Silver Nanowires as Electron Transfer Mediators in Electrochemical Catechol Biosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Preparation of AgNWs
2.4. Preparation of AgNWs-Tyr Modified Electrode
3. Results and Discussion
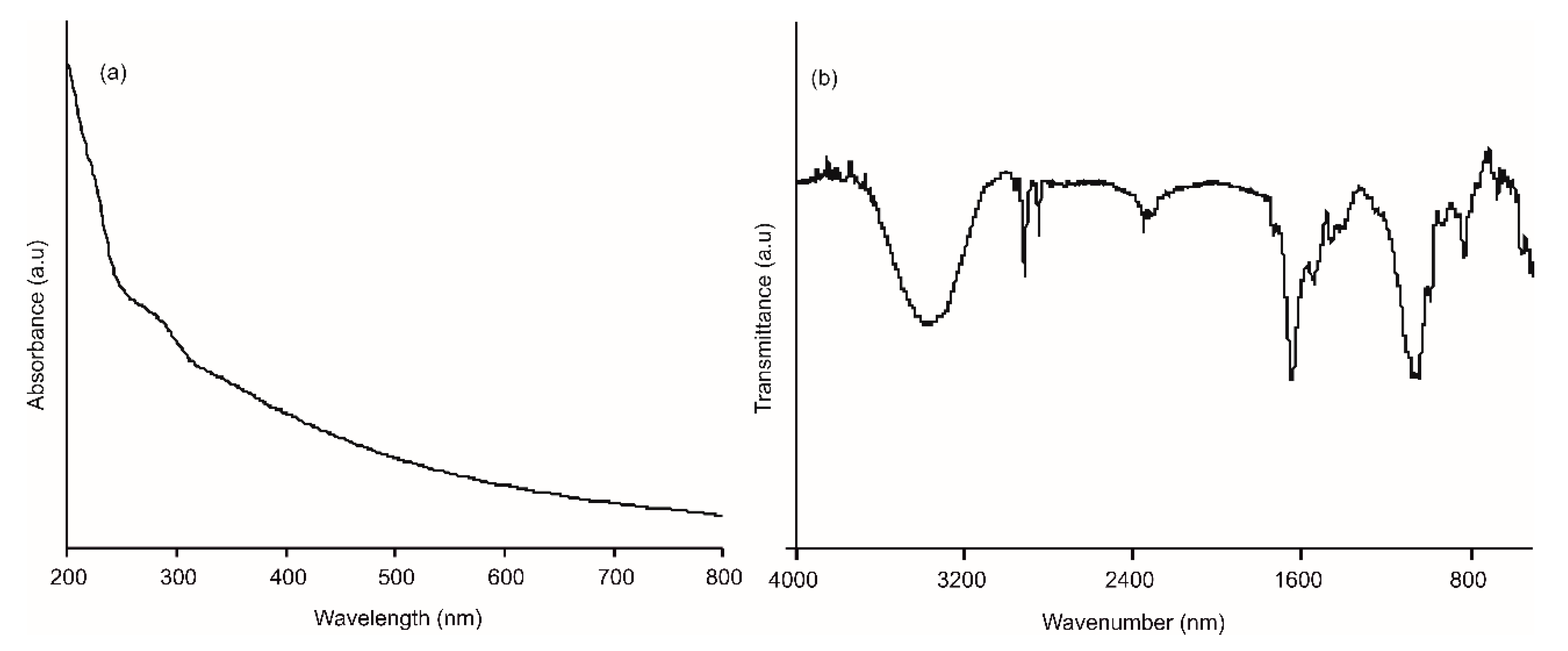
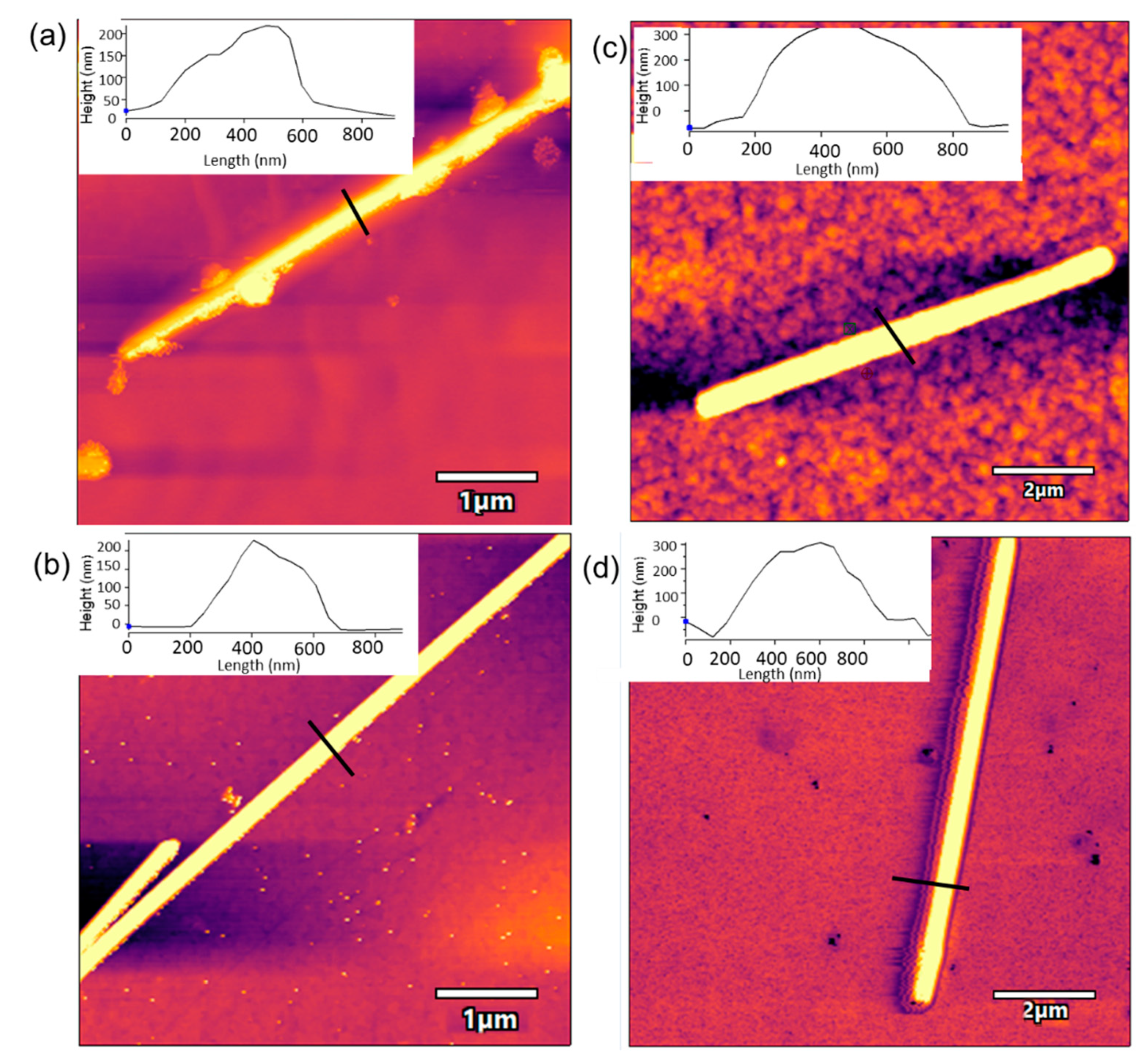
3.1. Characterization of AgNWs
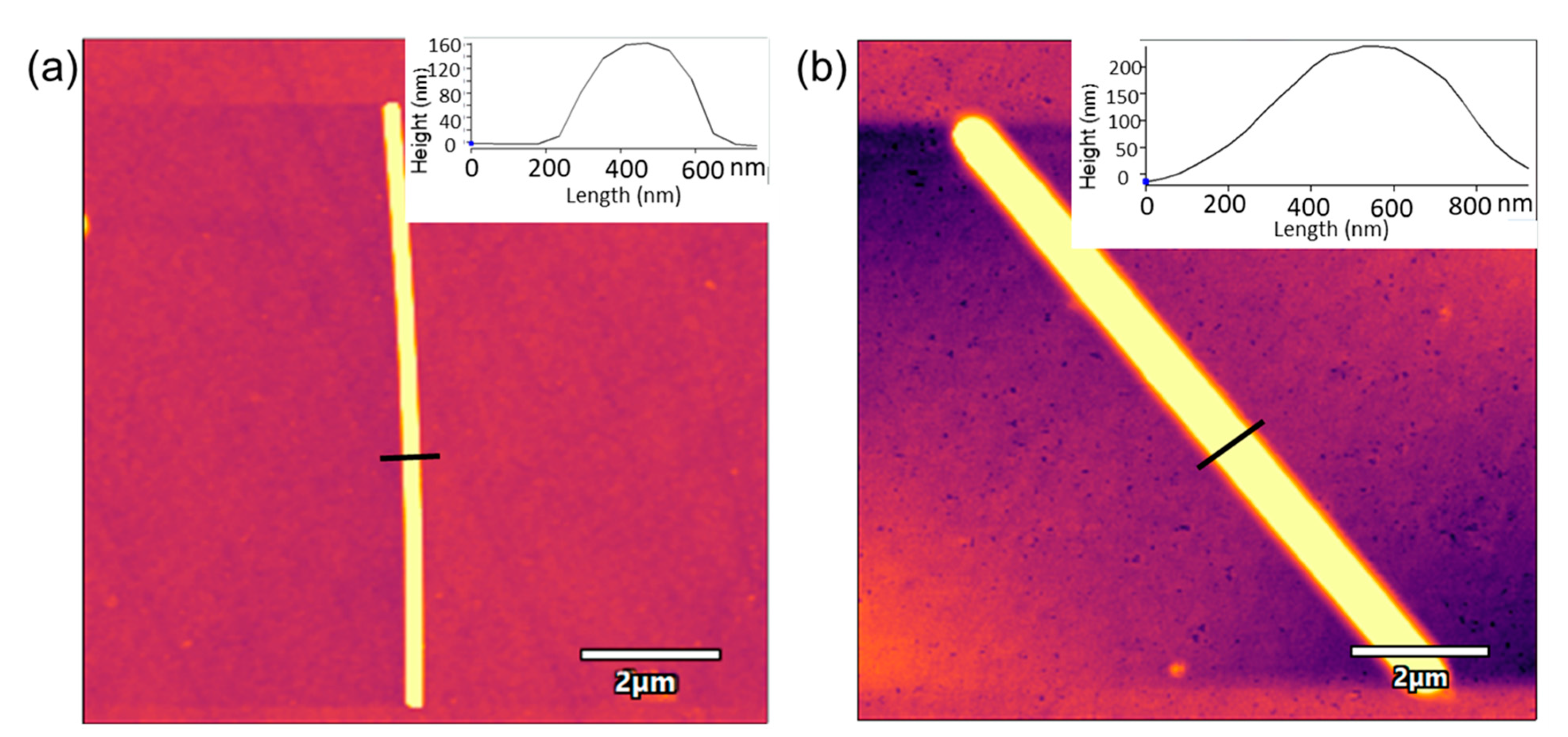
3.2. Characterization of the AgNWs-Tyr Biosensors: Spectroscopy and AFM
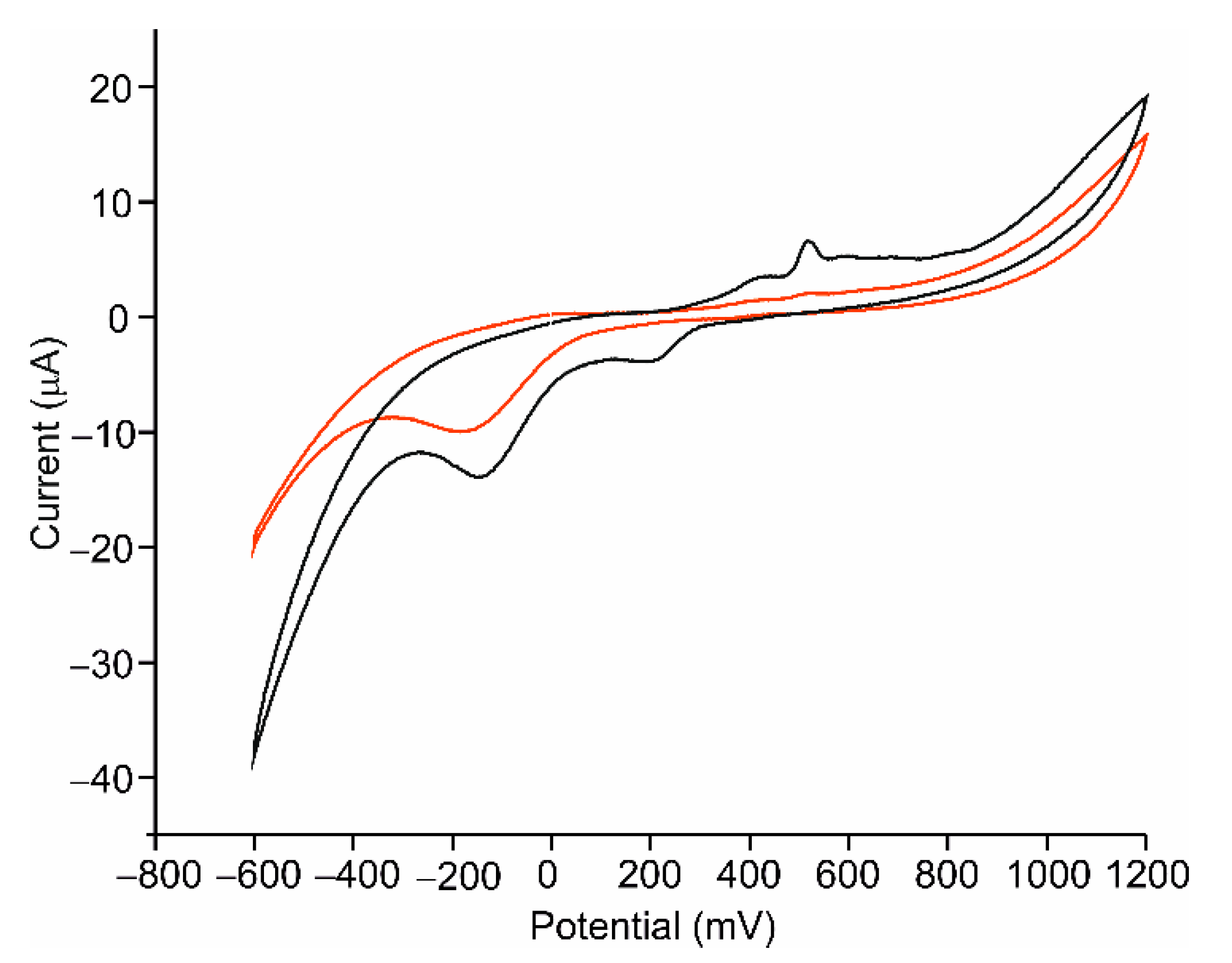
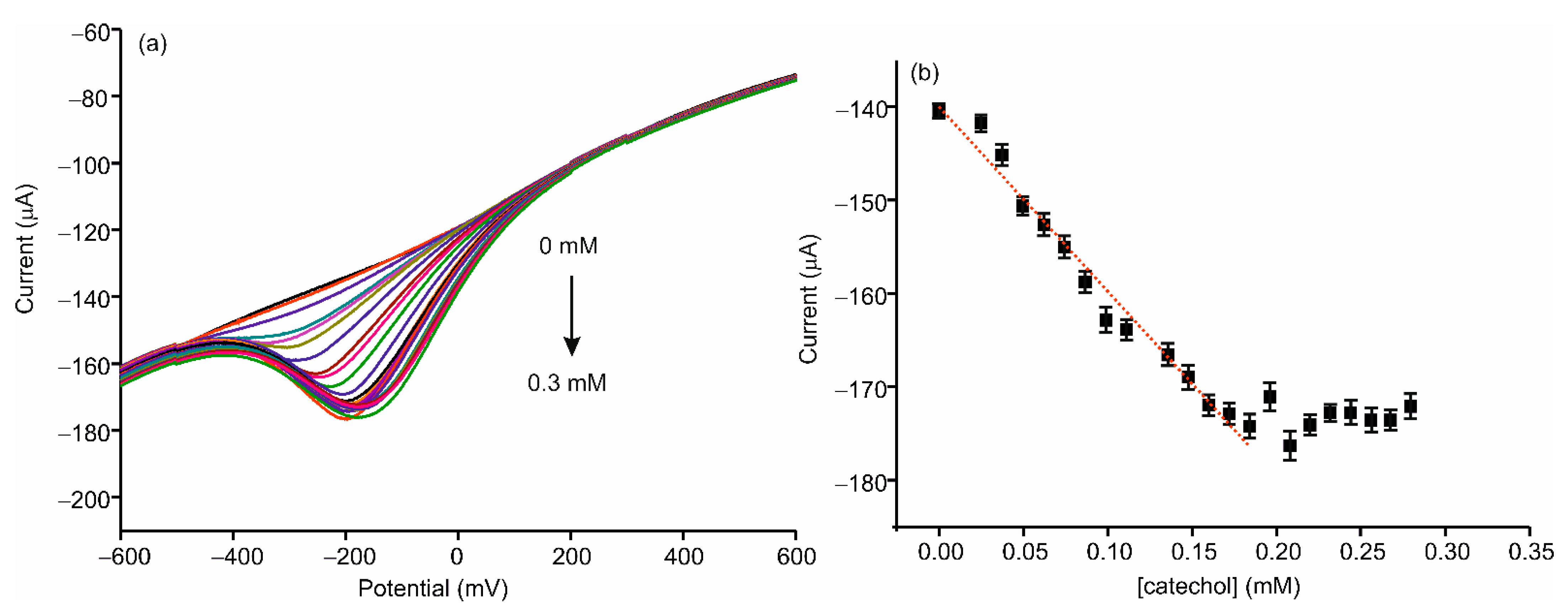
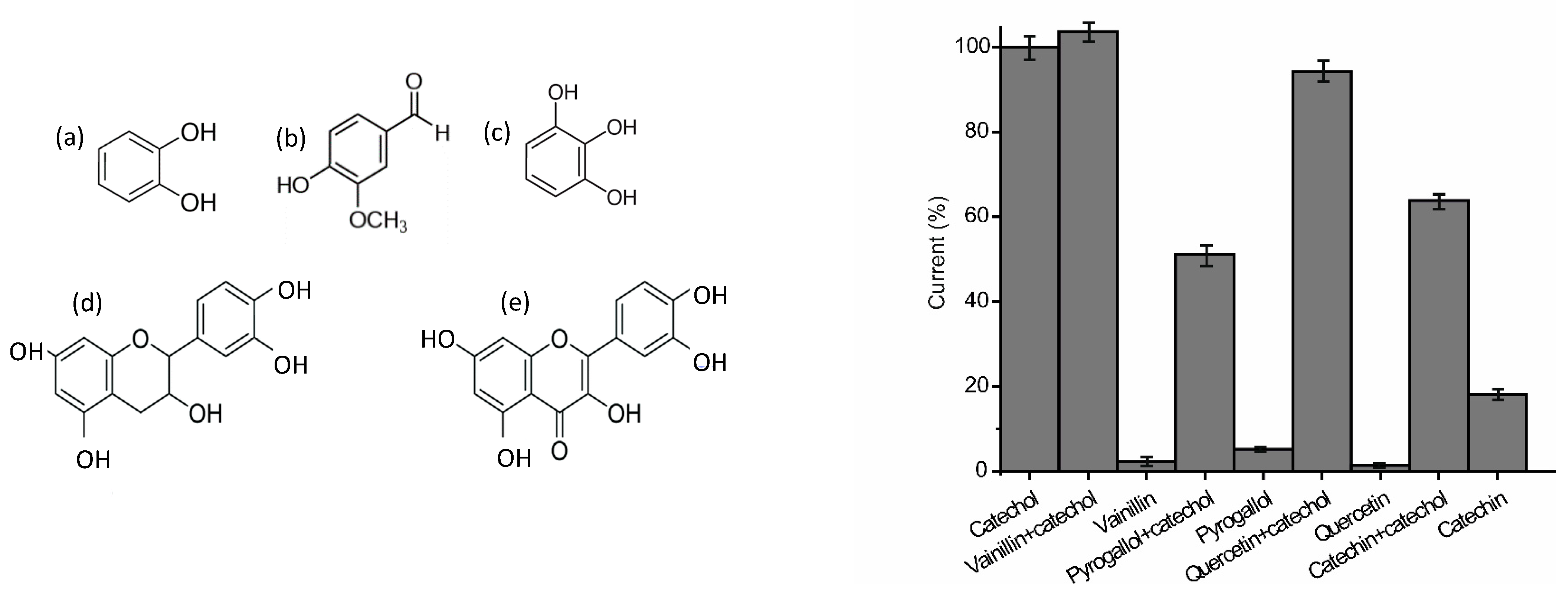
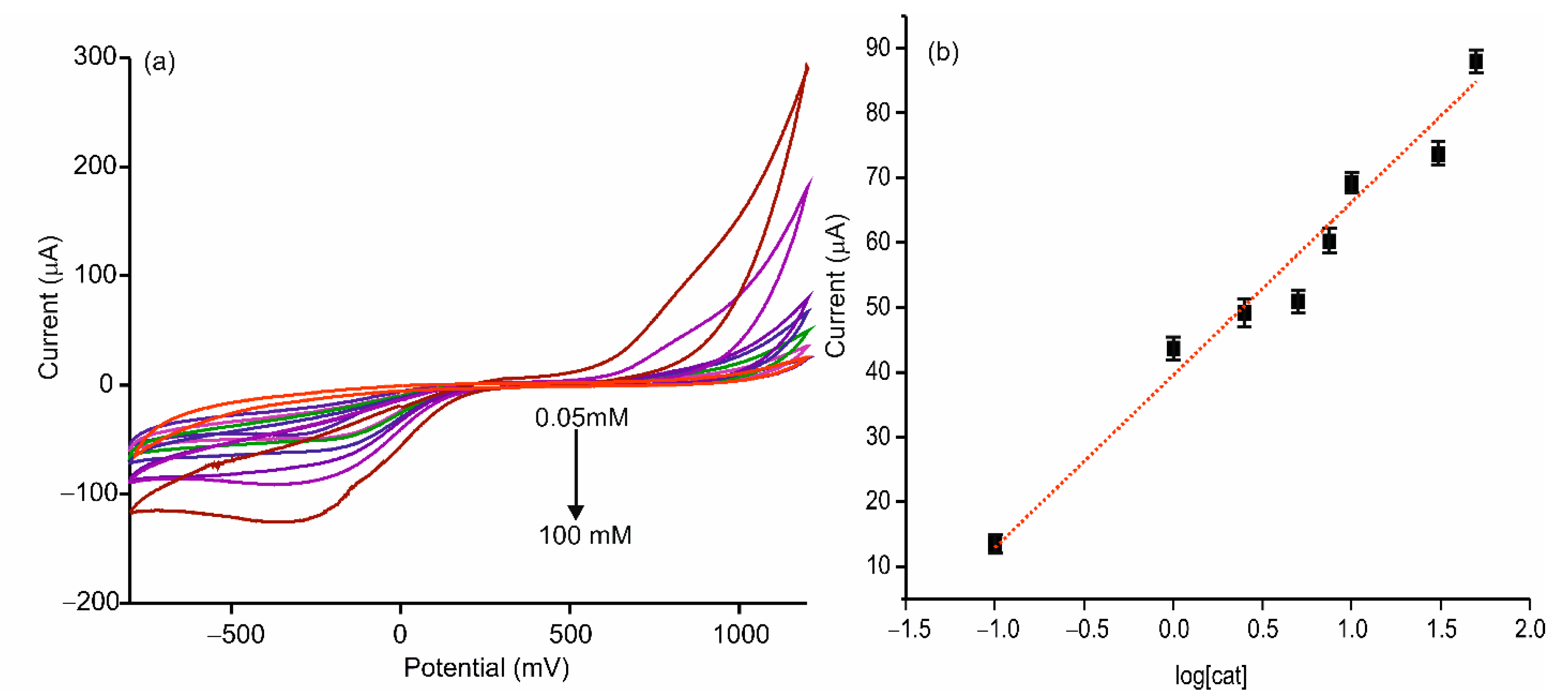
3.3. Electrochemical Characterization of the AgNWs-Tyr Biosensor
3.4. In Situ EC-AFM Studies. Reproducibility and Repeatability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nollet, L.M.L.; Gutierrez-Uribe, J.A. Phenolic Compounds in Food: Characterization and Analysis; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Prat-García, S.; Oliveira, J.; Del Alamo-Sanza, M.; De Freitas, V.; Nevares, I.; Mateus, N. Characterization of Anthocyanins and Anthocyanin-Derivatives in Red Wines during Ageing in Custom Oxygenation Oak Wood Barrels. Molecules 2020, 26, 64. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Piano, M. Electrochemical (Bio) Sensors for Environmental and Food Analyses. Biosensors 2018, 8, 57. [Google Scholar] [CrossRef]
- Lv, M.; Liu, Y.; Geng, J.; Kou, X.; Xin, Z.; Yang, D. Engineering Nanomaterials-Based Biosensors for Food Safety Detection. Biosens. Bioelectron. 2018, 106, 122–128. [Google Scholar] [CrossRef]
