A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal
Abstract
1. Introduction
- The suggested technique did not demand the post-processing of the ECG signal.
- It does not need handcrafted feature extraction.
- The proposed model has lower computational complexity than the previous models used to classify arrhythmia types.
2. Materials and Methods
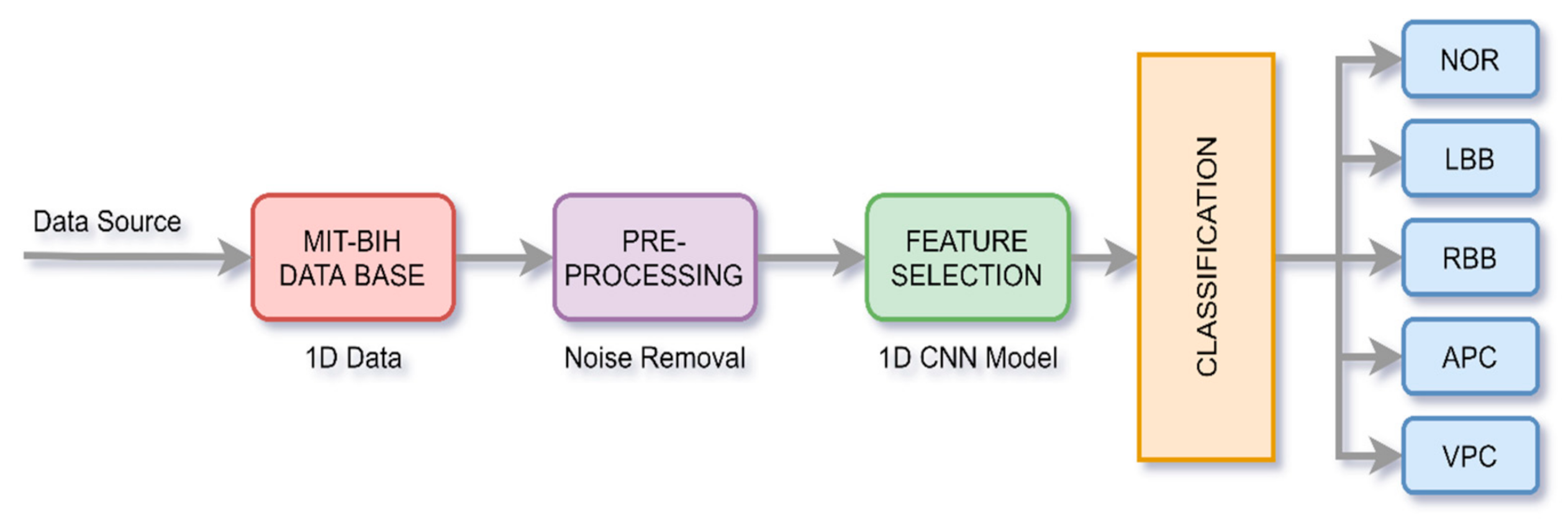
2.1. Proposed System for Arrhythmia Classification Using 1D CNN
2.1.1. Pre-processing of Data
2.1.2. 1D CNN Architecture
2.1.3. Cost Function
2.2. Method for Arrhythmia Classification Using 2D CNN
2.2.1. Pre-Processing
Generation of 2D Images
2.2.2. Augmentation of Data
2.2.3. Architecture of 2D CNN Model
2.2.4. Activation Function
2.2.5. Cost Function
2.2.6. Validation of Data
3. Experiments and Results
3.1. Data Set
3.2. Experimental Procedure
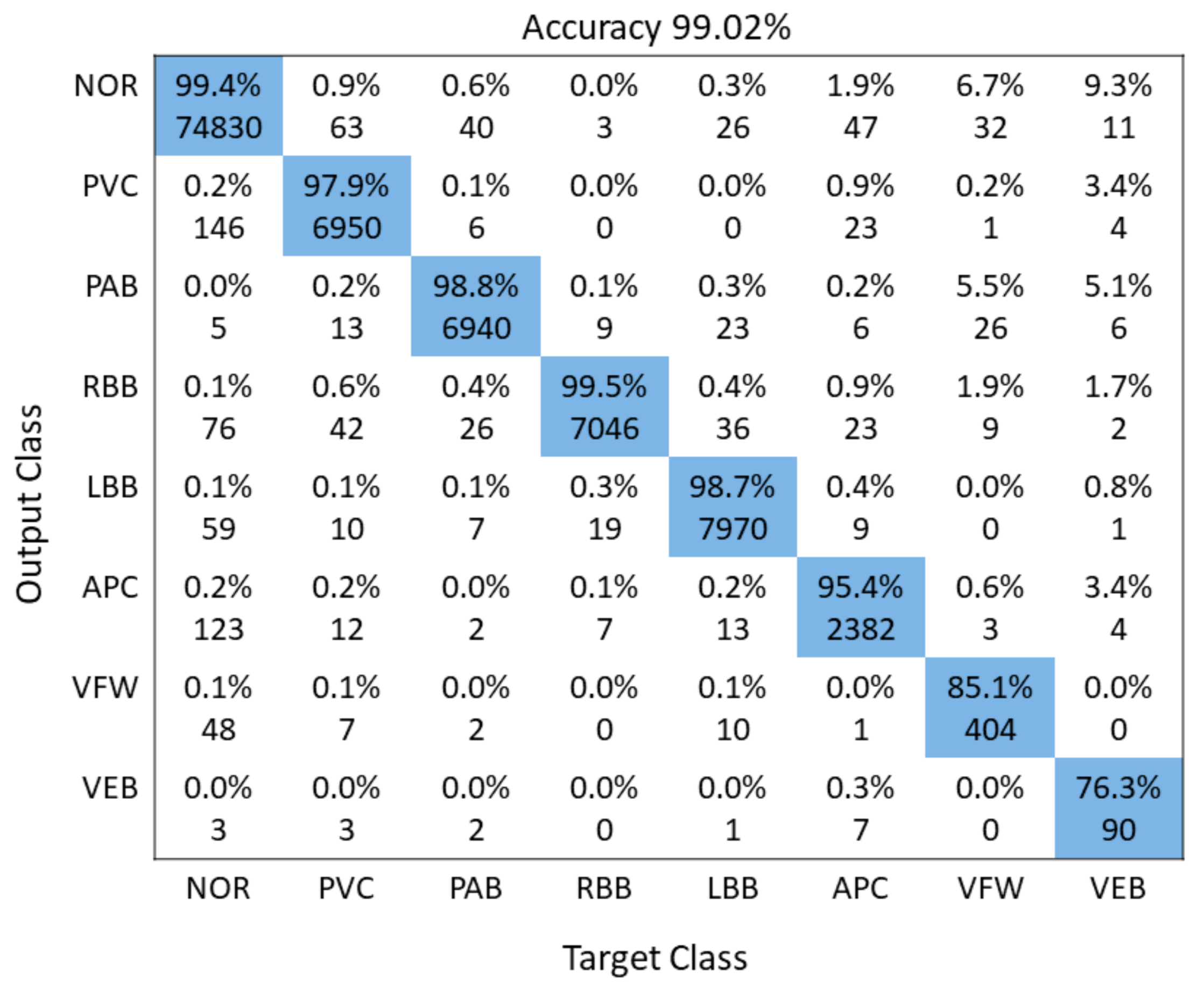
3.3. Performance Evaluation
4. Discussions of Both Models
4.1. 1-D CNN Comparison with Other Algorithms
4.2. 2-D CNN Comparison with Other Algorithms
5. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Lackland, D.T.; Weber, M.A. Global Burden of Cardiovascular Disease and Stroke: Hypertension at the Core. Can. J. Cardiol. 2015, 31, 569–571. [Google Scholar] [CrossRef]
- Evans, G.F.; Shirk, A.; Muturi, P.; Soliman, E.Z. Feasibility of using mobile ECG recording technology to detect atrial fibril-lation in low-resource settings. Glob. Heart 2017, 12, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zeng, N.; He, Y. Morphological Arrhythmia Automated Diagnosis Method Using Gray-Level Co-Occurrence Matrix Enhanced Convolutional Neural Network. IEEE Access 2019, 7, 67123–67129. [Google Scholar] [CrossRef]
- Laguna, P.; Cortes, J.P.M.; Pueyo, E. Techniques for Ventricular Repolarization Instability Assessment From the ECG. Proc. IEEE 2016, 104, 392–415. [Google Scholar] [CrossRef]
- Satija, U.; Ramkumar, B.; Manikandan, M.S. A New Automated Signal Quality-Aware ECG Beat Classification Method for Unsupervised ECG Diagnosis Environments. IEEE Sensors J. 2018, 19, 277–286. [Google Scholar] [CrossRef]
- Lynn, H.M.; Pan, S.B.; Kim, P. A Deep Bidirectional GRU Network Model for Biometric Electrocardiogram Classification Based on Recurrent Neural Networks. IEEE Access 2019, 7, 145395–145405. [Google Scholar] [CrossRef]
- Chu, Y.; Shen, H.; Huang, K. ECG Authentication Method Based on Parallel Multi-Scale One-Dimensional Residual Network with Center and Margin Loss. IEEE Access 2019, 7, 51598–51607. [Google Scholar] [CrossRef]
- Kim, H.; Chun, S.Y. Cancelable ECG Biometrics Using Compressive Sensing-Generalized Likelihood Ratio Test. IEEE Access 2019, 7, 9232–9242. [Google Scholar] [CrossRef]
