Development of an Automated Optical Inspection System for Rapidly and Precisely Measuring Dimensions of Embedded Microchannel Structures in Transparent Bonded Chips
Abstract
1. Introduction
2. Microfabrication of Microfluidic Device and Concept of AOI System
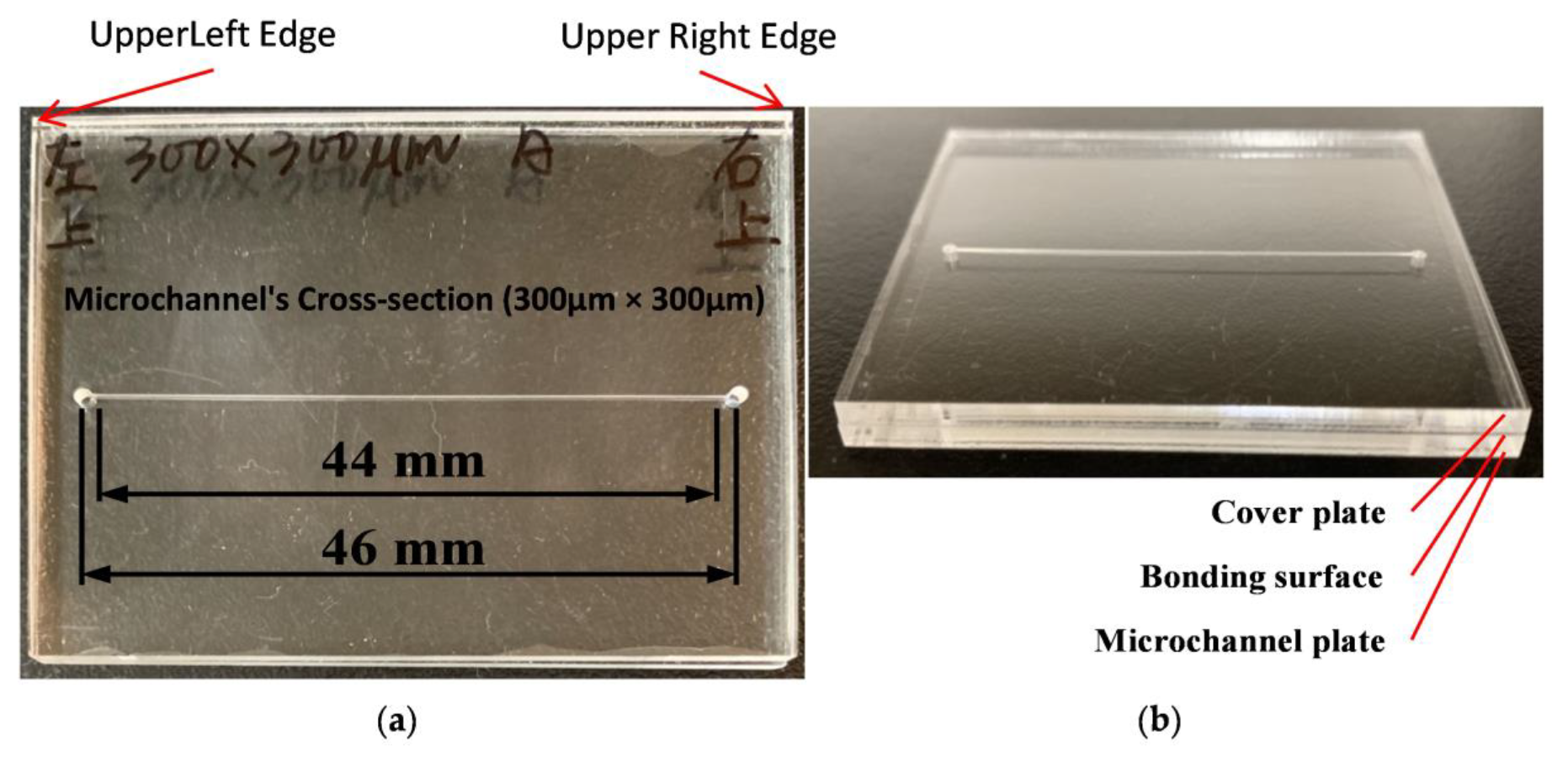
2.1. Microfabrication of Microfluidic Chip
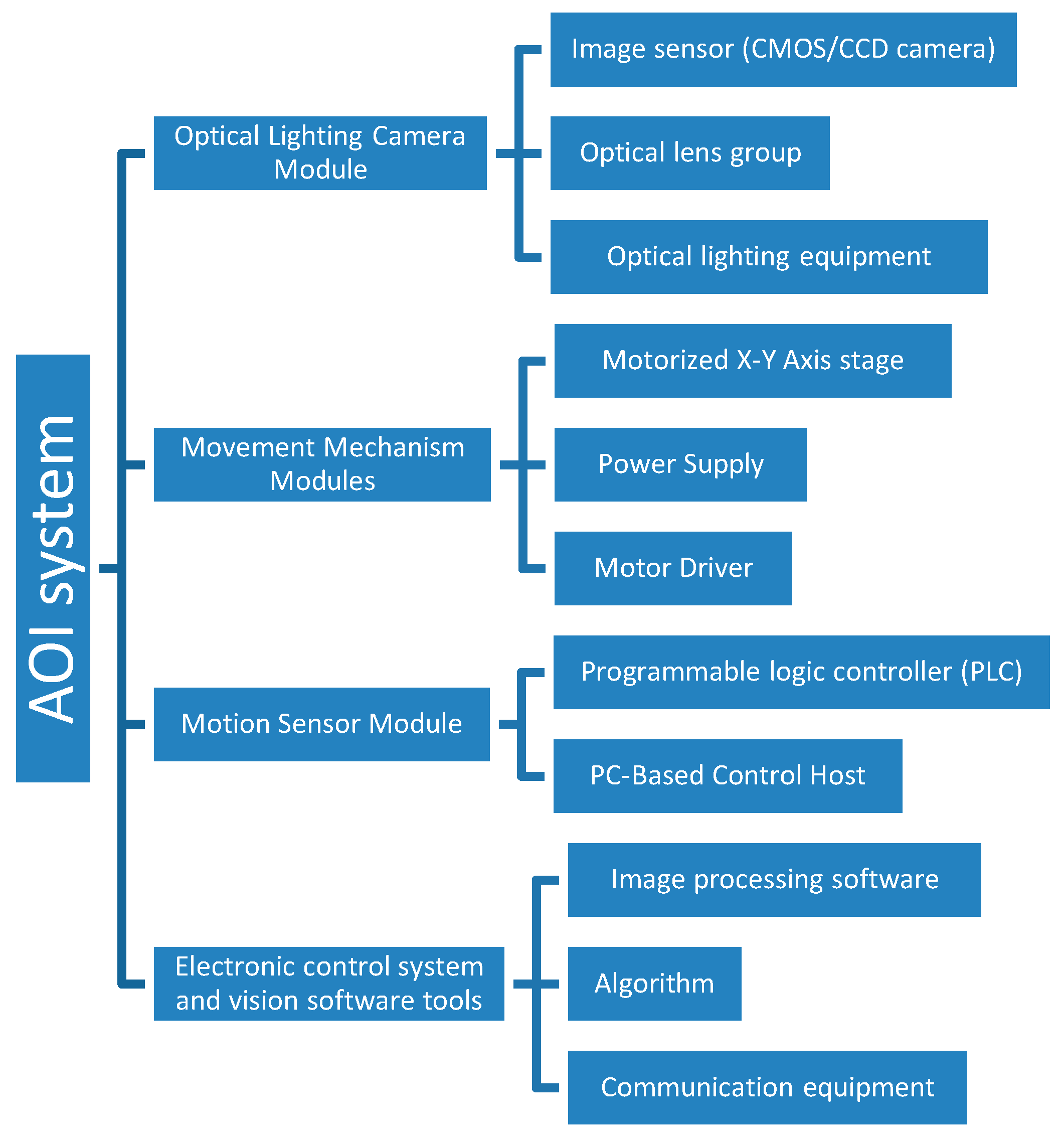
