas) 2006, 42, 823–828. [Google Scholar] [PubMed]
- Dipietro, L.; Sabatini, A.M.; Dario, P. Evaluation of an instrumented glove for hand-movement acquisition. J. Rehabil. Res. Dev. 2003, 40, 179–189. [Google Scholar] [CrossRef]
- O’Flynn, B.; Torres, J.; Connolly, J.; Condell, J.; Curran, K.; Gardiner, P. Novel smart sensor glove for arthritis rehabiliation. In Proceedings of the 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA, 6–9 May 2013; pp. 1–6. [Google Scholar]
- Connolly, J.; Condell, J.; O’Flynn, B.; Sanchez, J.T.; Gardiner, P. IMU Sensor-Based Electronic Goniometric Glove for Clinical Finger Movement Analysis. IEEE Sens. J. 2018, 18, 1273–1281. [Google Scholar] [CrossRef]
- Lin, B.S.; Lee, I.J.; Chen, J.L. Novel Assembled Sensorized Glove Platform for Comprehensive Hand Function Assessment by Using Inertial Sensors and Force Sensing Resistors. IEEE Sens. J. 2020, 20, 3379–3389. [Google Scholar] [CrossRef]
- de Pasquale, G. Glove-based systems for medical applications: Review of recent advancements. J. Text. Eng. Fash. Technol. 2018, 4, 286–295. [Google Scholar] [CrossRef]
- Kumar, S.; Sultan, M.J.; Ullah, A.; Zameer, S.; Siddiqui, S.; Sami, S.K. Human Machine Interface Glove Using Piezoresistive Textile Based Sensors. IOP Conf. Ser. Mater. Sci. Eng. 2018, 414, 012041. [Google Scholar] [CrossRef]
- Mori, Y.; Toyonaga, M. Data-glove for japanese sign language training system with gyro-Sensor. In Proceedings of the 2018 Joint 10th International Conference on Soft Computing and Intelligent Systems (SCIS) and 19th International Symposium on Advanced Intelligent Systems (ISIS), Toyama, Japan, 5–8 December 2018; pp. 1354–1357. [Google Scholar]
- Pham, T.; Pathirana, P.N.; Trinh, H.; Fay, P. A non-contact measurement system for the range of motion of the hand. Sensors 2015, 15, 18315–18333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Flynn, B.; Sachez-Torres, J.; Tedesco, S.; Downes, B.; Connolly, J.; Condell, J.; Curran, K. Novel Smart Glove Technology as a Biomechanical Monitoring Tool. Sens. Transducers 2015, 193, 23–32. [Google Scholar]
- Lin, B.S.; Lee, I.J.; Chiang, P.Y.; Huang, S.Y.; Peng, C.W. A Modular Data Glove System for Finger and Hand Motion Capture Based on Inertial Sensors. J. Med. Biol. Eng. 2019, 39, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Ruffing, V. Rheumatoid Arthritis Signs and Symptoms. Johns Hopkins Arthritis Center. 2020. Available online: https://www.hopkinsarthritis.org/arthritis-info/rheumatoid-arthritis/ra-symptoms (accessed on 15 June 2020).
- Netto, A.P. Hand Pain and Rheumatoid Arthritis (RA). Veritas Health. 2015. Available online: https://www.arthritis-health.com/types/rheumatoid/hand-pain-and-rheumatoid-arthritis-ra (accessed on 6 November 2020).
- Fang, B.; Sun, F.; Liu, H.; Guo, D. A Novel Data Glove Design Based on Inertial and Magnetic Sensors. Int. J. Swarm Intell. Evol. Comput. 2015, 4, 1–2. [Google Scholar]
- Ding, S.; Schumacher, M. Sensor monitoring of physical activity to improve glucose management in diabetic patients: A review. Sensors 2016, 16, 589. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Yadav, L.; Singhal, M.; Sachan, R.; Goyal, H.; Taparia, K.; Gulati, R.; Singh, A.; Trivedi, G. Smart glove for sign language communications. In Proceedings of the 2016 International Conference on Accessibility to Digital World (ICADW), Guwahati, India, 16–18 December 2016; pp. 27–31. [Google Scholar]
- Braun, A.; Wichert, R.; Kuijper, A.; Fellner, D.W. A benchmarking model for sensors in smart environments. In European Conference on Ambient Intelligence; Springer: Cham, Switzerland, 2014; Volume 8850, pp. 242–257. [Google Scholar]
- Wang, Q.; Markopoulos, P.; Yu, B.; Chen, W.; Timmermans, A. Interactive wearable systems for upper body rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Routhier, F.; Duclos, N.C.; Lacroix, É.; Lettre, J.; Turcotte, E.; Hamel, N.; Michaud, F.; Duclos, C.; Archambault, P.S.; Bouyer, L.J. Clinicians’ perspectives on inertial measurement units in clinical practice. PLoS ONE 2020, 15, e0241922. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.I.; Majumder, S.; Mondal, T.; Cowan, D.; Naseh, S.; Deen, M.J. Monitoring methods of human body joints: State-of-the-art and research challenges. Sensors 2019, 19, 2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.A.; Zaidan, B.B.; Zaidan, A.A.; Salih, M.M.; Lakulu, M.M.B. A review on systems-based sensory gloves for sign language recognition state of the art between 2007 and 2017. Sensors 2018, 18, 2208. [Google Scholar] [CrossRef] [Green Version]
- Condell, J.; Connolly, J.; Young, W. Action Sense. 2020. Available online: https://www.actionsense.org/ (accessed on 1 November 2020).
- Flex Point. 2020. Available online: https://www.flexpoint.com/bend-sensor (accessed on 1 October 2020).
- Saggio, G.; Lagati, A.; Orengo, G. Shaping Resistive Bend Sensors to Enhance Readout Linearity. ISRN Electron. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Components 101. Flexpoint characteristics. Components 101. Available online: https://components101.com/sensors/flex-sensor-working-circuit-datasheet (accessed on 19 January 2021).
- Wang, Q.; Liu, Y. Review of optical fiber bending/curvature sensor. Meas. J. Int. Meas. Confed. 2018, 130, 161–176. [Google Scholar] [CrossRef]
- Remouche, M.; Georges, F.; Meyrueis, P. Stress Sensing by an Optical Fiber Sensor: Method and Process for the Characterization of the Sensor Response Depending on Several Designs. Opt. Photonics J. 2013, 3, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, O.V.; Chertoriyskiy, A.A. Fiber-Optic Bend Sensor Based on Double Cladding Fiber. J. Sens. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Tyndall National Institute. VR Glove. Tyndall National Institute. 2017. Available online: https://www.tyndall.ie/funded-programmes/vr-glove/ (accessed on 20 November 2020).