- Dokur, Z.; Olmez, T.; Yazgan, E. Comparison of discrete wavelet and Fourier transforms for ECG beat classification. Electron. Lett. 1999, 35, 1502. [Google Scholar] [CrossRef]
- Banerjee, S.; Mitra, M. Application of Cross Wavelet Transform for ECG Pattern Analysis and Classification. IEEE Trans. Instrum. Meas. 2014, 63, 326–333. [Google Scholar] [CrossRef]
- Melgani, F.; Bazi, Y. Classification of electrocardiogram signals with support vector machines and particle swarm optimization. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, C.; Karthigaikumar, P.; Paul, A.; Satheeskumaran, S.; Kumar, R. ECG signal preprocessing and SVM classifi-er-based abnormality detection in remote healthcare applications. IEEE Access 2018, 6, 9767–9773. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Wang, Y.; Wang, L. Arrhythmia Recognition and Classification Using ECG Morphology and Segment Feature Analysis. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 16, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Ray, K.C. ECG Signal Analysis Using DCT-Based DOST and PSO Optimized SVM. IEEE Trans. Instrum. Meas. 2017, 66, 470–478. [Google Scholar] [CrossRef]
- Pasolli, E.; Melgani, F. Active Learning Methods for Electrocardiographic Signal Classification. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, X.; Wu, Z.; Yang, C.; Bai, B.; Yang, Q. Classification of Atrial Fibrillation Recurrence Based on a Convolution Neural Network With SVM Architecture. IEEE Access 2019, 7, 77849–77856. [Google Scholar] [CrossRef]
- Swathi, O.N.; Ganesan, M.; Lavanya, R. R peak detection and feature extraction for the diagnosis of heart diseases. In Proceedings of the 2017 International Conference on Advances in Computing, Communications and Informatics (ICACCI), Udupi, India, 13–16 September 2017; Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 2017; pp. 2388–2391. [Google Scholar]
- Parhi, K.K. VLSI Digital Signal Processing Systems: Design and Implementation; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Medjahed, S.A.; Ouali, M.; Saadi, T.A.; Benyettou, A. An Optimization-Based Framework for Feature Selection and Parameters Determination of SVMs. Int. J. Inf. Technol. Comput. Sci. 2015, 7, 1–9. [Google Scholar] [CrossRef]
- Huertas-Fernandez, I.; Garcia-Gomez, F.J.; Garcia-Solis, D.; Benitez-Rivero, S.; Marin-Oyaga, V.A.; Jesus, S.; Cáceres-Redondo, M.T.; Lojo, J.A.; Martín-Rodríguez, J.F.; Carrillo, F.; et al. Machine learning models for the differential diagnosis of vascular parkinsonism and Parkin-son’s disease using [123 I] FP-CIT SPECT. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 112–119. [Google Scholar] [CrossRef]