2.2. AOI System Design and Concept
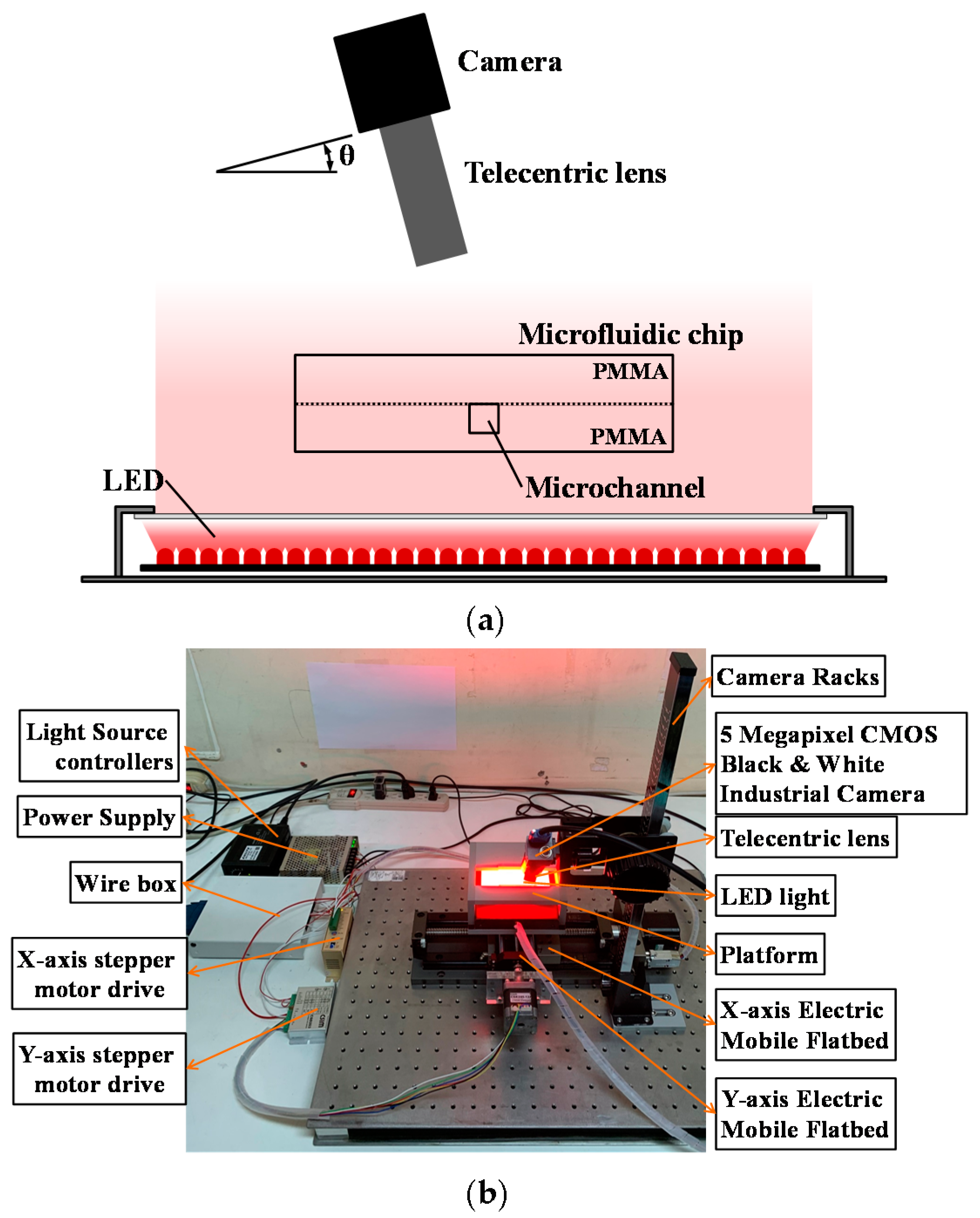
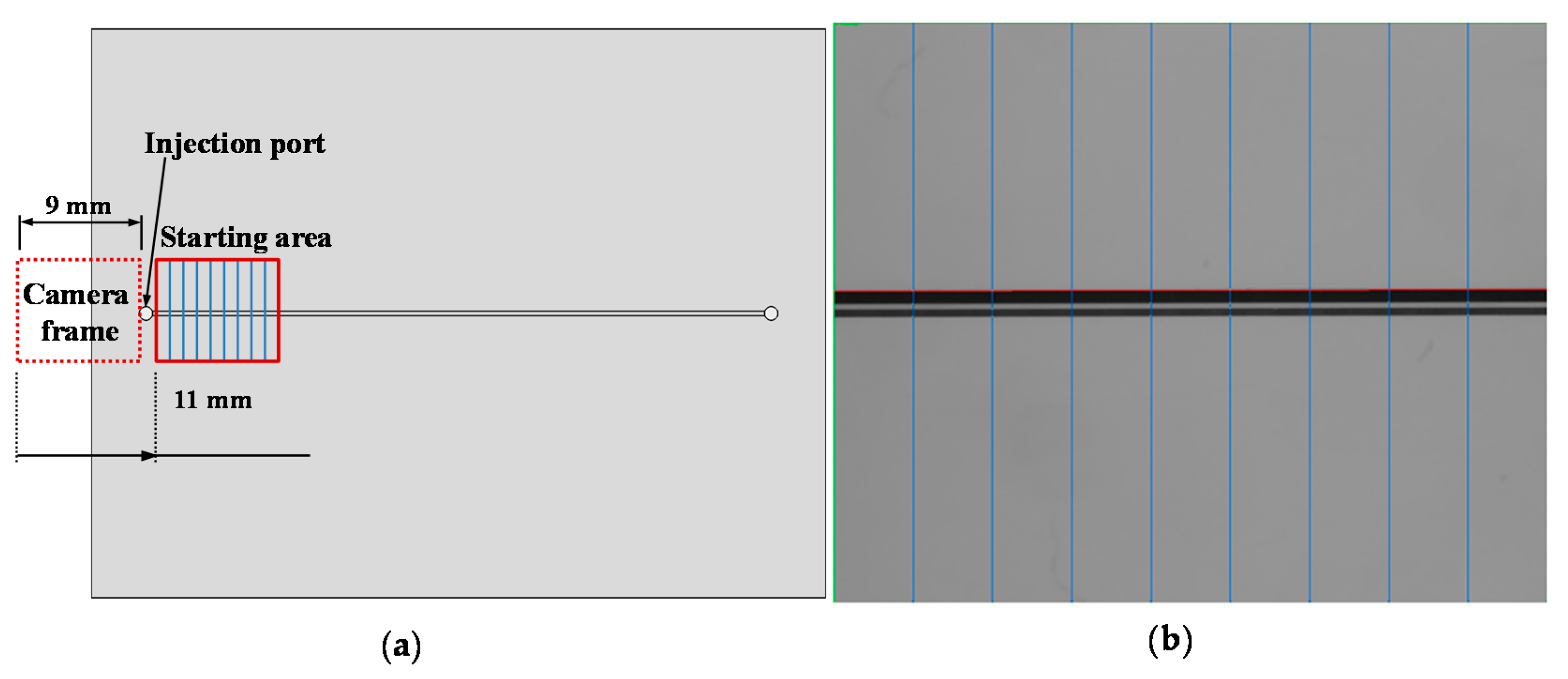
2.2.1. AOI System Setting
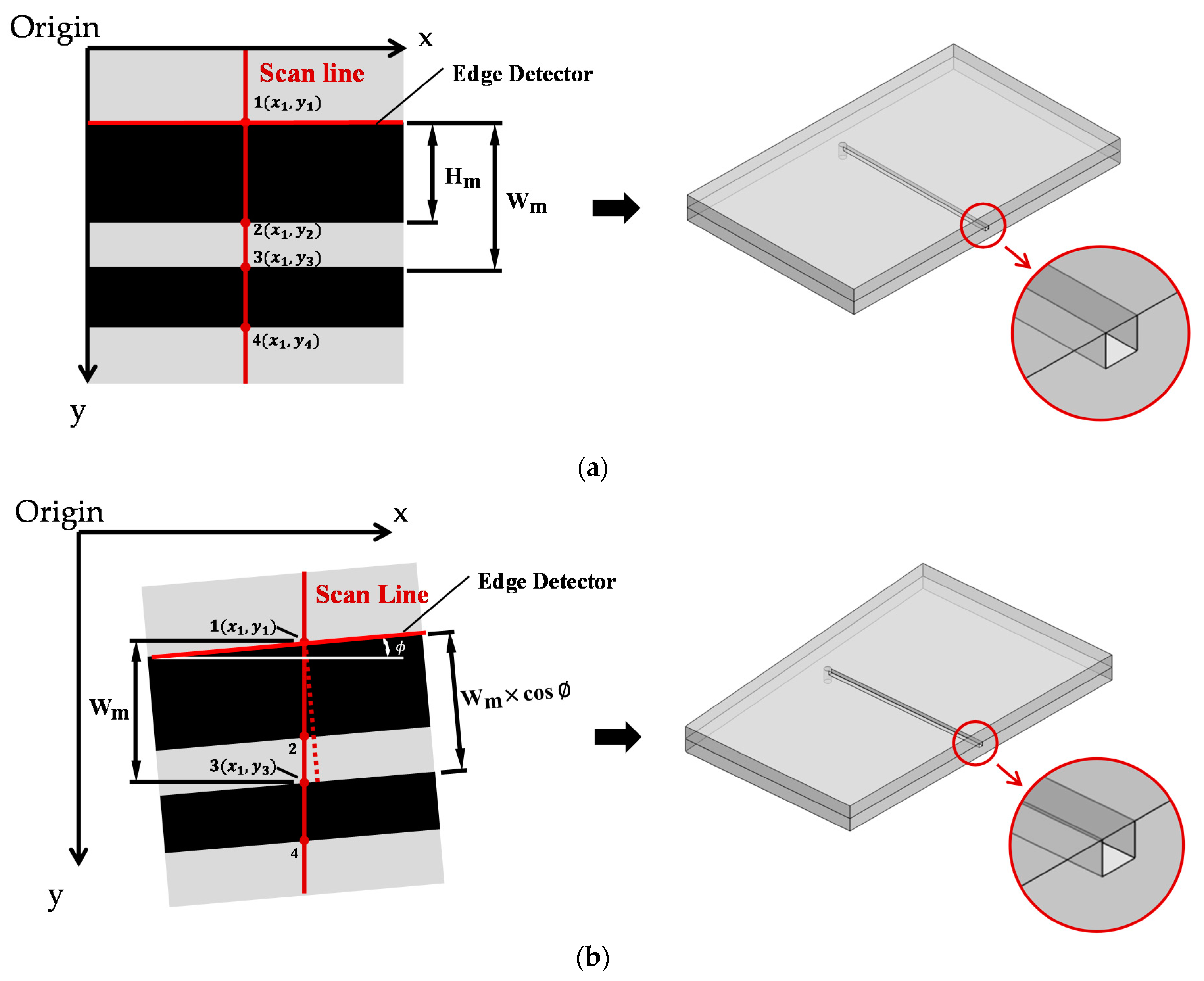