- Tyndall National Institute. Tyndall IMU version 2. Tyndall National Institute. 2015. Available online: https://www.tyndall.ie/biomechanics-and-motion-monitoring (accessed on 12 January 2021).
- 5th Dimention Technoligies. 5DT Hand Book. 5DT. 2020. Available online: http://5dt.com/downloads/dataglove/ultra/5DT Data Glove Ultra - Manual.pdf. (accessed on 20 November 2020).
- Ceruti, M.; Duffy, L.; Phan, H.; Eppele, K. Hall Effect Glove. US Patent US 8.421.448 B1, 16 April 2013. [Google Scholar]
- Honeywell. Hall efffect sensor SS495B. Honeywell. 2020. Available online: https://sensing.honeywell.com/honeywell-sensing-sensors-linear-hall-effect-ics-ss490-series-datasheet-005843-2-en.pdf. (accessed on 21 January 2021).
- Lee, Y.Y.; Wu, R.H.; Xu, S.T. Applications of linear Hall-effect sensors on angular measurement. In Proceedings of the 2011 IEEE International Conference on Control Applications (CCA), Denver, CO, USA, 28–30 September 2011; pp. 479–482. [Google Scholar]
- Abraham, L.; Urru, A.; Normani, N.; Wilk, M.P.; Walsh, M.; O’flynn, B. Hand tracking and gesture recognition using lensless smart sensors. Sensors 2018, 18, 2834. [Google Scholar] [CrossRef] [Green Version]
- StretchSense. StretchSense-MoCap Pro. StrectchSense. 2020. Available online: https://stretchsense.com/mocap-pro-supersplay-motion-capture-gloves/ (accessed on 23 November 2020).
- TEGARA. StretchSense MoCap Pro SuperSplay Gloves, a glove for hand motion capture equipped with a high-precision sensor that detects expansion and contraction. 2020. Available online: https://www.tegakari.net/en/2020/08/stretchsense-mocap-pro-supersplay/ (accessed on 27 December 2020).
- Shin Jeong Park. StretchSense Characteristics. 2020. Available online: https://softroboticstoolkit.com/stretchsense (accessed on 19 January 2021).
- Neely, J.S.; Restle, P.J. Capacitive Bend Sensor. US Patent US5610528A, 11 March 1997. [Google Scholar]
- Analog Devices. ADXL345. 2020. Available online: https://www.sparkfun.com/datasheets/Sensors/Accelerometer/ADXL345.pdf (accessed on 21 January 2021).
- Analog Devices. ADXL335. 2020. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/adxl335.pdf (accessed on 21 January 2021).
- TDX Invensense. MPU-6050. 2020. Available online: https://invensense.tdk.com/wp-content/uploads/2015/02/MPU-6000-Datasheet1.pdf (accessed on 19 January 2021).
- TDX Invensense. Motion tracking MPU-6050. 2020. Available online: https://invensense.tdk.com/products/motion-tracking/6-axis/mpu-6050/ (accessed on 19 January 2021).
- ST Electronics. LSM9DS1. 2020. Available online: https://www.st.com/resource/en/datasheet/lsm9ds1.pdf (accessed on 20 January 2021).
- TDX Invensense. MPU-9250. 2020. Available online: https://invensense.tdk.com/wp-content/uploads/2015/02/PS-MPU-9250A-01-v1.1.pdf (accessed on 19 January 2021).
- Sbernini, L.; Quitadamo, L.R.; Riillo, F.; di Lorenzo, N.; Gaspari, A.L.; Saggio, G. Sensory-Glove-Based Open Surgery Skill Evaluation. IEEE Trans. Human-Machine Syst. 2018, 48, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Farnell. Arduino Uno Datasheet. 2013. Available online: https://www.farnell.com/datasheets/1682209.pdf (accessed on 4 October 2020).
- Wu, H.; Guo, H.; Su, Z.; Shi, M.; Chen, X.; Cheng, X.; Han, M.; Zhang, H. Fabric-based self-powered noncontact smart gloves for gesture recognition. R. Soc. Chem. 2018, 6, 20277–20288. [Google Scholar] [CrossRef]
- Sturman, D.J.; Zeltzer, D. A Survey of Glove-based Input. IEEE Sens. J. 1994, 14, 30–39. [Google Scholar] [CrossRef]
- Kessler, G.D.; Hodges, L.F.; Walker, N. Evaluation of the CyberGlove as a Whole-Hand Input Device. ACM Trans. Comput. Interact. 1995, 2, 263–283. [Google Scholar] [CrossRef]
- Cyber Glove System. Cyberglove III. 2020. Available online: http://www.cyberglovesystems.com/cyberglove-iii/ (accessed on 12 January 2021).
- Pascal Lee, An Astronaut Smart Glove to Explore the Moon, Mars and Beyond. 2019. Available online: https://www.seti.org/press-release/astronaut-smart-glove-explore-moon-mars-and-beyond (accessed on 20 September 2020).
- 5th Dimention Technoligies, 5DT. 2020. Available online: https://5dt.com/5dt-data-glove-ultra/ (accessed on 25 December 2020).
- Haroon, A.; Fergus, P.; Shaheed, A.; Merabti, M. A wireless home and body sensor network platform for the early detection of arthritis. In Proceedings of the 2010 7th IEEE Consumer Communications and Networking Conference, Las Vegas, NV, USA, 9–12 January 2010; pp. 1–5. [Google Scholar]
- Neofect. Rapael smart glove. 2020. Available online: https://www.neofect.com/us/blog/stroke-rehabilitation-is-now-fun-thanks-to-rapael-smart-glove (accessed on 12 December 2020).
- Rico, P. Meditech, LLC. 2020. Available online: https://irp-cdn.multiscreensite.com/68072aa0/files/uploaded/RAPAEL Catalogue_Eng.pdf (accessed on 27 December 2020).
- Manus. Manus Prime 2 Xsens. 2020. Available online: https://manus-vr.com/xsens-gloves (accessed on 24 December 2020).
- Manus. Manus Prime II Xsens. 2020. Available online: https://www.tegakari.net/en/2020/06/manus_prime_2/ (accessed on 23 December 2020).
- Carbonaro, N.; Mura, G.D.; Lorussi, F.; Paradiso, R.; de Rossi, D.; Tognetti, A. Exploiting wearable goniometer technology for motion sensing gloves. IEEE J. Biomed. Heal. Inform. 2014, 18, 1788–1795. [Google Scholar] [CrossRef]
- Griffith, E. Ingress Protection (IP) IP68 requirements. 2020. Available online: https://www.pcmag.com/how-to/dust-resistant-waterproof-making-sense-of-gadget-ratings (accessed on 1 July 2020).