- Salvatore, C.; Cerasa, A.; Battista, P.; Gilardi, M.C.; Quattrone, A.; Castiglioni, I. Magnetic resonance imaging biomarkers for the early diagnosis of Alzheimer’s disease: A machine learning approach. Front. Neurosci. 2015, 9, 307. [Google Scholar] [CrossRef]
- Dreiseitl, S.; Ohno-Machado, L.; Kittler, H.; Vinterbo, S.; Billhardt, H.; Binder, M. A Comparison of Machine Learning Methods for the Diagnosis of Pigmented Skin Lesions. J. Biomed. Inform. 2001, 34, 28–36. [Google Scholar] [CrossRef]
- Kononenko, I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef]
- Maglogiannis, I.G. Emerging Artificial Intelligence Applications in Computer Engineering: Real Word ai Systems with Applications in Ehealth, Hci, Information Retrieval and Pervasive Technologies; Ios Press: Amsterdam, The Netherlands, 2007; Volume 160. [Google Scholar]
- Prusty, M.R.; Chakraborty, J.; Jayanthi, T.; Velusamy, K. Performance comparison of supervised machine learning algorithms for multiclass transient classification in a nuclear power plant. In Proceedings of the International Conference on Swarm, Evolu-tionary, and Memetic Computing, Bhubaneswar, India,18–20 December 2014; Springer: Berlin/Heidelberg, Germany, 2014; pp. 111–122. [Google Scholar]
- Song, M.H.; Lee, J.; Cho, S.P.; Lee, K.J.; Yoo, S.K. Support vector machine based arrhythmia classification using reduced features. Int. J. Control Autom. Syst. 2005, 3, 571. [Google Scholar]
- Das, M.K.; Ari, S. ECG Beats Classification Using Mixture of Features. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Santur, Y.; Santur, S.G. Knowledge Mining Approach For Healthy Monitoring From Pregnancy Data With Big Volumes. Int. J. Intell. Syst. Appl. Eng. 2016, 4, 141–145. [Google Scholar] [CrossRef]
- Santur, Y.; Karakose, M.; Akin, E. Random Forest Based Diagnosis Approach for Rail Fault Inspection in Railways. In Proceedings of the International Conference on Electrical and Electronics Engineering (Eleco 2015), Bursa, Turkey, 26–28 November 2015; pp. 714–719. [Google Scholar]
- Ullah, A.; Anwar, S.M.; Bilal, M.; Mahmoud, R.M. Classification of Arrhythmia by Using Deep Learning with 2-D ECG Spec-tral Image Representation. Remote Sens. 2020, 12, 1685. [Google Scholar] [CrossRef]
- Acharya, U.R.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M.; Gertych, A.; San Tan, R. A Deep Convolutional Neural Network Model to Classify Heartbeats. Comput. Biol. Med. 2017, 89, 389–396. [Google Scholar] [CrossRef]
- Pyakillya, B.; Kazachenko, N.; Mikhailovsky, N. Deep Learning for ECG Classification. J. Phys. Conf. Ser. 2017, 913, 012004. [Google Scholar] [CrossRef]
- Alajlan, N.; Bazi, Y.; Melgani, F.; Malek, S.; Bencherif, M.A. Detection of premature ventricular contraction arrhythmias in electrocardiogram signals with kernel methods. Signal Image Video Process. 2014, 8, 931–942. [Google Scholar] [CrossRef]