- Forzani, E.S.; Solís, V.M.; Calvo, E.J. Electrochemical Behavior of Polyphenol Oxidase Immobilized in Self-Assembled Structures Layer by Layer with Cationic Polyallylamine. Anal. Chem. 2000, 72, 5300–5307. [Google Scholar] [CrossRef]
- Apetrei, C.; Rodríguez-Méndez, M.; De Saja, J.A. Amperometric Tyrosinase Based Biosensor Using An Electropolymerized Phosphate-Doped Polypyrrole Film as An Immobilization Support. Application for Detection of Phenolic Compounds. Electrochim. Acta 2011, 56, 8919–8925. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Garcia-Hernandez, C.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Promoting Laccase Sensing Activity for Catechol Detection Using LBL Assemblies of Chitosan/Ionic Liquid/Phthalocyanine as Immobilization Surfaces. Bioelectrochemistry 2020, 132, 107407. [Google Scholar] [CrossRef]
- Fartas, F.M.; Abdullah, J.; Yusof, N.A.; Sulaiman, Y.; Saiman, M.I. Biosensor Based on Tyrosinase Immobilized on Graphene-Decorated Gold Nanoparticle/Chitosan for Phenolic Detection in Aqueous. Sensors 2017, 17, 1132. [Google Scholar] [CrossRef]
- Wee, Y.; Park, S.; Kwon, Y.H.; Ju, Y.; Yeon, K.-M.; Kim, J. Tyrosinase-immobilized CNT Based Biosensor for Highly-Sensitive Detection of Phenolic Compounds. Biosens. Bioelectron. 2019, 132, 279–285. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Rodriguez-Mendez, M.L.; Apetrei, C.; de Saja, J.A. Tyrosinase-immobilized CNT based biosensor for highly-sensitive detection of phenolic compounds. Sens. Actuat. B Chem. 2013, 177, 138–144. [Google Scholar]
