Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gold Nanoparticle Synthesis
2.3. Sensor Fabrication
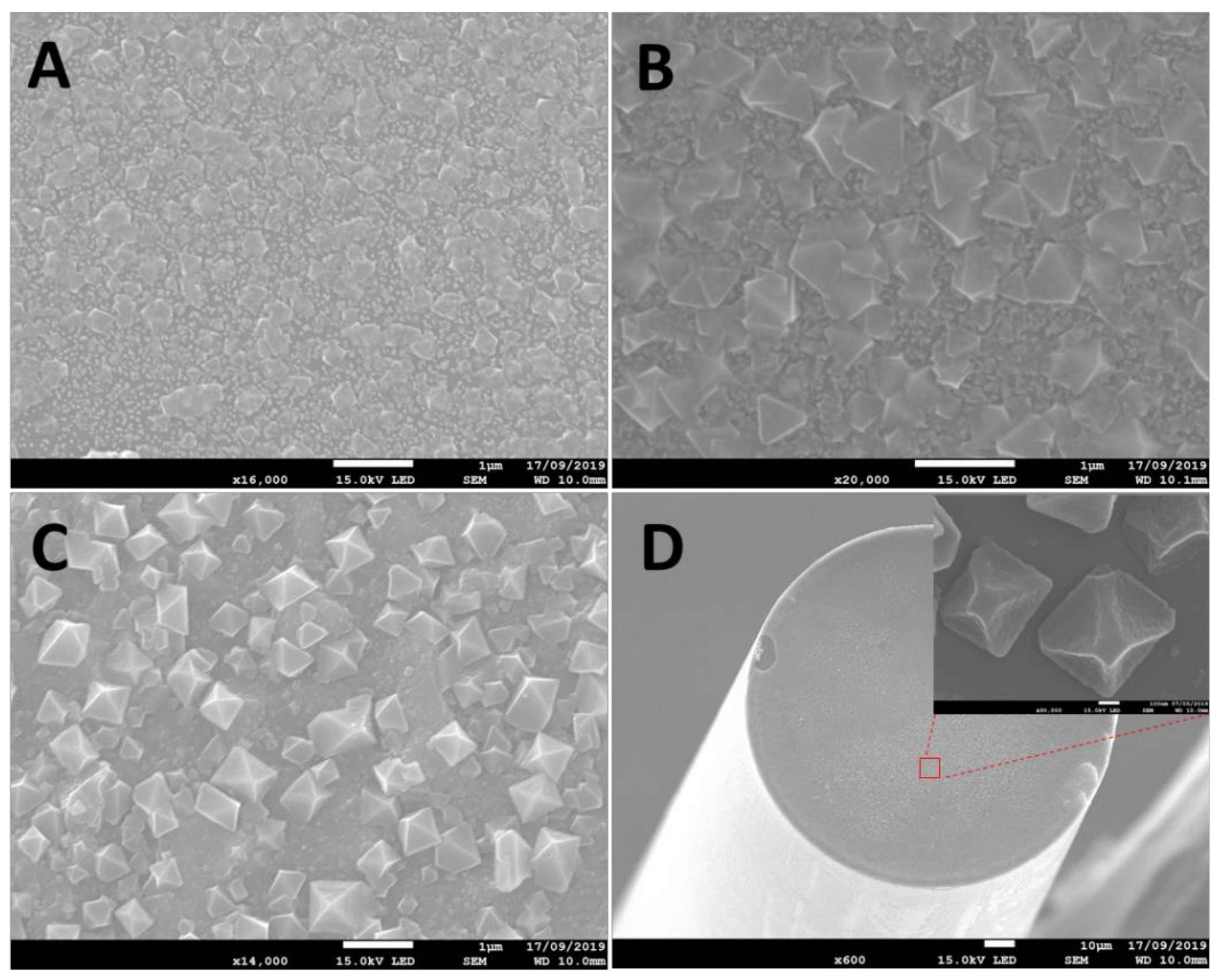
2.4. Microscopy
2.5. Experimental Set-Up and Organic Vapour Measurements
3. Results and Discussion