- Rehman, S.U.; Waqas, M.; Tu, S.; Koubaa, A.; Rehman, O.; Ahmad, J.; Hanif, M.; Han, Z. Deep Learning Techniques for Future Intelligent Cross-Media Retrieval. arXiv 2020, arXiv:2008.01191. [Google Scholar]
- Alonso-Atienza, F.; Morgado, E.; Fernandez-Martinez, L.; García-Alberola, A.; Rojo-Álvarez, J.L. Detection of Life-Threatening Arrhythmias Using Feature Selection and Support Vector Machines. IEEE Trans. Biomed. Eng. 2013, 61, 832–840. [Google Scholar] [CrossRef]
- Alvarado, A.S.; Lakshminarayan, C.; Principe, J.C. Time-Based Compression and Classification of Heartbeats. IEEE Trans. Biomed. Eng. 2012, 59, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Homaeinezhad, M.R.; Atyabi, S.A.; Tavakkoli, E.; Toosi, H.N.; Ghaffari, A.; Ebrahimpour, R. ECG arrhythmia recognition via a neuro-SVM–KNN hybrid clas- sifier with virtual QRS image-based geometrical features. Expert Syst. Appl. 2012, 39, 2047–2058. [Google Scholar] [CrossRef]
- Javadi, M.; Arani, S.A.A.A.; Sajedin, A.; Ebrahimpour, R. Classification of ECG arrhythmia by a modular neural network based on Mixture of Experts and Negatively Correlated Learning. Biomed. Signal Process. Control. 2013, 8, 289–296. [Google Scholar] [CrossRef]
- Längkvist, M.; Karlsson, L.; Loutfi, A. Sleep Stage Classification Using Unsupervised Feature Learning. Adv. Artif. Neural Syst. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Sameni, R.; Shamsollahi, M.B.; Jutten, C.; Clifford, G.D. A Nonlinear Bayesian Filtering Framework for ECG Denoising. IEEE Trans. Biomed. Eng. 2007, 54, 2172–2185. [Google Scholar] [CrossRef] [PubMed]
- Tracey, B.H.; Miller, E.L. Nonlocal Means Denoising of ECG Signals. IEEE Trans. Biomed. Eng. 2012, 59, 2383–2386. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Y.; Pan, X.; Gao, X. Parallel-type fractional zero-phase filtering for ECG signal denoising. Biomed. Signal Process. Control. 2015, 18, 36–41. [Google Scholar] [CrossRef]
- Yadav, S.K.; Sinha, R.; Bora, P.K. Electrocardiogram signal denoising using non-local wavelet transform domain filtering. IET Signal Process. 2015, 9, 88–96. [Google Scholar] [CrossRef]
- Rehman, S.U.; Waqas, M.; Huang, Y.; Rehman, O.U.; Ahmad, B.; Ahmad, S. Unsupervised pre-trained filter learning approach for efficient convolution neural network. Neurocomputing 2019, 365, 171–190. [Google Scholar] [CrossRef]
- Rehman, S.U.; Tu, S.; Huang, Y.; Magurawalage, C.M.S.; Chang, C.-C. Optimization of CNN through Novel Training Strategy for Visual Classification Problems. Entropy 2018, 20, 290. [Google Scholar] [CrossRef]