- Rocha, D.P.; Dornellas, R.M.; Cardoso, R.M.; Narciso, L.C.; Silva, M.N.; Nossol, E.; Richter, E.M.; Munoz, R.A. Chemically Versus Electrochemically Reduced Graphene Oxide: Improved Amperometric and Voltammetric Sensors of Phenolic Compounds on Higher Roughness Surfaces. Sens. Actuators B Chem. 2018, 254, 701–708. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.-L.; Liang, Y.; Liu, W. A Graphene-Based Electrochemical Sensor for Rapid Determination of Phenols in Water. Sensors 2013, 13, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Qiao, H.; Li, D.; Luo, L.; Chen, K.; Wei, Q. Laccase Biosensor Based on Electrospun Copper/Carbon Composite Nanofibers for Catechol Detection. Sensors 2014, 14, 3543–3556. [Google Scholar] [CrossRef] [PubMed]
- Mattos, G.J.; Moraes, J.T.; Barbosa, E.C.M.; Camargo, P.H.; Dekker, R.F.; Barbosa-Dekker, A.M.; Sartori, E.R. Laccase Stabilized on β-D-Glucan Films on the Surface of Carbon Black/Gold Nanoparticles: A New Platform for Electrochemical Biosensing. Bioelectrochemistry 2019, 129, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and Direct Amperometric Analysis of Polyphenols in Beers Using Tyrosinase-Modified Screen-Printed Gold Nanoparticles Biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Sandeep, S.; Santhosh, A.S.; Swamy, N.K.; Suresh, G.S.; Melo, J.S. Detection of Catechol using a Biosensor Based on Biosynthesized Silver Nanoparticles and Polyphenol Oxidase Enzymes. Port. Electrochim. Acta 2019, 37, 257–270. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Rodriguez-Mendez, M.L.; Sutter, P.; Tong, X.; Sutter, E. Nanoscale Au–In Alloy–Oxide Core–Shell Particles as Electrocatalysts for Efficient Hydroquinone Detection. J. Phys. Chem. C 2015, 119, 25100–25107. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Y.; Zhao, D.; Liu, G. Amperometric Tyrosinase Biosensor Based on Fe3O4 Nanoparticles–Chitosan Nanocomposite. Biosens. Bioelectron. 