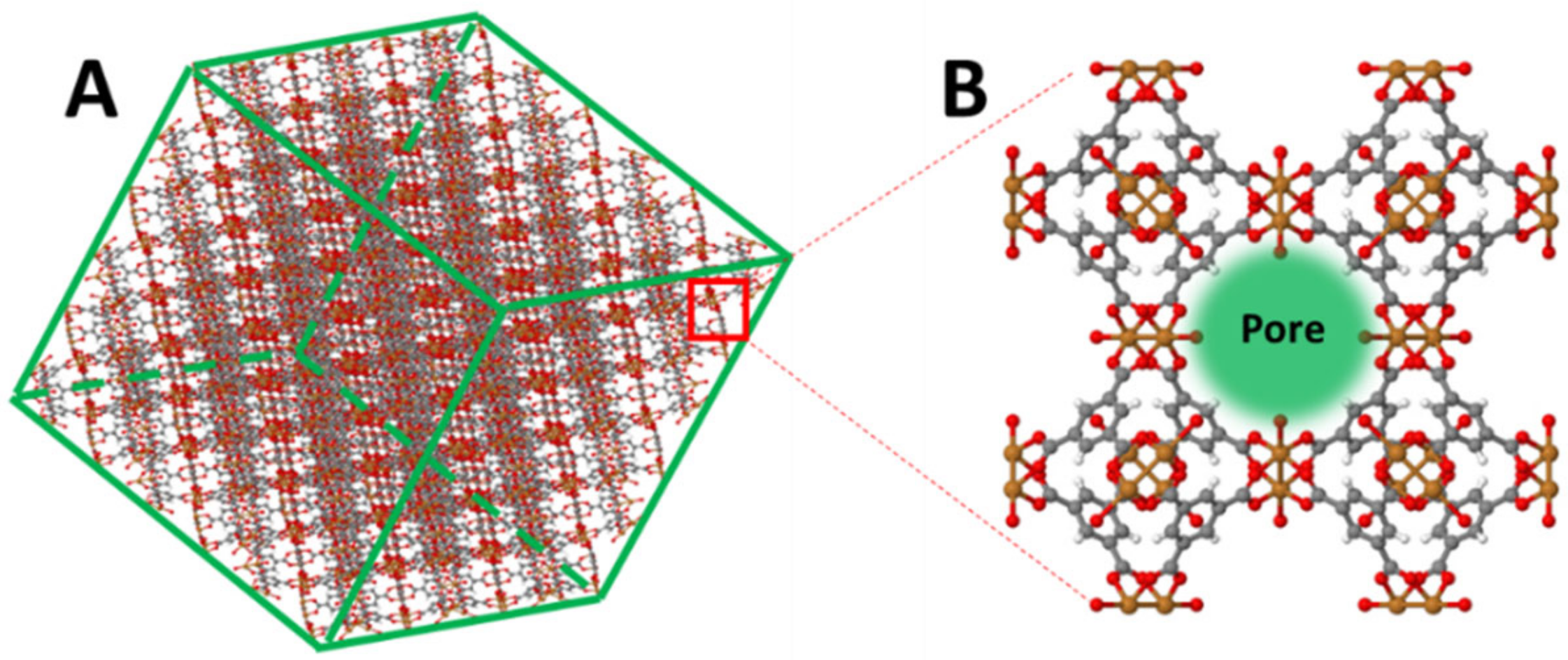
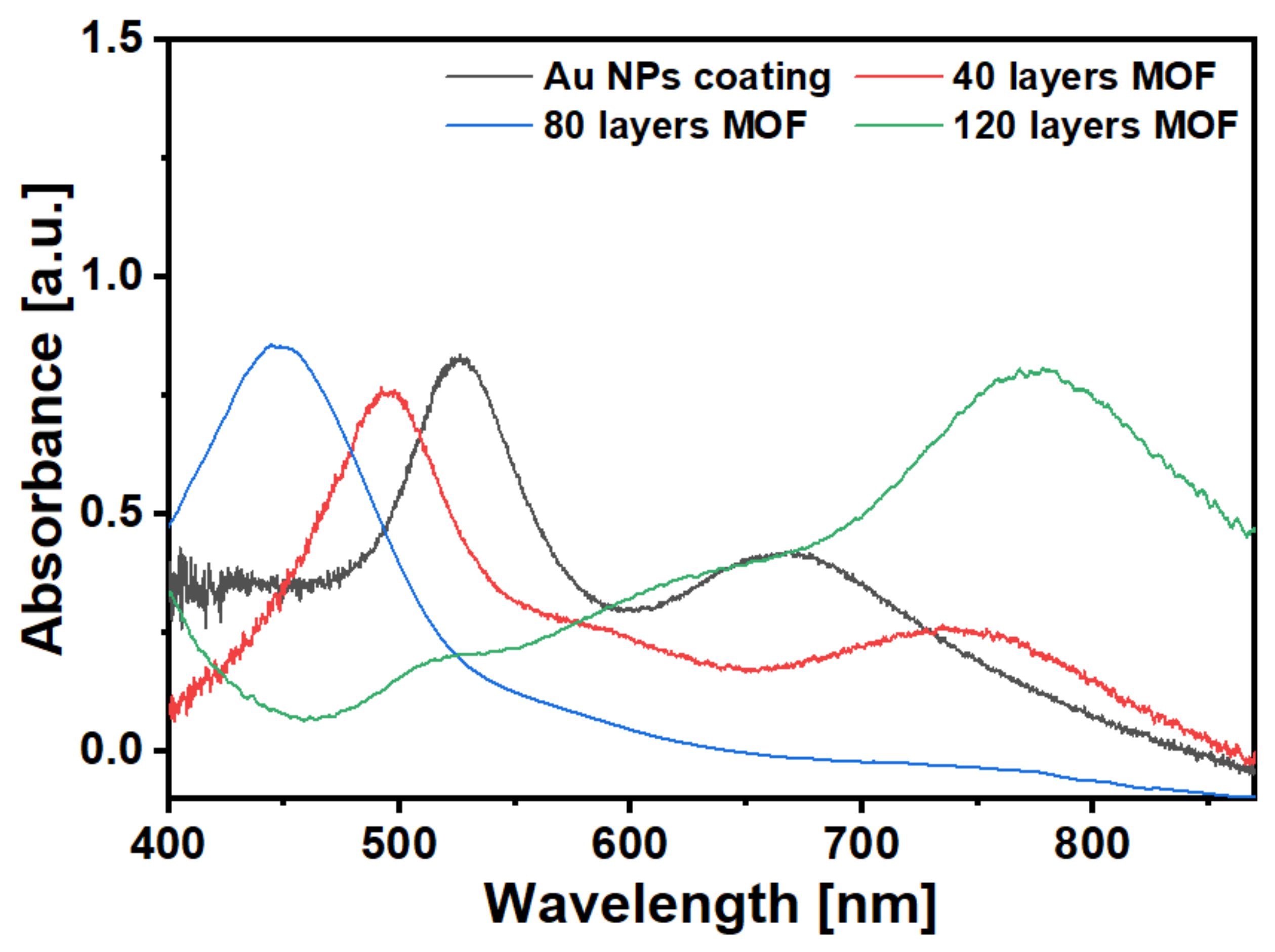
3.1. Characterisations of Gold Nanoparticles and HKUST-1 Films
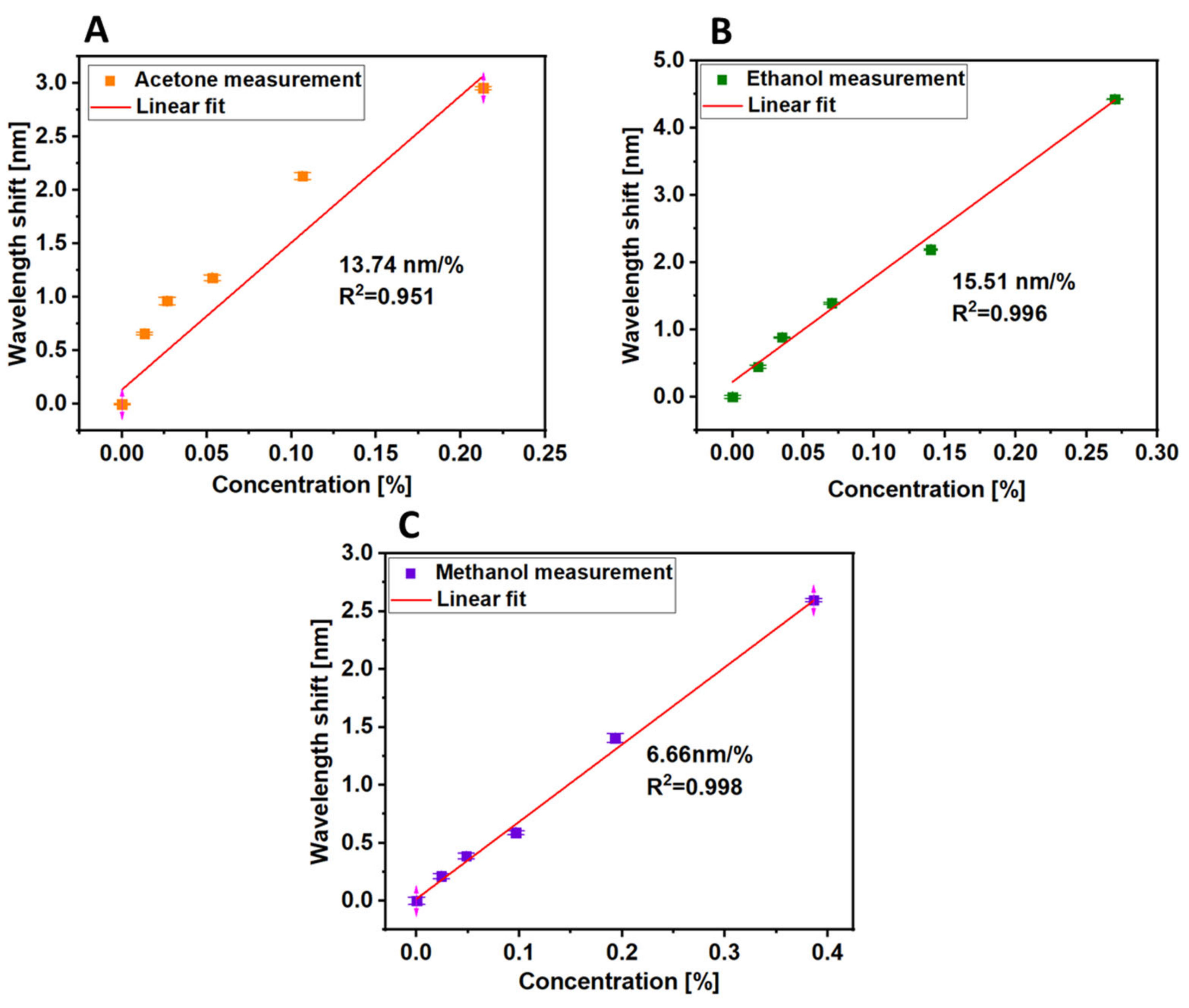
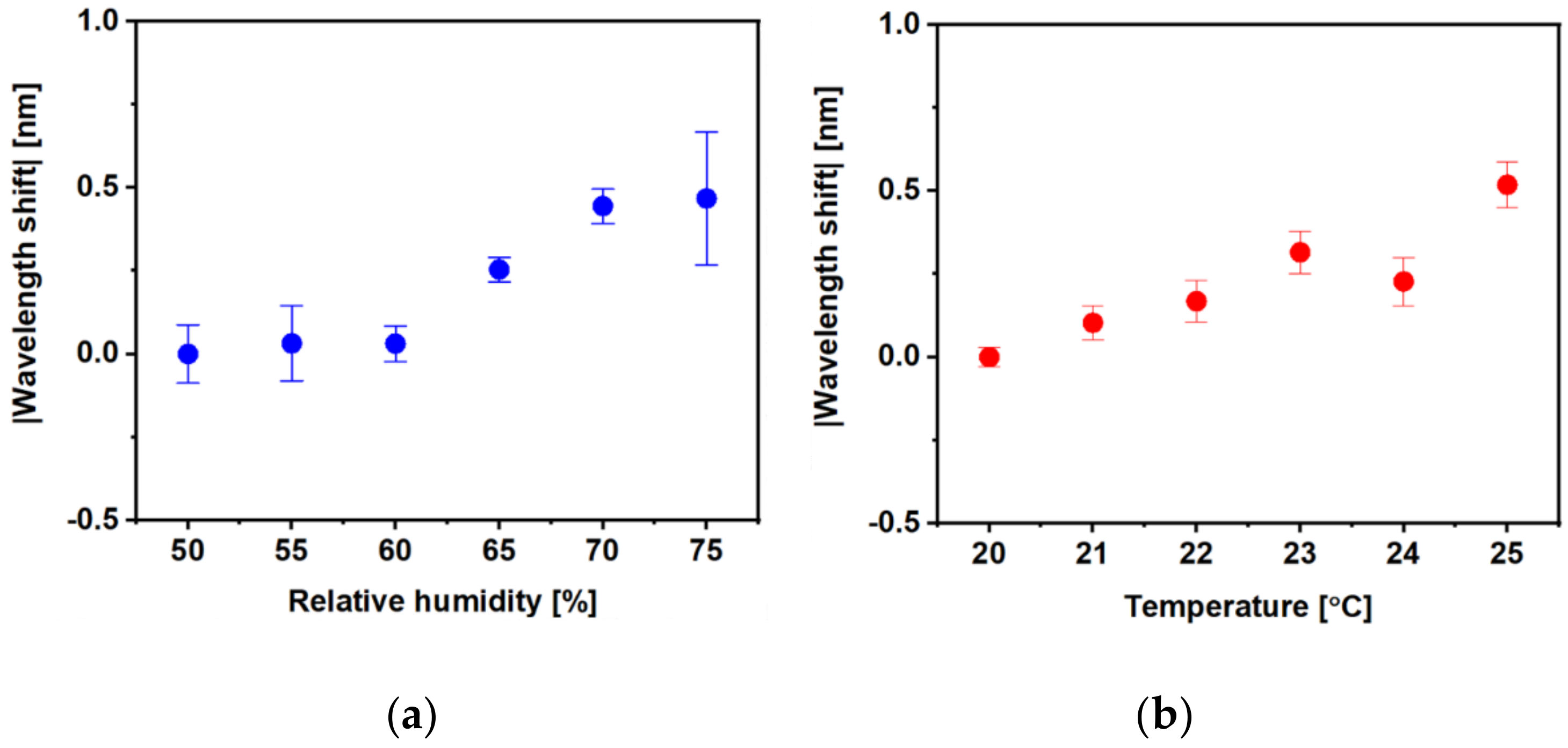
3.2. Volatile Organic Compound Sensing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, S.; Philip, B.; Mehendale, H. Volatile organic compounds. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 967–970. [Google Scholar]
- Drobek, M.; Figoli, A.; Santoro, S.; Navascués, N.; Motuzas, J.; Simone, S.; Algieri, C.; Gaeta, N.; Querze, L.; Trotta, A. PVDF-MFI mixed matrix membranes as VOCs adsorbers. Microporous Mesoporous Mater. 2015, 207, 126–133. [Google Scholar] [CrossRef]
- Liotta, L. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmospheric Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Volatile Organic Compounds Impact on Indoor Air Quality. 2017. Available online: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality (accessed on 25 January 2021).