- Rehman, S.U.; Tu, S.; Huang, G.; Liu, G. CSFL: A novel unsupervised convolution neural network approach for visual pattern classification. AI Commun. 2017, 30, 311–324. [Google Scholar] [CrossRef]
- Rehman, S.; Tu, S.; Huang, Y.; Yang, Z. Face recognition: A novel un-supervised convolutional neural network meth-od. In Proceedings of the 2016 IEEE International Conference of Online Analysis and Computing Science (ICOACS), Chongqing, China, 28–29 May 2016; IEEE: Piscataway Township, NJ, USA, 2016; pp. 139–144. [Google Scholar]
- Available online: https://www.physionet.org/physiobank/database/mitdb/ (accessed on 15 October 2020).
- Tu, S.; Rehman, S.U.; Waqas, M.; Rehman, O.U.; Yang, Z.; Ahmad, B.; Halim, Z.; Zhao, W. Optimisation-based training of evolutionary convolution neural network for visual classification applications. IET Comput. Vis. 2020, 14, 259–267. [Google Scholar] [CrossRef]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Ghemawat, S. Tensorflow: Large-scale machine learning on heterogeneous distributed systems. arXiv 2016, arXiv:1603.04467. [Google Scholar]
- Guler, I.; Ubeylı, E.D. ECG beat classifier designed by combined neural network model. Pattern Recognit. 2005, 38, 199–208. [Google Scholar] [CrossRef]
- Jiang, W.; Kong, S.G. Block-based neural networks for personalized ECG signal classification. IEEE Trans. Neural Netw. 2007, 18, 1750–1761. [Google Scholar] [CrossRef]
- Kiranyaz, S.; Ince, T.; Gabbouj, M. Real-Time Patient-Specific ECG Classification by 1-D Convolutional Neural Networks. IEEE Trans. Biomed. Eng. 2015, 63, 664–675. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, J.; Yoon, C. An Automated ECG Beat Classification System Using Convolutional Neural Networks. In Proceedings of the 2016 6th International Conference on IT Convergence and Security (ICITCS), Prague, Czech Republic, 26–26 September 2016; pp. 1–5. [Google Scholar]
- Gutiérrez-Gnecchi, J.A.; Morfin-Magaña, R.; Lorias-Espinoza, D.; Tellez-Anguiano, A.D.C.; Reyes-Archundia, E.; Méndez-Patiño, A.; Castañeda-Miranda, R. DSP-based arrhythmia classification using wavelet transform and probabilistic neural network. Biomed. Signal Process. Control. 2017, 32, 44–56. [Google Scholar] [CrossRef]
- Rajkumar, A.; Ganesan, M.; Lavanya, R. Arrhythmia classification on ECG using Deep Learning. In Proceedings of the 2019 5th International Conference on Advanced Computing & Communication Systems (ICACCS), Coimbatore, India, 15–16 March 2019; Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 2019; pp. 365–369. [Google Scholar]
- Inan, O.T.; Giovangrandi, L.; Kovacs, G.T. Robust neural-network-based classification of premature ventricular contractions using wavelet transform and timing interval features. IEEE Trans. Biomed. Eng. 2006, 53, 2507–2515. [Google Scholar] [CrossRef]