- Initiative, N.S. Nanotechnology for Sensors and Sensors for Nanotechnology: Improving and Protecting Health, Safety, and the Environment. Nanotechnol. Signat. Initiat. 2012, 1–11. [Google Scholar]
- Goncu-Berk, G.; Topcuoglu, N. A Healthcare Wearable for Chronic Pain Management. Design of a Smart Glove for Rheumatoid Arthritis. Des. J. 2017, 20, S1978–S1988. [Google Scholar] [CrossRef]
- Stilli, A.; Cremoni, A.; Bianchi, M.; Ridolfi, A.; Gerii, F.; Vannetti, F.; Wurdemann, H.A.; Allotta, B.; Althoefer, K. AirExGlove-A novel pneumatic exoskeleton glove for adaptive hand rehabilitation in post-stroke patients. In Proceedings of the 2018 IEEE International Conference on Soft Robotics (RoboSoft), Livorno, Italy, 24–28 April 2018; pp. 579–584. [Google Scholar]
- Fujiwara, E.; Miyatake, D.Y.; Santos, M.F.M.D.; Suzuki, C.K. Development of a glove-based optical fiber sensor for applications in human-robot interaction. In Proceedings of the 2013 8th ACM/IEEE International Conference on Human-Robot Interaction (HRI), Tokyo, Japan, 3–6 March 2013; pp. 123–124. [Google Scholar]