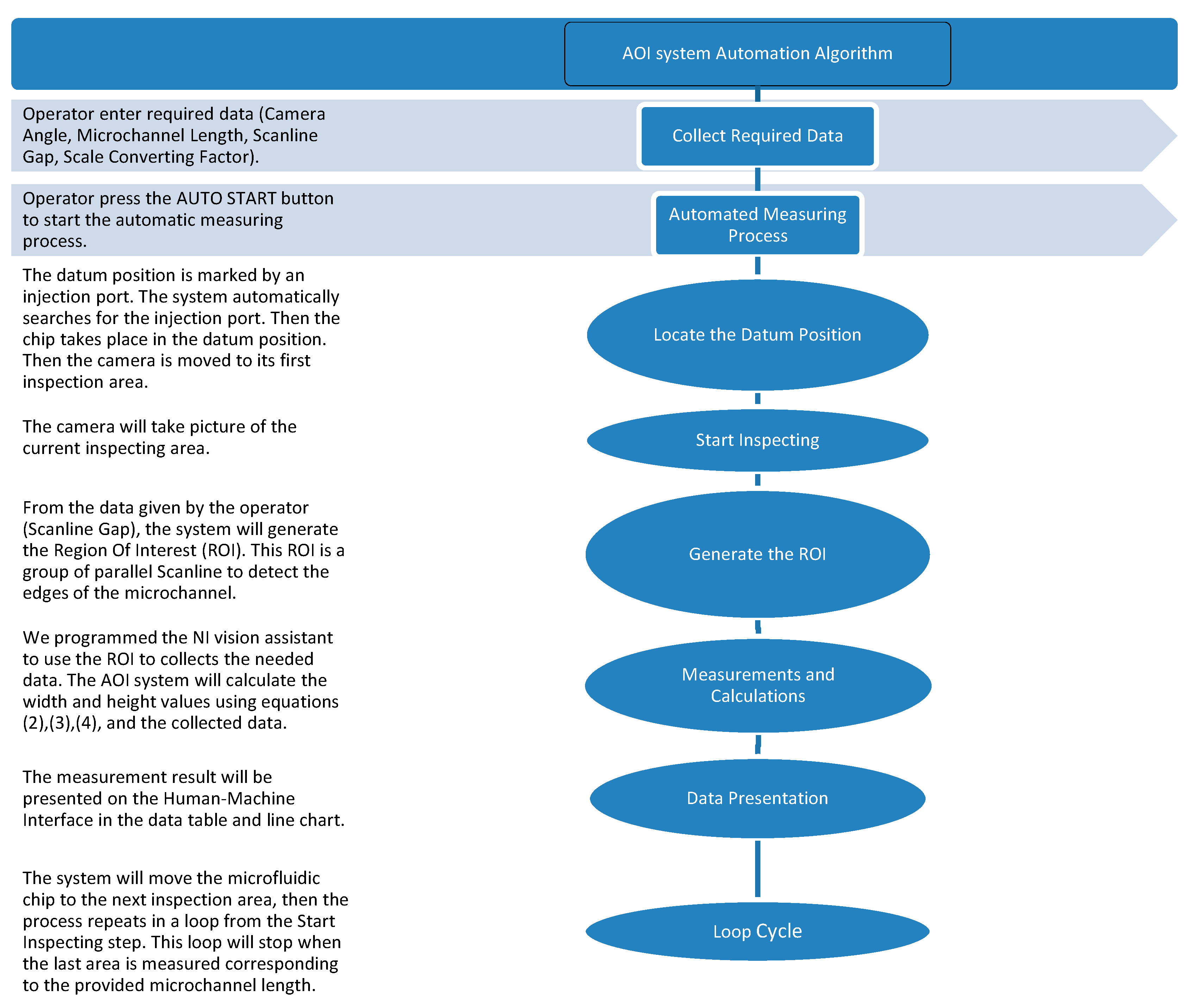
2.2.2. System Design and Algorithms
3. Experiment Procedure
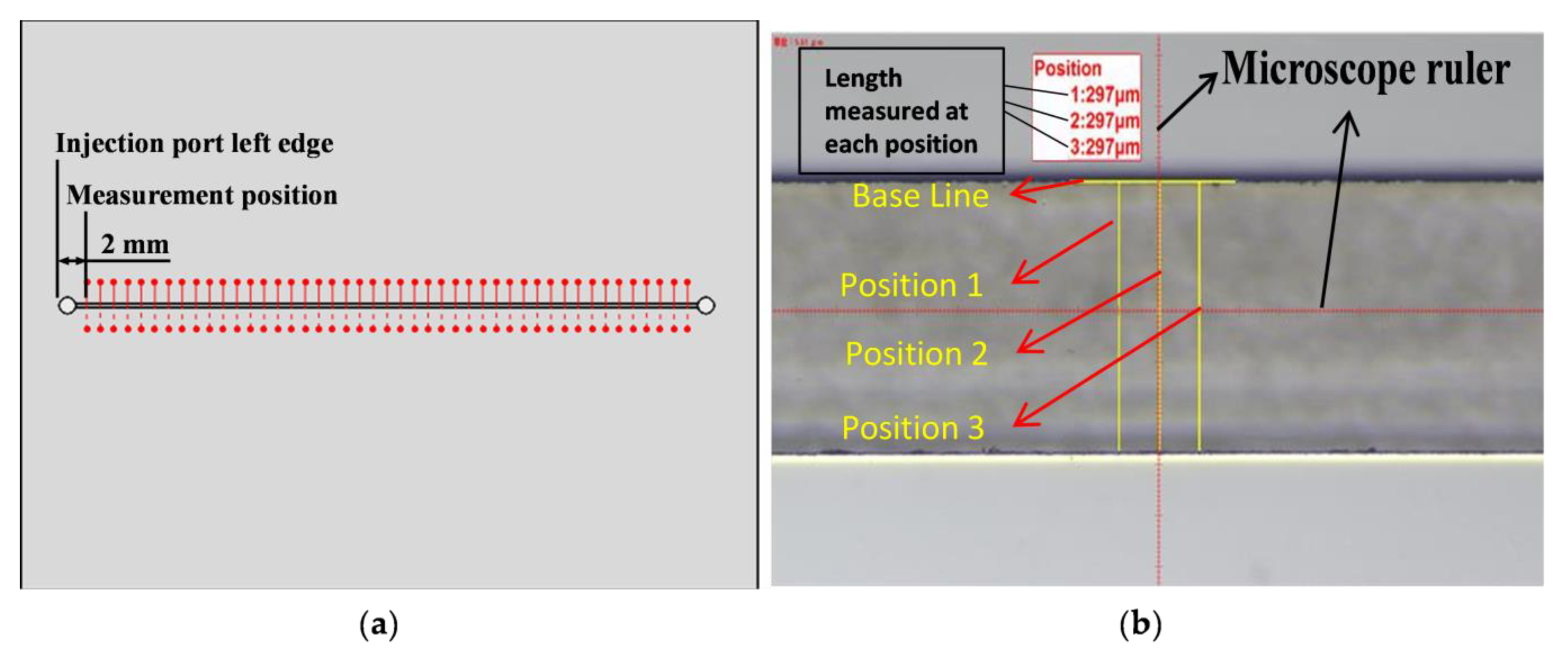
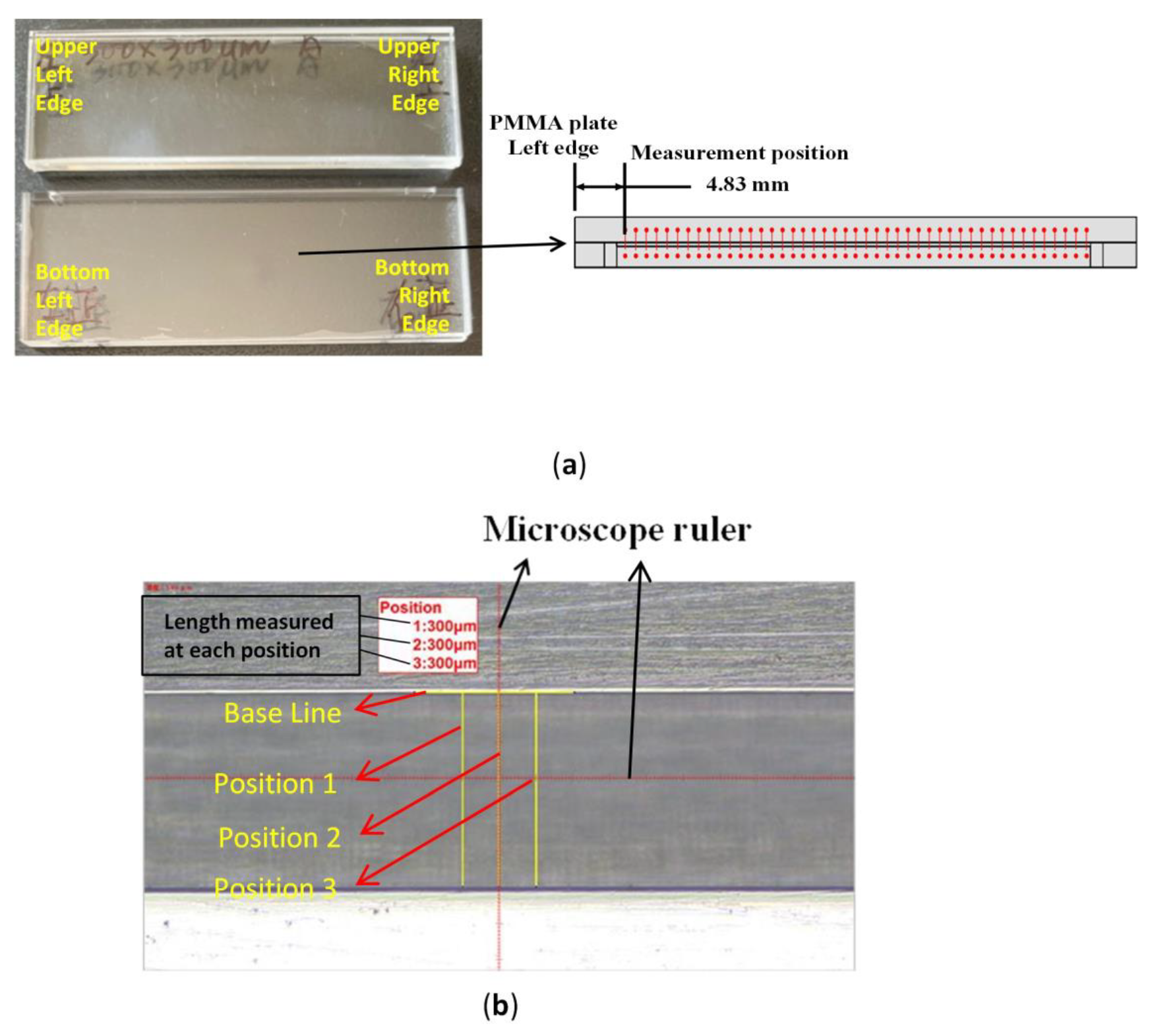