- Zadeh, A.E.; Khazaee, A. High Efficient System for Automatic Classification of the Electrocardiogram Beats. Ann. Biomed. Eng. 2011, 39, 996–1011. [Google Scholar] [CrossRef]
- Martis, R.J.; Acharya, U.R.; Mandana, K.; Ray, A.; Chakraborty, C. Cardiac decision making using higher order spectra. Biomed. Signal Process. Control. 2013, 8, 193–203. [Google Scholar] [CrossRef]
- Joshi, N.P.; Topannavar, P.S. Support vector machine-based heartbeat classification. In Proceedings of the 4th IRF International Conference, Bangalore, India, 4 May 2014; pp. 140–144. [Google Scholar]
- Ur Rehman, S.; Huang, Y.; Tu, S.; Basharat, A. Learning a Semantic Space for Modeling Images, Tags and Feelings in Cross-Media Search. In Pacific-Asia Conference on Knowledge Discovery and Data Mining; Springer: Cham, Switzerland, 2019; pp. 65–76. [Google Scholar]
- Ince, T.; Kiranyaz, S.; Gabbouj, M. A Generic and Robust System for Automated Patient-Specific Classification of ECG Signals. IEEE Trans. Biomed. Eng. 2009, 56, 1415–1426. [Google Scholar] [CrossRef]
- Dutta, S.; Chatterjee, A.; Munshi, S. Correlation technique and least square support vector machine combine for frequency domain based ECG beat classification. Med Eng. Phys. 2010, 32, 1161–1169. [Google Scholar] [CrossRef]
- Kumar, R.G.; Kumaraswamy, Y.S. Investigating cardiac arrhythmia in ECG using random forest 400 classification. Int. J. Comput. Appl. 2012, 37, 31–34. [Google Scholar]
- Ince, T.; Kiranyaz, S.; Eren, L.; Askar, M.; Gabbouj, M. Real-time motor fault detection by 1-D 404 convolutional neu-ral networks. IEEE Trans. Ind. Electron. 2016, 6311, 7067–7075. [Google Scholar] [CrossRef]
- Nanjundegowda, R.; Meshram, V.; Jain University. Dayananda Sagar University Arrhythmia Detection Based on Hybrid Features of T-wave in Electrocardiogram. Int. J. Intell. Eng. Syst. 2018, 11, 153–162. [Google Scholar] [CrossRef]
- Zhai, X.; Tin, C. Automated ECG Classification Using Dual Heartbeat Coupling Based on Convolutional Neural Network. IEEE Access 2018, 6, 27465–27472. [Google Scholar] [CrossRef]
- Rangappa, V.G.; Prasad, S.V.A.V.; Agarwal, A. Classification of Cardiac Arrhythmia stages using Hybrid Features Extraction with K-Nearest Neighbour classifier of ECG Signals. Learning 2018, 11, 21–32. [Google Scholar] [CrossRef]
- Izci, E.; Ozdemir, M.A.; Degirmenci, M.; Akan, A. Cardiac Arrhythmia Detection from 2D ECG Images by Using Deep Learning Technique. In 2019 Medical Technologies Congress (TIPTEKNO); Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 2019; pp. 1–4. [Google Scholar]





| Layer # | Type | Kernel Size | Stride | # Kernel |
|---|---|---|---|---|
| 1 | 1D Conv | 5 | 1 | 128 |
| 2 | Pooling | 2 | 2 | - |
| 3 | 1D Conv | 5 | 1 | 128 |