- Innovation Channels. Smart Glove to Make Stroke Rehab More Effective and Affordable. Innovation Enterprise Channel. 2020. Available online: https://channels.theinnovationenterprise.com/articles/smart-glove-to-make-stroke-rehab-more-effective-and-affordable-sgtmsrmeaa (accessed on 27 December 2020).
- Henderson, J.; Condell, J.; Connolly, J.; Kelly, D.; Curran, K. Reliability and Validity of Clinically Accessible Smart Glove Technologies to Measure Joint Range of Motion. Sensors 2021, 21, 1555. [Google Scholar] [CrossRef]

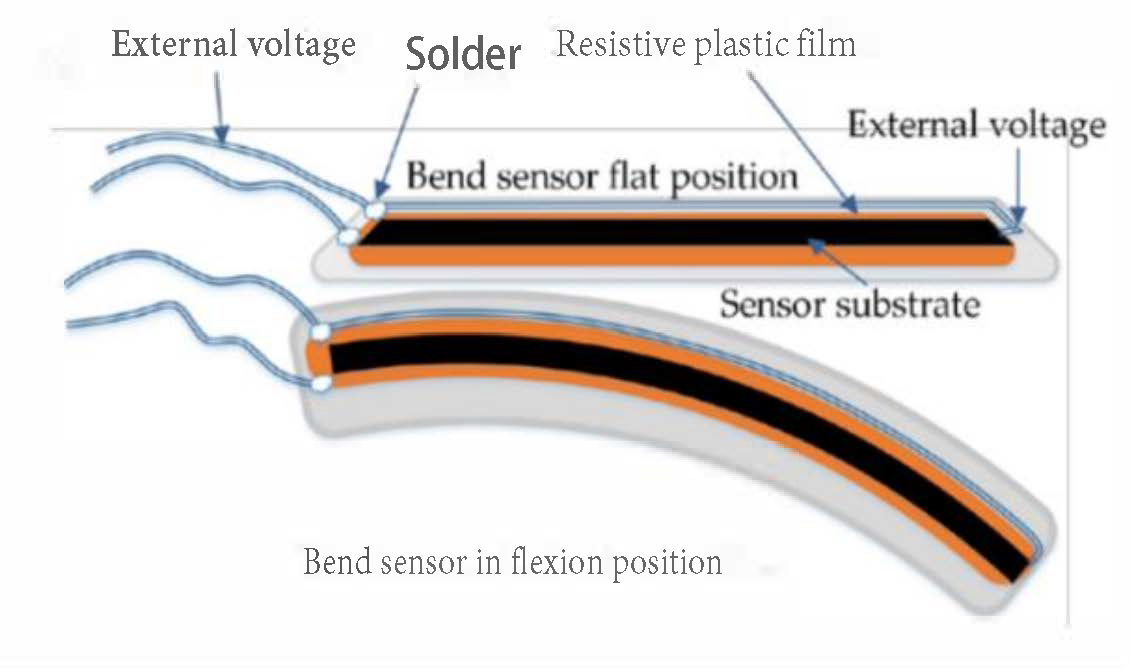
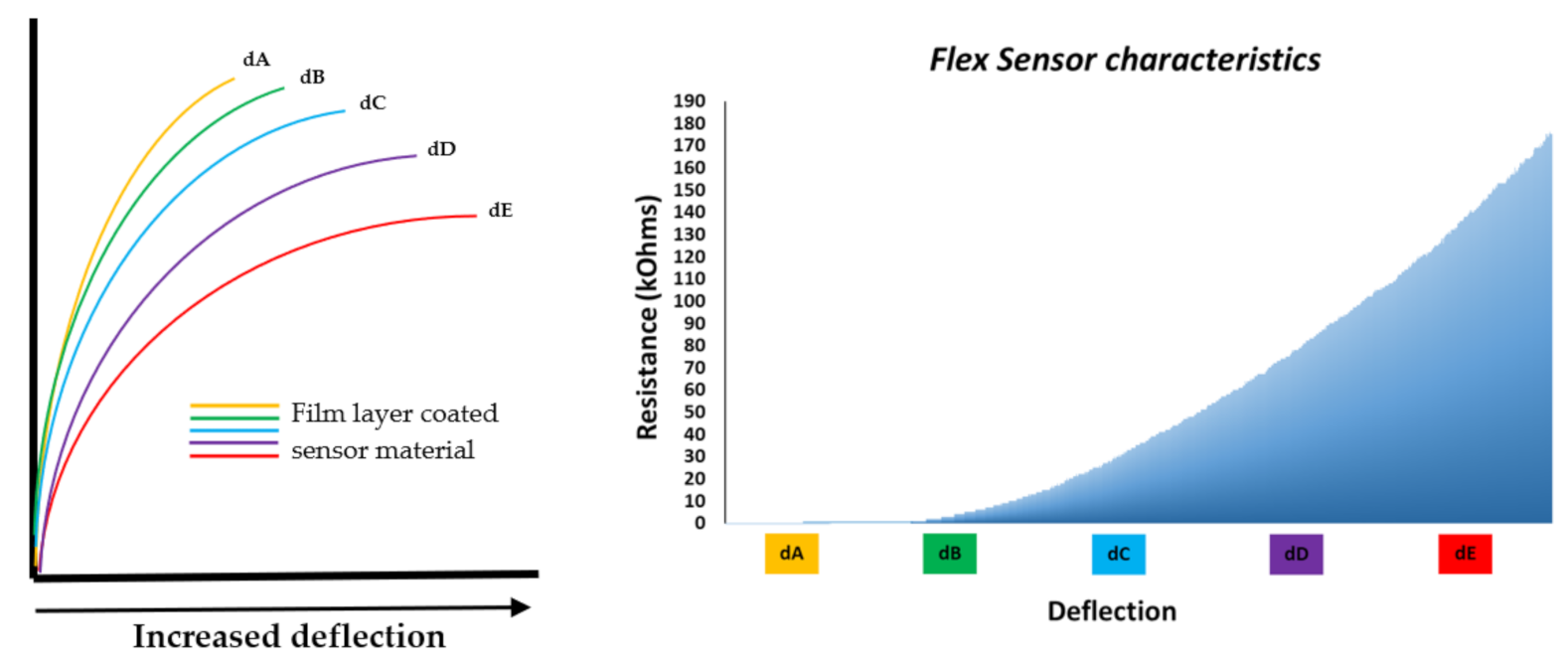
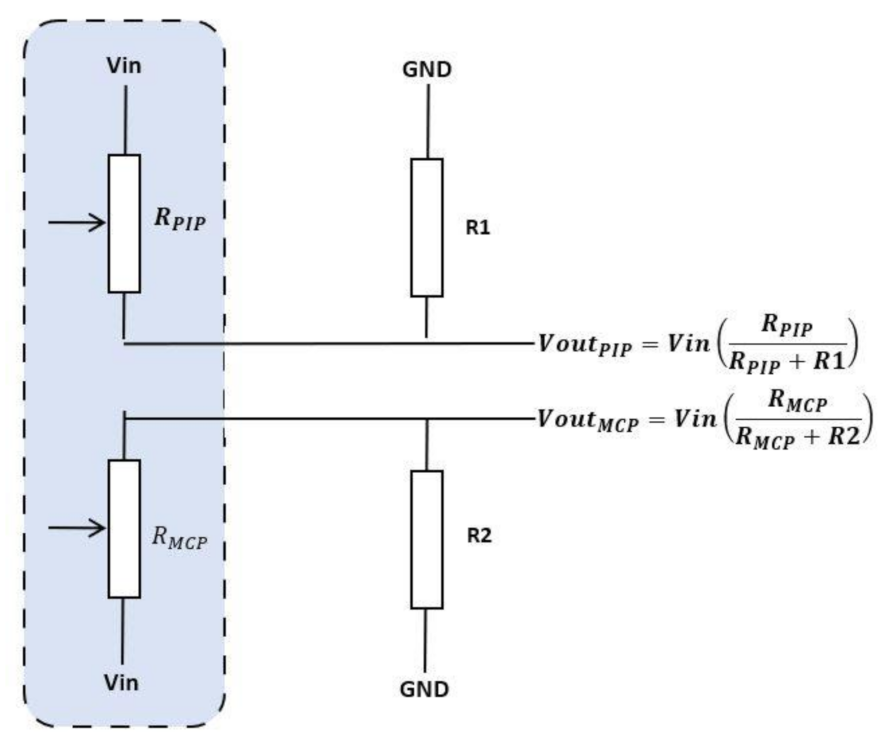
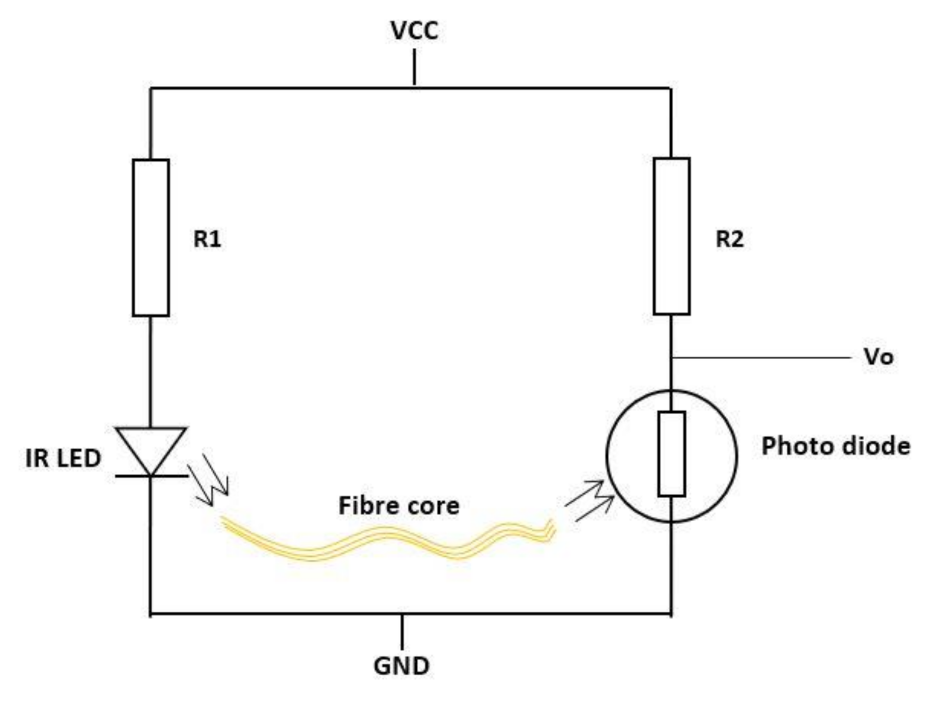

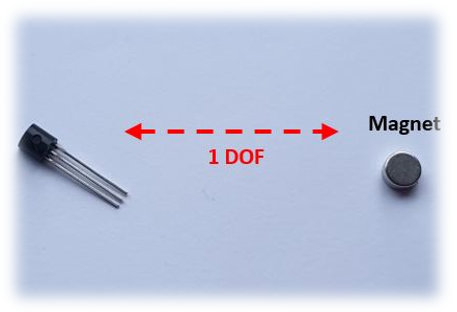
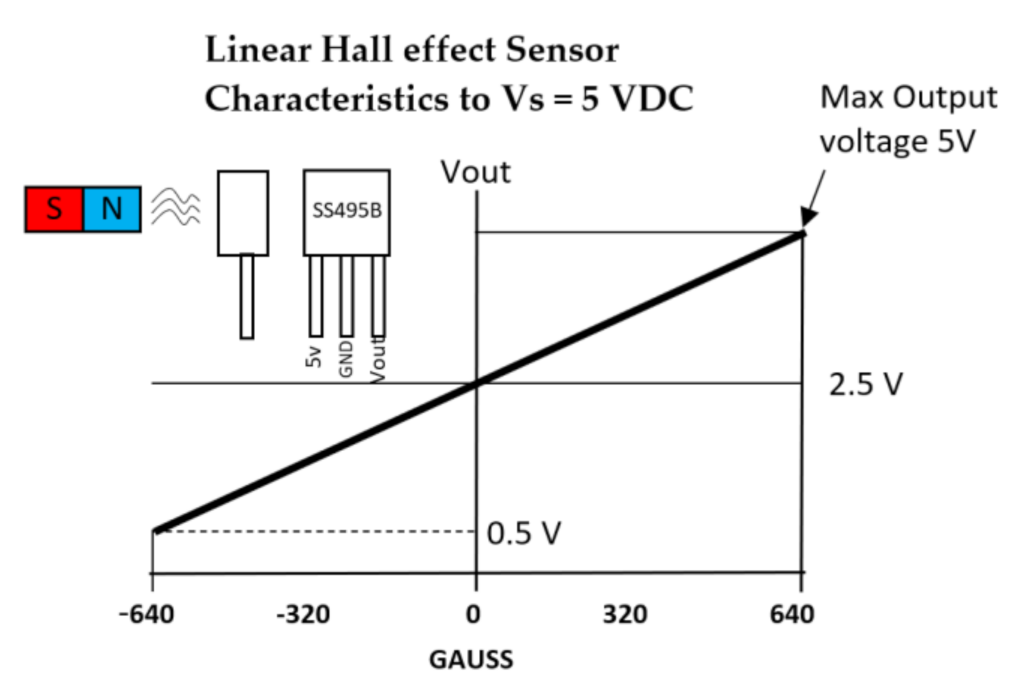
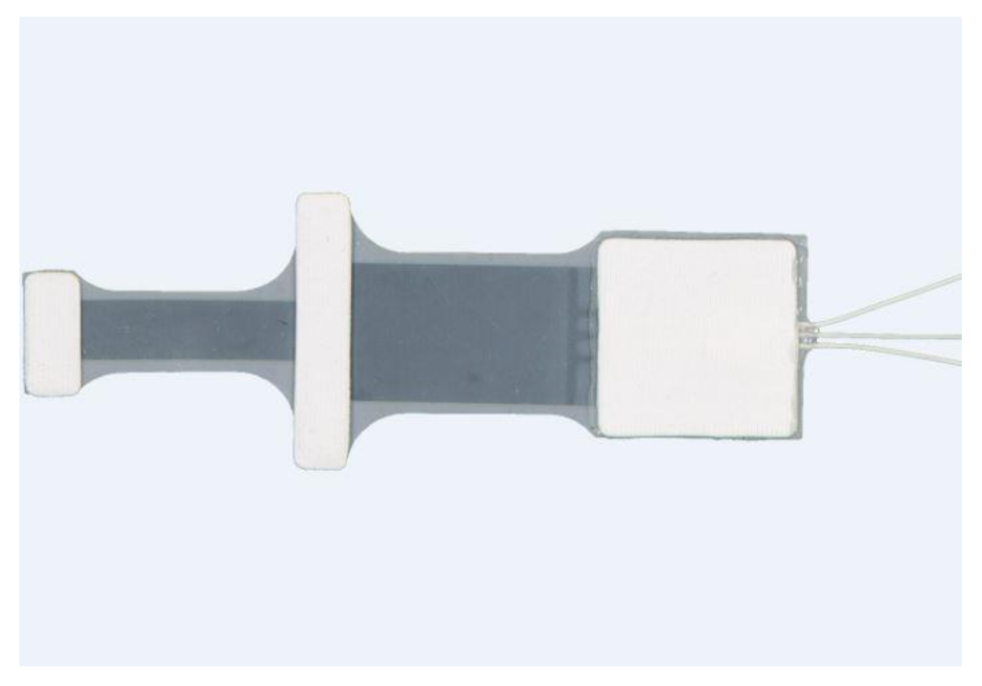
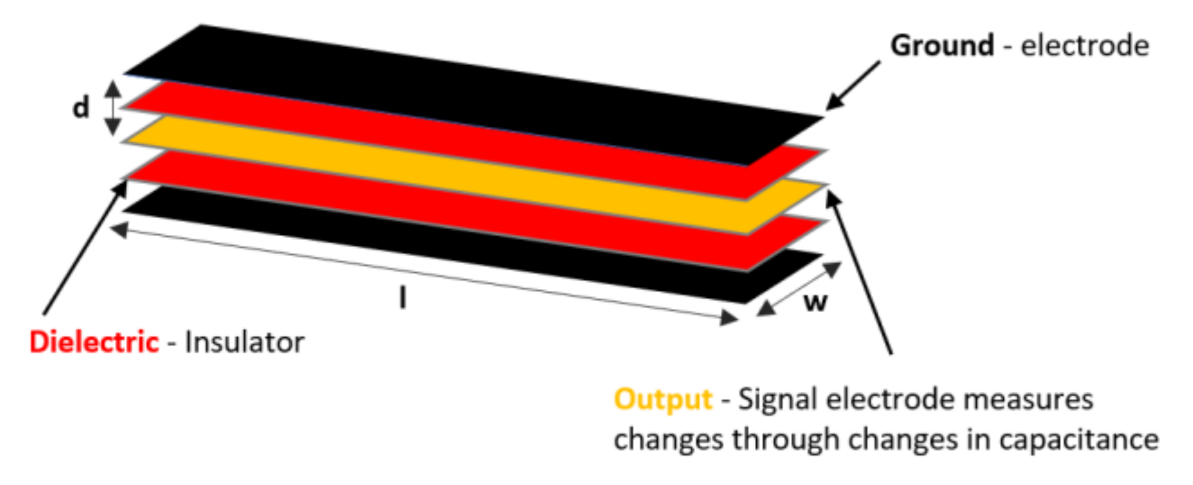