- Deng, C.; Zhang, X.; Li, N. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography–mass spectrometry. J. Chromatogr. B 2004, 808, 269–277. [Google Scholar] [CrossRef]
- Sponring, A.; Filipiak, W.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Analysis of volatile organic compounds (VOCs) in the headspace of NCI-H1666 lung cancer cells. Cancer Biomark. 2011, 7, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Brunner, C.; Szymczak, W.; Höllriegl, V.; Mörtl, S.; Oelmez, H.; Bergner, A.; Huber, R.M.; Hoeschen, C.; Oeh, U. Discrimination of cancerous and non-cancerous cell lines by headspace-analysis with PTR-MS. Anal. Bioanal. Chem. 2010, 397, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Schallschmidt, K. Comparison of volatile organic compounds from lung cancer patients and healthy controls—Challenges and limitations of an observational study. J. Breath Res. 2016, 10, 046007. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Corradi, M.; Mazzone, P.; Mutti, A. Lung cancer biomarkers in exhaled breath. Expert Rev. Mol. Diagn. 2011, 11, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [PubMed]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Anderson, J.C. Measuring breath acetone for monitoring fat loss: Review. Obesity 2015, 23, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits, 4th ed.; Health and Safety Executive: Bootle, UK, 2020. [Google Scholar]
- Kitson, F.G.; Larsen, B.S.; McEwen, C.N. Gas Chromatography and Mass Spectrometry: A Practical Guide; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of surface enhanced raman spectroscopy (SERS) in therapeutic drug monitoring (TDM). A critical review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, A.-S.; Zou, B.; Zhang, H.-X.; Yan, K.-L.; Lin, Y. Advances of metal–organic frameworks for gas sensing. Polyhedron 2018, 154, 83–97. [Google Scholar] [CrossRef]
- Nijem, N.; Fürsich, K.; Kelly, S.T.; Swain, C.; Leone, S.R.; Gilles, M.K. HKUST-1 thin film layer-by-layer liquid phase epitaxial growth: Film properties and stability dependence on layer number. Cryst. Growth Des. 2015, 15, 2948–2957. [Google Scholar] [CrossRef]
- Gutiérrez-Sevillano, J.J.; Vicent-Luna, J.M.; Dubbeldam, D.; Calero, S. Molecular mechanisms for adsorption in Cu-BTC metal organic framework. J. Phys. Chem. C 2013, 117, 11357–11366. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lu, P.; Culp, J.T.; Ohodnicki, P.R. Metal–organic framework thin film coated optical fiber sensors: A novel waveguide-based chemical sensing platform. ACS Sensors 2018, 3, 386–394. [Google Scholar] [CrossRef]
- Hromadka, J.; Tokay, B.; Correia, R.; Morgan, S.P.; Korposh, S. Carbon dioxide measurements using long period grating optical fibre sensor coated with metal organic framework HKUST-1. Sensors Actuators B Chem. 2018, 255, 2483–2494. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Tu, M.; Sun, T.; Grattan, K. LSPR optical fibre sensors based on hollow gold nanostructures. Sensors Actuators B Chem. 2014, 191, 37–44. [Google Scholar] [CrossRef]
- Gowri, A.; Sai, V. Development of LSPR based U-bent plastic optical fiber sensors. Sensors Actuators B: Chem. 2016, 230, 536–543. [Google Scholar] [CrossRef]
- Chen, J.; Shi, S.; Su, R.; Qi, W.; Huang, R.; Wang, M.; Wang, L.; He, Z. Optimization and application of reflective LSPR optical fiber biosensors based on silver nanoparticles. Sensors 2015, 15, 12205–12217. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Stavila, V.; Volponi, J.V.; Katzenmeyer, A.M.; Dixon, M.C.; Allendorf, M.D. Kinetics and mechanism of metal–organic framework thin film growth: Systematic investigation of HKUST-1 deposition on QCM electrodes. Chem. Sci. 2012, 3, 1531–1540. [Google Scholar] [CrossRef]
- Terrie, P.E.; Boguski, K. Understanding Units of Measurement. Available online: https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.files/fileid/14285 (accessed on 16 February 2021).