2008, 23, 1781–1787. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Rassas, I.; Minot, S.; Bessueille, F.; Rodriguez-Mendez, M.L.; Errachid, A.; Jaffrezic-Renault, N. Voltammetric Sensor Based on Electrodeposited Molecularly Imprinted Chitosan Film on BDD Electrodes for Catechol Detection in Buffer and in Wine Samples. Mater. Sci. Eng. C 2020, 110, 110667. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; González-Gil, A.; Rodriguez-Valentin, J.; Garcia-Hernandez, C.; Martín, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. The Role of the Surface Structure. Sensors 2020, 20, 2152. [Google Scholar]
- Sun, D.; Yang, D.; Wei, P.; Liu, B.; Chen, Z.; Zhang, L.; Lu, J. One-Step Electrodeposition of Silver Nanostructures on 2D/3D Metal-Organic Framework ZIF-67: Comparison and Application in Electrochemical Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2020, 12, 41960–41968. [Google Scholar] [CrossRef]
- Bagal-Kestwal, D.; Pan, M.; Chiang, B.-H. Electrically Nanowired-Enzymes for Probe Modification and Sensor Fabrication. Biosens. Bioelectron. 2018, 121, 223–235. [Google Scholar] [CrossRef]
- Lee, H.-J.; Oh, S.; Cho, K.-Y.; Jeong, W.-L.; Lee, D.-S.; Park, S.-J. Spontaneous and Selective Nanowelding of Silver Nanowires by Electrochemical Ostwald Ripening and High Electrostatic Potential at the Junctions for High-Performance Stretchable Transparent Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 14124–14131. [Google Scholar] [CrossRef]