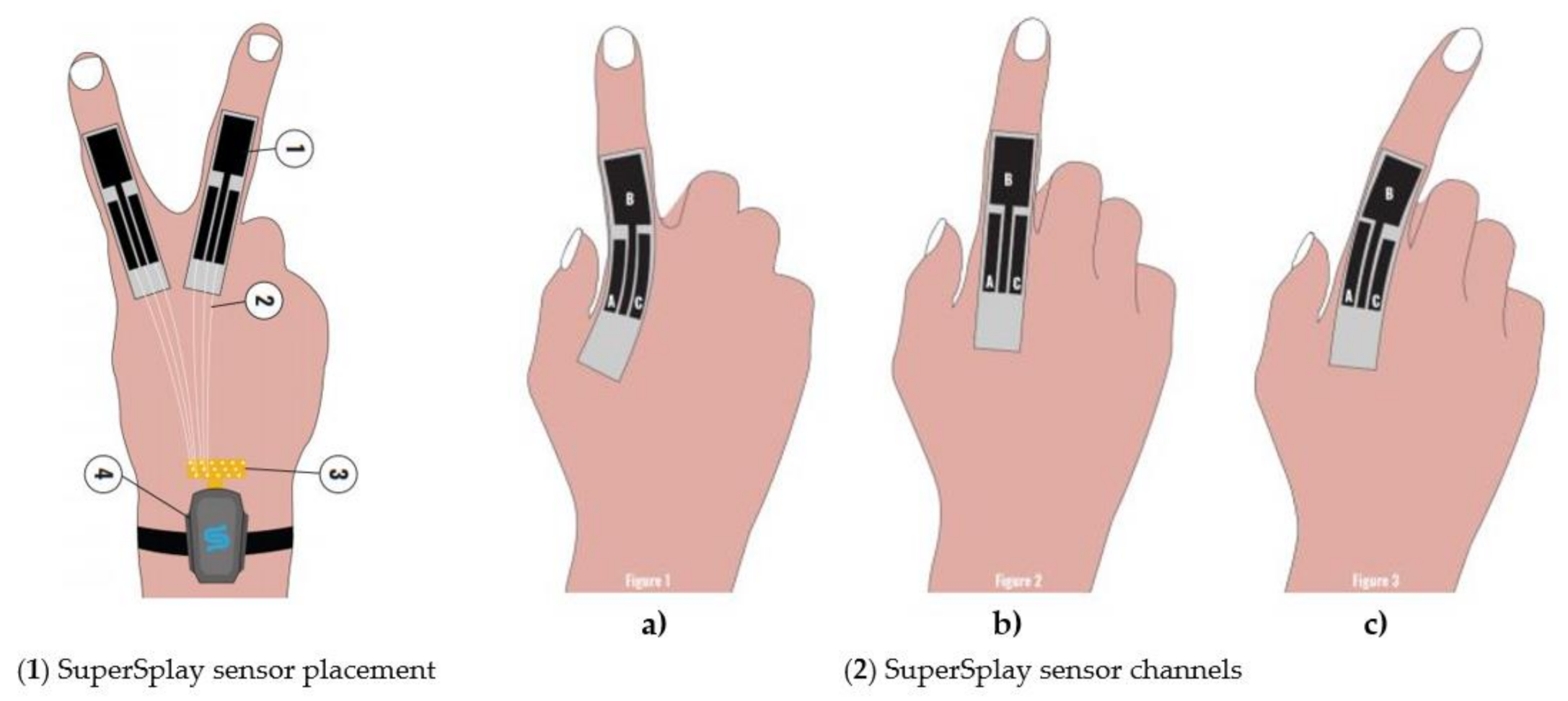

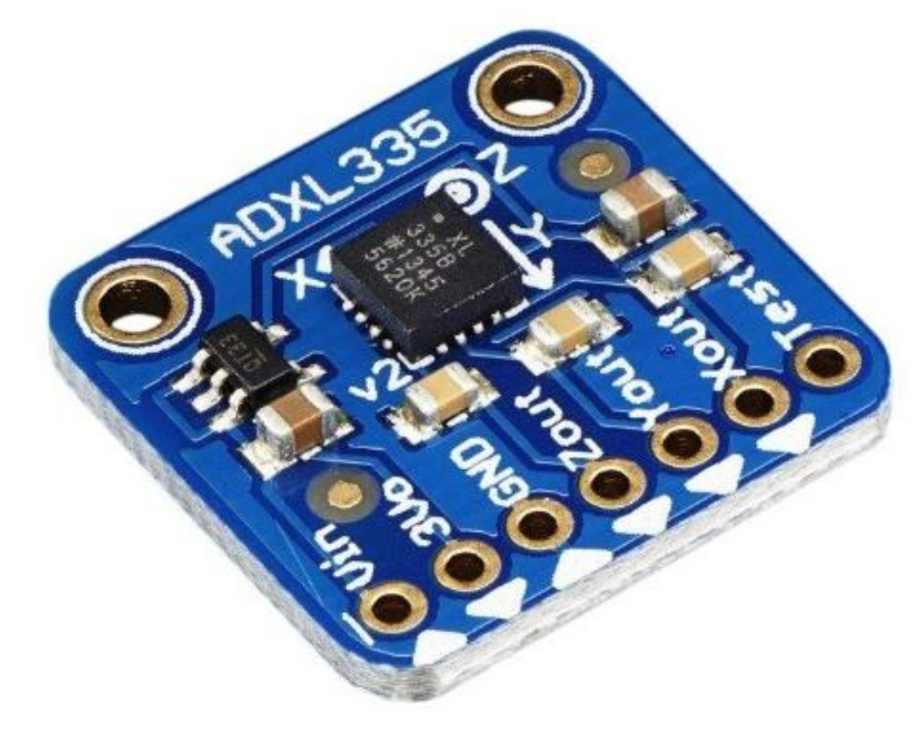
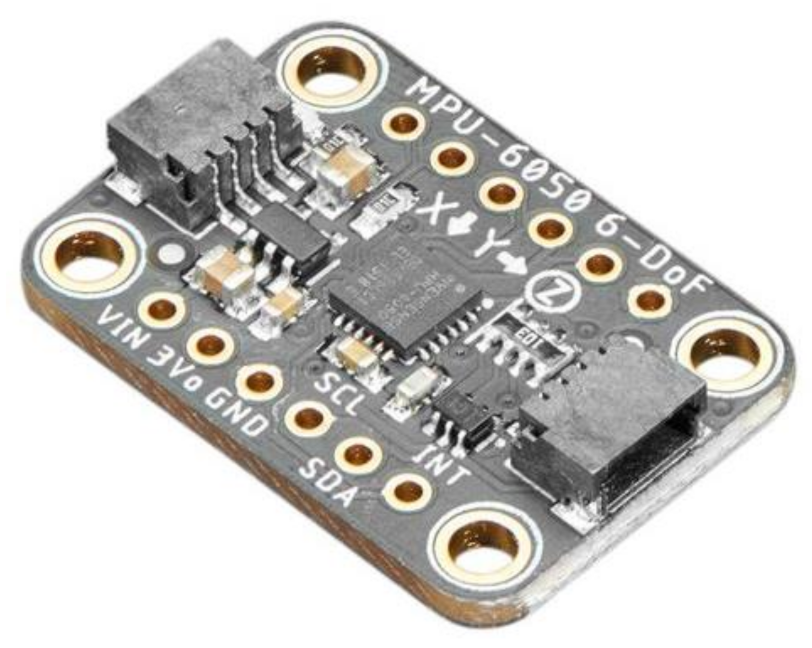






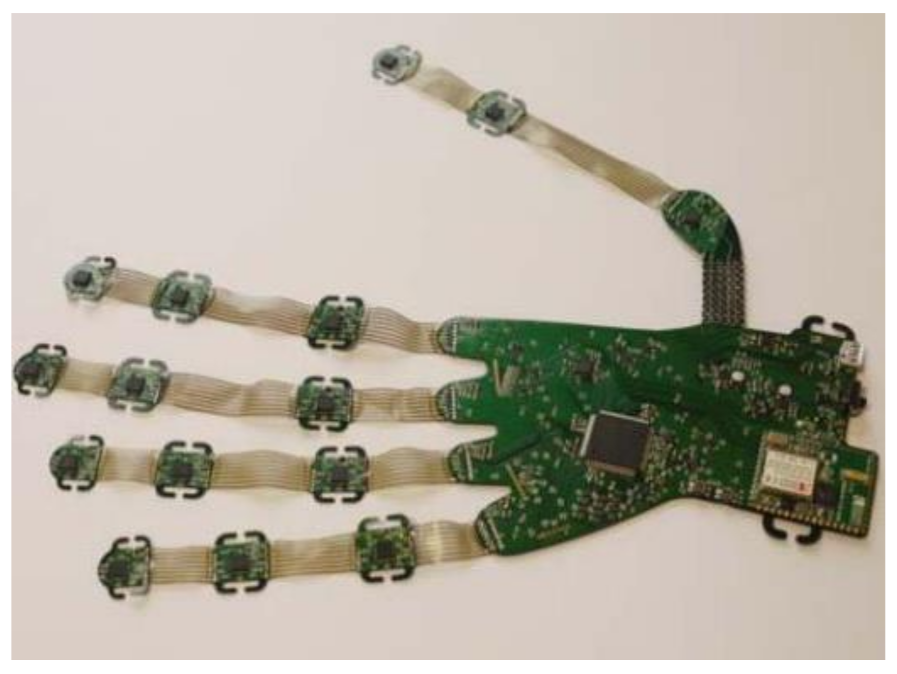
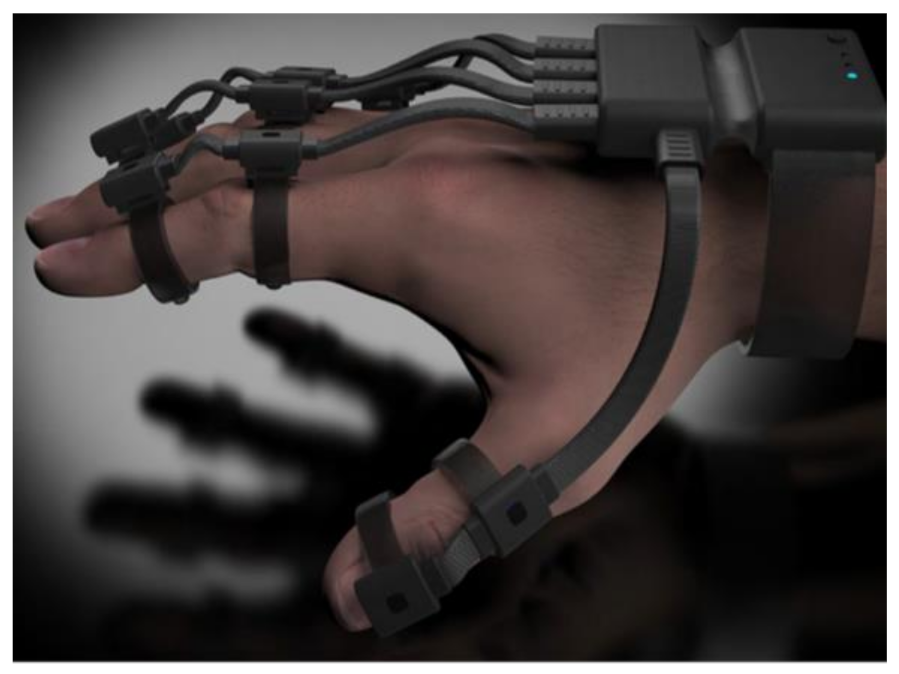




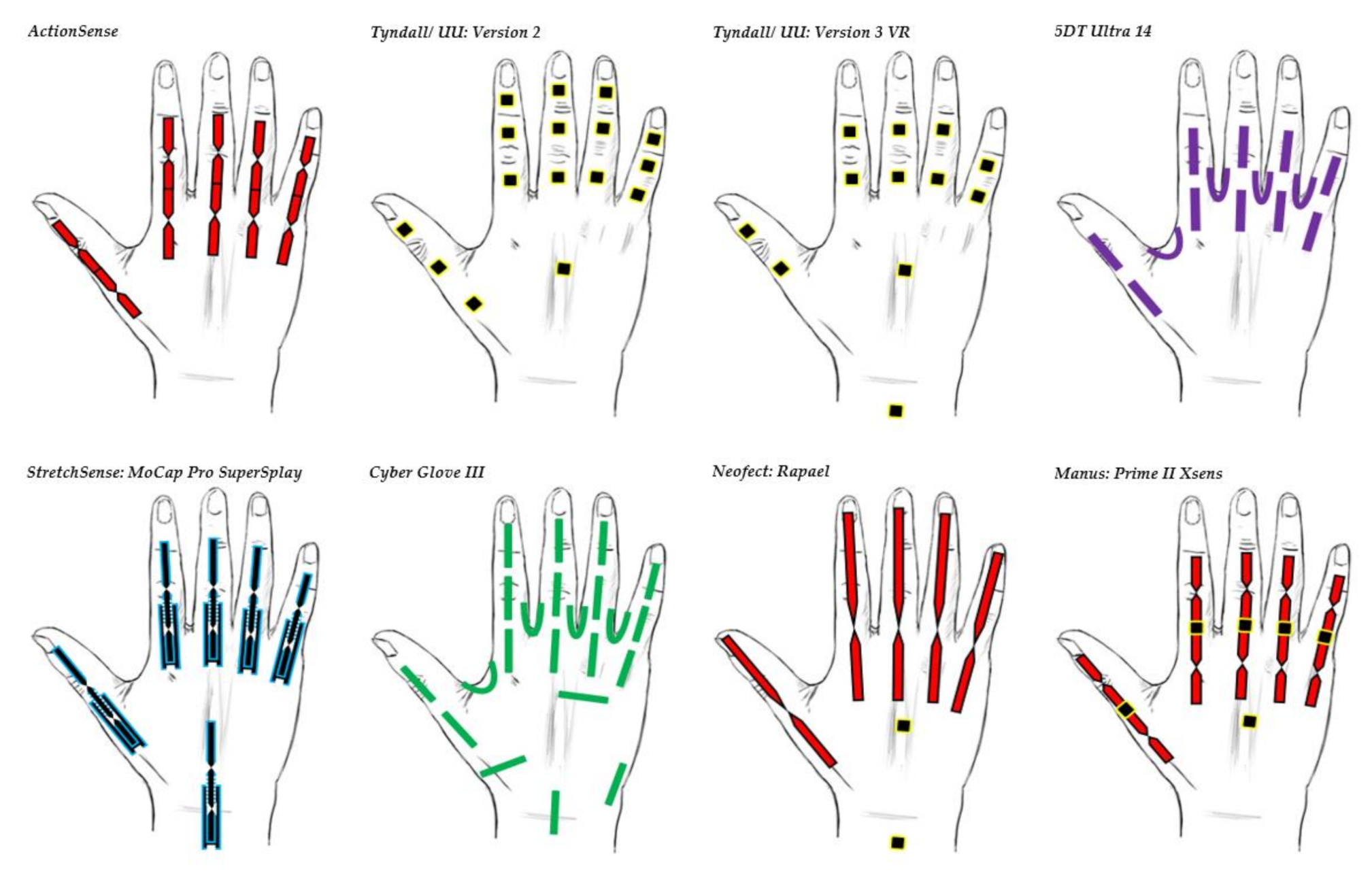
| Figure | Device/Sensor | Cost Device | Individual Sensor | DOF | Voltage Supply | Output Type |
|---|---|---|---|---|---|---|
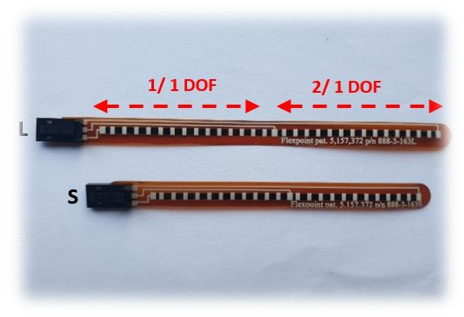
| Figure 5. | Flexpoint 2 in 1 sensor | N/A | £5–£20 | 1 DOF | 3.3 V–12 V | Analog |
| Figure 10. | Fibre optic sensor | N/A | - | 1 DOF | IR = 1.2 V Photodiode = 2.5 V | Analog |
| Figure 11. | Hall effect sensor | N/A | £1–£5 | 1 DOF | 4.5 V–10.5 V | Analog, Ratiometric |
| Figure 13. | StretchSense capacitive sensor | N/A | - | 3 DOF | 0–3 V | Analog |
| Figure 18. | Adafruit MPU-6050 | £10 | £7 | 6 DOF Acc & Gyro | 2.375 V–3.46 V | Digital, I2C (400 kHz) 16-bit |
| Figure 19. | SparkFun LSM9DS1 | £10 | £2.78 | 9 DOF Acc & Gyro & Mag | 1.9 V–3.6 V | Digital, I2C (400 kHz)/SPI 16-bit |