3.1. Camera Angle Setting Determination
3.2. Microchannel Dimensions Determination Using Microscope
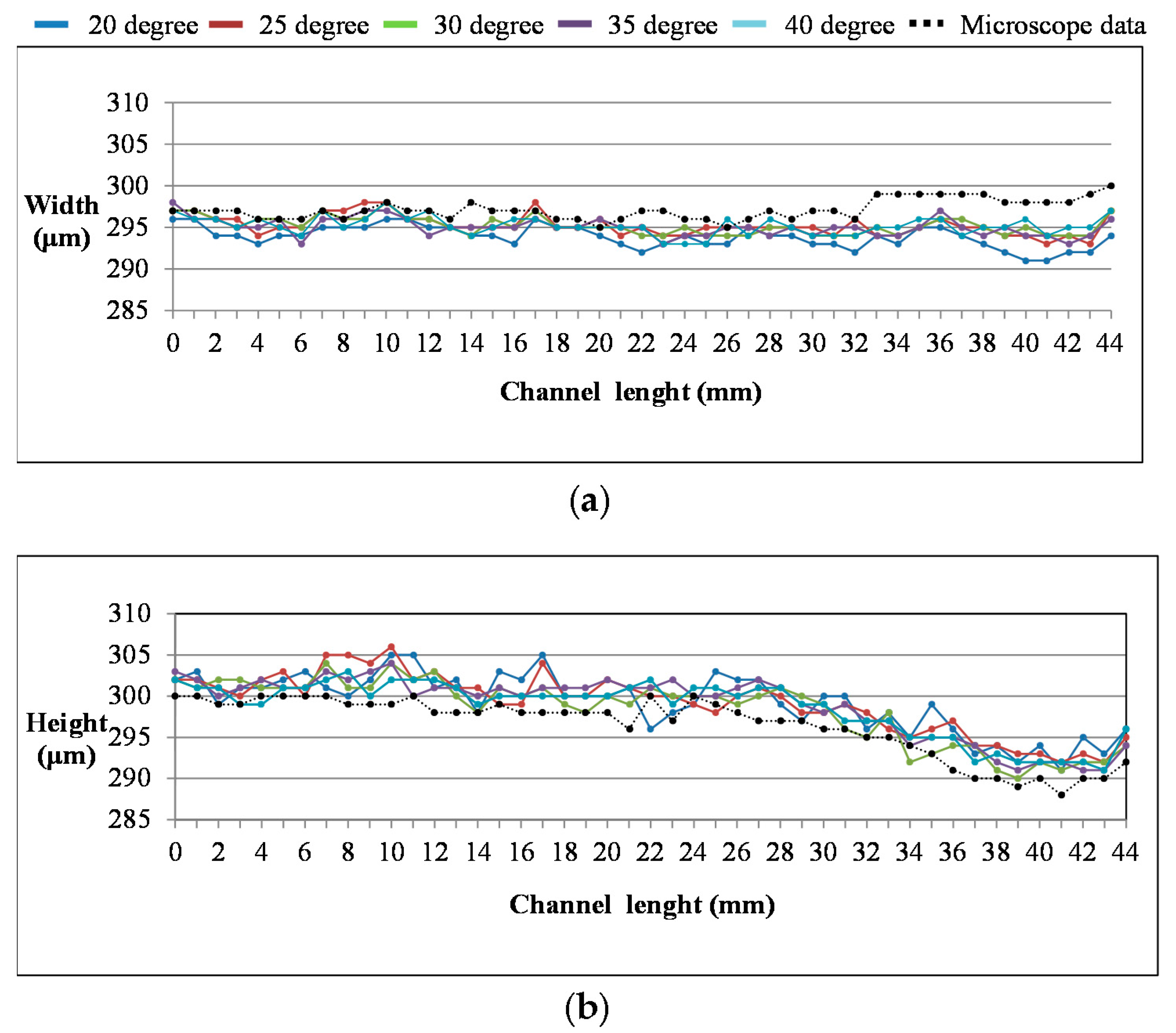
4. Result and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reports and Data, Biochip Market by Product (DNA Chip, Lab-on-Chip, Protein Chip, and Others), by Technology (Microfluidics and Microarray), by Substrate Materials, by Applications and by End-Users and Segment Forecasts, 2016–2026. 2019. Available online: https://www.reportsanddata.com/report-detail/biochip-market (accessed on 6 May 2019).