- He, W.; Ye, C. Flexible Transparent Conductive Films on the Basis of Ag Nanowires: Design and Applications: A Review. J. Mater. Sci. Technol. 2015, 31, 581–588. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, C.; Li, L.; Zhang, C.-W.; Liu, J.; Yu, H.-D.; Huang, W. All Paper-Based Flexible and Wearable Piezoresistive Pressure Sensor. ACS Appl. Mater. Interfaces 2019, 11, 25034–25042. [Google Scholar] [CrossRef]
- Yogeswaran, U.; Chen, S.-M. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef]
- Cherevko, S.; Chung, C.-H. Gold Nanowire Array Electrode for Non-Enzymatic Voltammetric and Amperometric Glucose Detection. Sens. Actuators B Chem. 2009, 142, 216–223. [Google Scholar] [CrossRef]
- Xu, L.; Hou, Y.; Zhang, M.; Cheng, T.; Li, Y.-H.; Yao, C.; Wu, Q. Electrochemical Sensor Based on A Silver Nanowires Modified Electrode for the Determination of Cholesterol. Anal. Methods 2015, 7, 5649–5653. [Google Scholar] [CrossRef]
- Zhang, C.; Govindaraju, S.; Giribabu, K.; Huh, Y.-S.; Yun, K. AgNWs-PANI Nanocomposite Based Electrochemical Sensor for Detection of 4-Nitrophenol. Sens. Actuators B Chem. 2017, 252, 616–623. [Google Scholar] [CrossRef]
- Wang, L.; Gao, X.; Jin, L.; Wu, Q.; Chen, Z.; Lin, X.-F. Amperometric Glucose Biosensor Based on Silver Nanowires and Glucose Oxidase. Sens. Actuators B Chem. 2013, 176, 9–14. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Chakaravarthy, S.; Hernandez-Rangel, A.; Prokhorov, E.; Luna-Bárcenas, G.; Esparza, R.; Meyyappan, M. Chitosan Supported Silver Nanowires as A Platform for Direct Electrochemistry and Highly Sensitive Electrochemical Glucose Biosensing. RSC Adv. 2016, 6, 20102–20108. [Google Scholar] [CrossRef]