- Amirjani, A.; Haghshenas, D.F. Ag nanostructures as the surface plasmon resonance (SPR)˗based sensors: A mechanistic study with an emphasis on heavy metallic ions detection. Sensors Actuators B Chem. 2018, 273, 1768–1779. [Google Scholar] [CrossRef]
- Liu, L.; He, C.; Morgan, S.P.; Correia, R.; Korposh, S. A fiber-optic localized surface plasmon resonance (LSPR) sensor anchored with metal organic framework (HKUST-1) film for acetone sensing. In Proceedings of the Seventh European Workshop on Optical Fibre Sensors, Limassol, Cypris, 1–4 October 2019; p. 111990Z. [Google Scholar]
- Hundal, G.; Hwang, Y.K.; Chang, J.-S. Formation of nanoporous and non-porous organic–inorganic hybrid materials incorporating α-Keggin phosphotungstate anion: X-ray crystal structure of a 3D polymeric complex [{Na6 (C9H5O6) 3 (H2O) 15}{PW12O40}]∞ with a ‘Ball-in-Bowl’type molecular structure. Polyhedron 2009, 28, 2450–2458. [Google Scholar] [CrossRef]
- Nan, J.; Dong, X.; Wang, W.; Jin, W.; Xu, N. Step-by-step seeding procedure for preparing HKUST-1 membrane on porous α-alumina support. Langmuir 2011, 27, 4309–4312. [Google Scholar] [CrossRef]
- Meilikhov, M.; Yusenko, K.; Schollmeyer, E.; Mayer, C.; Buschmann, H.-J.; Fischer, R.A. Stepwise deposition of metal organic frameworks on flexible synthetic polymer surfaces. Dalton Trans. 2011, 40, 4838–4841. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Shi, L.; Huang, H.; Cao, W.; Li, J.; Sun, L.; Jin, X.; Peng, X. Room temperature synthesis of free-standing HKUST-1 membranes from copper hydroxide nanostrands for gas separation. Chem. Commun. 2013, 49, 5666–5668. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ledezma, U.H.; Gurnani, P.; Albelha, T.; Thurecht, K.J.; Correia, R.G.; Morgan, S.P.; Patel, P.; Alexander, C.; Korposh, S. Surface polymer imprinted optical fibre sensor for dose detection of dabrafenib. Analyst 2020, 145, 4504–4511. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Korposh, S.; Correia, R.; Hayes-Gill, B.; Morgan, S. Optical fibre temperature sensor based on thermochromic liquid crystal. In Proceedings of the Seventh European Workshop on Optical Fibre Sensors, Limassol, Cypris, 1–4 October 2019; p. 1119908. [Google Scholar]
- He, C.; Korposh, S.; Hernandez, F.U.; Liu, L.; Correia, R.; Hayes-Gill, B.R.; Morgan, S.P. Real-time humidity measurement during sports activity using optical fibre sensing. Sensors 2020, 20, 1904. [Google Scholar] [CrossRef]
- Daglar, H.; Keskin, S. Recent advances, opportunities, and challenges in high-throughput computational screening of MOFs for gas separations. Coord. Chem. Rev. 2020, 422, 213470. [Google Scholar] [CrossRef]
- Farzi-Kahkesh, S.; Rahmani, M.B.; Fattah, A. Growth of novel α-MoO3 hierarchical nanostructured thin films for ethanol sensing. Mater. Sci. Semicond. Process. 2020, 120, 105263. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Liu, L.; Korposh, S.; Correia, R.; Morgan, S.P. Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework. Sensors 2021, 21, 1420. https://doi.org/10.3390/s21041420
He C, Liu L, Korposh S, Correia R, Morgan SP. Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework. Sensors. 2021; 21(4):1420. https://doi.org/10.3390/s21041420
Chicago/Turabian StyleHe, Chenyang, Liangliang Liu, Sergiy Korposh, Ricardo Correia, and Stephen P. Morgan. 2021. "Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework" Sensors 21, no. 4: 1420. https://doi.org/10.3390/s21041420
APA StyleHe, C., Liu, L., Korposh, S., Correia, R., & Morgan, S. P. (2021). Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework. Sensors, 21(4), 1420. https://doi.org/10.3390/s21041420