| Figure 20. | Adafruit MPU-9250 | £7 | £5–£8 | 9 DOF Acc & Gyro & Mag | 2.4 V–3.6 V | Digital, I2C (400 kHz)/ SPI 16-bit |
| Data Glove | Use | Market | Number of Sensors | Cost | Sensor Technology | Sensor Description | Joints Monitored(Refer to All Glove Image for Illustration) | Legend |
|---|---|---|---|---|---|---|---|---|
| 5DT Ultra 14 | Motion capture and animation | Commercial | 14 | £5000 | Fibre Optic | 5DT’s own sensor | All MCP & PIP joints of fingers. MCP & IP joints of thumb. Splay of all digits. |  |
| Tyndall/UU: Version 2 | Clinical | Research | 16 | £26,000 | IMU (9 DOF) | MPU-9150 | All MCP, PIP & DIP joints of fingers. IP, MCP & CMC joints of thumb. Splay of all digits. |  |
| Tyndall/UU: Version 3 VR | Clinical/VR | Research | 12 | £12,000 | IMU (9 DOF) | MPU-9250 | All MCP & PIP joints of fingers. IP & MCP joints of thumb. Splay of all digits & wrist movement. |  |
| Cyber Glove III | Motion capture environment | Commercial | 22 | $15,000 | Flex bend | Unknown | All MCP, PIP & DIP joints of fingers. IP, MCP & CMC joints of thumb. Splay of all digits, wrist & palm arch movement. |  |
| ActionSense | Clinical | Research | 5 | £400 | Flex bend | Flexpoint’s 2in1 combined sensor | All MCP & PIP joints of fingers. IP & MCP joints of thumb. |  |
| Neofect: Rapael | Clinical | Commercial | 5 Flex bend 2 IMU | $15,000 | Flex bend & IMU | Unknown | Fingers, wrist & forearm movement. |  |
| Manus: Prime II Xsens | Character animation | Commercial | 10 flex bend 5 IMU | $5000–$6000 | Flex bend & IMU (Combined sensor fusion) | Unknown | All MCP & PIP joints of fingers. IP, MCP & CMC joints of thumb. |  |
| StretchSesne: MoCap Pro SuperSplay | Film and game animation | Commercial | 6 | $7150 | Capacitive | StretchSense’s own SuperSplay sensor | All MCP & PIP joints of fingers. MCP & IP joints of thumb. Splay of all digits & wrist movement. |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, J.; Condell, J.; Connolly, J.; Kelly, D.; Curran, K. Review of Wearable Sensor-Based Health Monitoring Glove Devices for Rheumatoid Arthritis. Sensors 2021, 21, 1576. https://doi.org/10.3390/s21051576
Henderson J, Condell J, Connolly J, Kelly D, Curran K. Review of Wearable Sensor-Based Health Monitoring Glove Devices for Rheumatoid Arthritis. Sensors. 2021; 21(5):1576. https://doi.org/10.3390/s21051576
Chicago/Turabian StyleHenderson, Jeffrey, Joan Condell, James Connolly, Daniel Kelly, and Kevin Curran. 2021. "Review of Wearable Sensor-Based Health Monitoring Glove Devices for Rheumatoid Arthritis" Sensors 21, no. 5: 1576. https://doi.org/10.3390/s21051576
APA StyleHenderson, J., Condell, J., Connolly, J., Kelly, D., & Curran, K. (2021). Review of Wearable Sensor-Based Health Monitoring Glove Devices for Rheumatoid Arthritis. Sensors, 21(5), 1576. https://doi.org/10.3390/s21051576