- Yang, X.; Bai, J.; Wang, Y.; Jiang, X.; He, X. Hydrogen Peroxide and Glucose Biosensor Based on Silver Nanowires Synthesized by Polyol Process. Analyst 2012, 137, 4362–4367. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hou, Y.; Zhang, M.; Yang, X.; Jenkins, G.; Huang, W.; Yao, C.; Wu, Q. A Novel Electrochemical Biosensor for Detection of Cholesterol. Russ. J. Electrochem. 2016, 52, 239–244. [Google Scholar] [CrossRef]
- Schuette, W.M.; Buhro, W.E. Polyol Synthesis of Silver Nanowires by Heterogeneous Nucleation; Mechanistic Aspects Influencing Nanowire Diameter and Length. Chem. Mater. 2014, 26, 6410–6417. [Google Scholar] [CrossRef]
- Ma, J.; Zhan, M. Rapid Production of Silver Nanowires Based on High Concentration of AgNO3 Precursor and Use of FeCl3 as Reaction Promoter. RSC Adv. 2014, 4, 21060. [Google Scholar] [CrossRef]
- Ahmad, M.; Sun, H.; Hussain, M.; Karim, S.; Nisar, A.; Khan, M. Development of Silver Nanowires Based Highly Sensitive Amperometric Glucose Biosensor. Electroanalysis 2015, 27, 1498–1506. [Google Scholar] [CrossRef]
- Bin Johan, M.R.; Aznan, N.A.K.; Yee, S.T.; Ho, I.H.; Ooi, S.W.; Singho, N.D.; Aplop, F. Synthesis and Growth Mechanism of Silver Nanowires through Different Mediated Agents (CuCl2 and NaCl) Polyol Process. J. Nanomater. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K. Recent Strategies to Minimise Fouling in Electrochemical Detection Systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Chen, X. Synthesis of Uniform Silver Nanowires from AgCl Seeds for Transparent Conductive Films via Spin-Coating at Variable Spin-Speed. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 154–161. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. A Cyclic Voltammetry Method Suitable for Characterizing Antioxidant Properties of Wine and Wine Phenolics. J. Agric. Food Chem. 2001, 49, 1957–1965. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; García-Cabezón, C.; García-Hernández, C.; Bramorski, C.; Blanco, Y.; Martín, F.; Kawai, T.; De Saja, J.A.; Rodriguez-Mendez, M.L. Analysis of Organic Acids and Phenols of Interest in the Wine Industry Using Langmuir–Blodgett Films Based on Functionalized Nanoparticles. Anal. Chim. Acta 2015, 853, 572–578. [Google Scholar] [CrossRef]
- Cortina-Puig, M.; Noguer, T.; Marty, J.L.; Calas-Blanchard, C. Biosensors in Food Processing, Safety and Quality Control; Mutlu, M., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 257–272. [Google Scholar]
- Fu, J.; Li, D.; Li, G.; Huang, F.; Wei, Q. Carboxymethyl Cellulose Assisted Immobilization of Silver Nanoparticles onto Cellulose Nanofibers for the Detection of Catechol. J. Electroanal. Chem. 2015, 738, 92–99. [Google Scholar] [CrossRef]
- Karim, N.; Lee, H.J. Amperometric Phenol Biosensor Based on Covalent Immobilization of Tyrosinase on Au Nanoparticle Modified Screen Printed Carbon Electrodes. Talanta 2013, 116, 991–996. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, C.; García-Cabezón, C.; Martín, F.; De Saja, J.A.; Rodriguez-Mendez, M.L. Layered Composites of PEDOT/PSS/Nanoparticles and PEDOT/PSS/Phthalocyanines as Electron Mediators for Sensors and Biosensors. Beilstein J. Nanotechnol. 2016, 7, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.-M.G.; Hux, N.P.; Philips, S.J.; Towns, M.H. Michaelis–Menten Graphs, Lineweaver–Burk Plots, and Reaction Schemes: Investigating Introductory Biochemistry Students’ Conceptions of Representations in Enzyme Kinetics. J. Chem. Educ. 2019, 96, 1833–1845. [Google Scholar] [CrossRef]