| 4 | Pooling | 2 | 2 | - |
| 5 | Fully Connected | - | - | 520 |
| 6 | Output Layer | - | - | 5 |
| Layer # | Type | Kernel Size | Stride | # Kernel | Input Size |
|---|---|---|---|---|---|
| 1 | 2D Conv | 3 × 3 | 1 | 64 | 512 × 512 × 1 |
| 1 | Pooling | 2 × 2 | 2 | 512 × 512 × 64 | |
| 2 | 2D Conv | 3 × 3 | 1 | 128 | 256 × 256 × 64 |
| 2 | Pooling | 2 × 2 | 2 | 256 × 256 × 128 | |
| 3 | 2D Conv | 3 × 3 | 1 | 256 | 128 × 128 × 128 |
| 3 | Pooling | 2 × 2 | 2 | 128 × 128 × 256 | |
| 4 | 2D Conv | 3 × 3 | 1 | 512 | 64 × 64 × 256 |
| 4 | Pooling | 2 × 2 | 2 | 64 × 64 × 512 | |
| 5 | Fully Connected | 4096 | 16 × 16 × 512 | ||
| 6 | Output Layer | 8 | 4096 |
| S. No. | Reference | Year | #Class | Methods | Accuracy |
|---|---|---|---|---|---|
| 1 | [52] | 2007 | 5 | NN | 96.70% |
| 2 | [11] | 2008 | 6 | SVM | 89.72% |
| 3 | [53] | 2015 | 5 | 1D-CNN | 95.14% |
| 4 | [54] | 2016 | 5 | 1D-CNN | 92.60% |
| 5 | [55] | 2017 | 5 | PNN | 92.80% |
| 6 | [56] | 2018 | 5 | 1D-CNN | 90.00% |
| 7 | [57] | 2019 | 5 | 1D-CNN | 93.60% |
| 8 | [58] | 2011 | 5 | SVM, GA | 97.30% |
| 9 | [59] | 2013 | 5 | NN, SVM | 93.00% |
| 10 | [60] | 2014 | 5 | SVM | 86.40% |
| 11 | Proposed Technique | 2020 | 5 | 1D-CNN | 97.38% |
| No. | Reference | Year | #Class | Methods | Accuracy |
|---|---|---|---|---|---|
| 1 | [11] | 2008 | 6 | SVM | 91.67% |
| 2 | [61] | 2009 | 4 | FFNN | 96.94% |
| 3 | [62] | 2009 | 5 | PCA, ANN | 98.30% |
| 4 | [63] | 2010 | 3 | LS-SVM | 95.82% |
| 5 | [64] | 2012 | 3 | RFT | 92.16% |
| 6 | [65] | 2016 | 5 | 1D-CNN | 96.40% |
| 7 | [66] | 2018 | 2 | DWT, DNN | 98.33% |
| 8 | [67] | 2018 | 5 | 2D-CNN | 96.05% |
| 9 | [68] | 2018 | 2 | KNN | 98.40% |
| 10 | [69] | 2019 | 5 | 2D CNN | 97.42% |
| 11 | [57] | 2019 | 7 | 1D CNN | 93.60% |
| 12 | Proposed Technique | 2020 | 8 | 2D-CNN | 99.02% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Rehman, S.u.; Tu, S.; Mehmood, R.M.; Fawad; Ehatisham-ul-haq, M. A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal. Sensors 2021, 21, 951. https://doi.org/10.3390/s21030951
Ullah A, Rehman Su, Tu S, Mehmood RM, Fawad, Ehatisham-ul-haq M. A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal. Sensors. 2021; 21(3):951. https://doi.org/10.3390/s21030951
Chicago/Turabian StyleUllah, Amin, Sadaqat ur Rehman, Shanshan Tu, Raja Majid Mehmood, Fawad, and Muhammad Ehatisham-ul-haq. 2021. "A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal" Sensors 21, no. 3: 951. https://doi.org/10.3390/s21030951
APA StyleUllah, A., Rehman, S. u., Tu, S., Mehmood, R. M., Fawad, & Ehatisham-ul-haq, M. (2021). A Hybrid Deep CNN Model for Abnormal Arrhythmia Detection Based on Cardiac ECG Signal. Sensors, 21(3), 951. https://doi.org/10.3390/s21030951