| Biosensor Description | R2 | Sensitivity (µA mM−1) | LOD (M) | Linear Range (µM) | Ref. |
|---|---|---|---|---|---|
| AgNWs-Tyr | 0.976 | 197.9 | 2.7·10−6 | 25–172 | This work |
| Lac/AgNPs/cellulose/GCE | 0.999 | 11.5 | 1.64·10−6 | 5–3650 | [43] |
| Gr/PPy/AgNPs/PPO | -- | 13.66 | 4.70·10−7 | 1–15 | [16] |
| Tyr-AuNPs-SPCE | 0.993 | 0.55 | 1.2·10−6 | 2.5–20 | [15] |
| Tyr-AuNPs-DHP/GCE | 0.999 | 115 | 1.7·10−5 | 2.5–95 | [44] |
| PEDOT/PSS/AuNPs-Tyr | 0.971 | -- | 2.8·10−6 | 90–1500 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvo-Comino, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Silver Nanowires as Electron Transfer Mediators in Electrochemical Catechol Biosensors. Sensors 2021, 21, 899. https://doi.org/10.3390/s21030899
Salvo-Comino C, Martin-Pedrosa F, Garcia-Cabezon C, Rodriguez-Mendez ML. Silver Nanowires as Electron Transfer Mediators in Electrochemical Catechol Biosensors. Sensors. 2021; 21(3):899. https://doi.org/10.3390/s21030899
Chicago/Turabian StyleSalvo-Comino, Coral, Fernando Martin-Pedrosa, Cristina Garcia-Cabezon, and Maria Luz Rodriguez-Mendez. 2021. "Silver Nanowires as Electron Transfer Mediators in Electrochemical Catechol Biosensors" Sensors 21, no. 3: 899. https://doi.org/10.3390/s21030899
APA StyleSalvo-Comino, C., Martin-Pedrosa, F., Garcia-Cabezon, C., & Rodriguez-Mendez, M. L. (2021). Silver Nanowires as Electron Transfer Mediators in Electrochemical Catechol Biosensors. Sensors, 21(3), 899. https://doi.org/10.3390/s21030899