- Feynman, R.P. There is plenty of room at the bottom. Caltech Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Nasseri, B.; Soleimania, N.; Rabieec, N.; Kalbasid, A.; Karimie, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Zhang, F.; Wang, C.; Xie, R.; Liang, Q. Recent progress in lab-on-a-chip for pharmaceutical analysis and pharmacological/toxicological test. Trends Anal. Chem. 2019, 117, 215–2302. [Google Scholar] [CrossRef]
- Bruijns, B.; van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic devices for forensic DNA analysis: A review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, A.; Harmon, J.; Zhou, Y.; Li, S.; Nakagawa, Y.; Hayashi, M.; Mikami, H.; Lei, C.; Goda, K. AI on a chip. Lab Chip 2020, 20, 3074–3090. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Chen, G. Fabrication, modification, and application of poly (methyl methacrylate) microfluidic chips. Electrophoresis 2008, 29, 1801–1814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Chen, G. Hot embossing and thermal bonding of poly (methyl methacrylate) microfluidic chips using positive temperature coefficient ceramic heater. Anal. Bioanal. Chem. 2011, 401, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, B.; Zare, R.N. Construction of microfluidic chips using polydimethylsiloxane for adhesive bonding. Lab Chip 2005, 5, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Duong, L.H. Novel solvent bonding method for thermoplastic microfluidic chips. Sens. Actuators B Chem. 2016, 237, 556–562. [Google Scholar] [CrossRef]
- Li, S.; Xu, Z.; Mazzeo, A.D.; Burns, D.J.; Fu, G.; Dirckx, M.; Shilpiekandula, V.; Chen, X.; Nayak, N.C.; Wong, E. Review of production of microfluidic devices: Material, manufacturing and metrology. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 7–11 April 2008. [Google Scholar]
- Reyes, D.R.; Halter, M.; Hwang, J. Dimensional metrology of lab-on-a-chip internal structures: A comparison of optical coherence tomography with confocal fluorescence microscopy. J. Microsc. 2015, 259, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kolekar1, R.; Torgerson1, D.; Viner2, J.; Gale, B.; Ameel, T. Depth measurement in fully enclosed microchannels using laser interferometry. Meas. Sci. Technol. 2012, 23, 087004. [Google Scholar] [CrossRef]
- Kotz, F.; Arnold, K.; Wagner, S.; Bauer, W.; Keller, N.; Nargang, T.M.; Helmer, D.; Rapp, B.E. Liquid PMMA: A high-resolution polymethylmethacrylate negative photoresist as enabling material for direct printing of microfluidic chips. Adv. Eng. Mater. 2018, 20, 1700699. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, L.; Li, Z.; Lu, B. Fabrication of PDMS microfluidic chips used in rapid diagnosis by micro jetting. Multimed. Tools Appl. 2018, 77, 3761–3774. [Google Scholar] [CrossRef]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.-E.; Ahn, S.-K.; Kang, J.H. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip 2019, 19, 3706–3713. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Nikitopoulos, D.E.; Soper, S.A.; Murphy, M.C. Temperature distribution effects on micro-CFPCR performance. Biomed. Microdevices 2008, 10, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Ji, W.; Liu, S.; Chen, Q.; Wu, B.; Coleman, R.; Fan, X. Silicon-based solvent immersion imprint lithography for rapid polystyrene microfluidic chip prototyping. Sens. Actuators B Chem. 2017, 248, 311–317. [Google Scholar] [CrossRef]
- Tanio, N.; Nakanishi, T. Physical aging and refractive index of poly (methyl methacrylate) glass. Polym. J. 2006, 38, 814–818. [Google Scholar] [CrossRef]
- Broadwell, I.; Fletcher, P.D.I.; Haswell, S.J.; McCreedy, T.; Zhang, X. Quantitative 3-dimensional profiling of channel networks within transparent ‘lab-on-a-chip’ microreactors using a digital imaging method. Lab Chip 2001, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]


| Degree | Width | Height | ||
|---|---|---|---|---|
| Average Error (%) | Standard Deviation (%) | Average Error (%) | Standard Deviation (%) | |
| 20 | 2.35 | 0.62 | 2.35 | 0.58 |
| 25 | 2.01 | 0.53 | 2.34 | 0.58 |
| 30 | 1.67 | 0.51 | 1.68 | 0.44 |
| 35 | 1.68 | 0.51 | 1.69 | 0.45 |
| 40 | 1.67 | 0.46 | 1.69 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-C.; Lin, Y.-T.; Truong, C.-M.; Chen, P.-S.; Chiang, H.-K. Development of an Automated Optical Inspection System for Rapidly and Precisely Measuring Dimensions of Embedded Microchannel Structures in Transparent Bonded Chips. Sensors 2021, 21, 698. https://doi.org/10.3390/s21030698
Chen P-C, Lin Y-T, Truong C-M, Chen P-S, Chiang H-K. Development of an Automated Optical Inspection System for Rapidly and Precisely Measuring Dimensions of Embedded Microchannel Structures in Transparent Bonded Chips. Sensors. 2021; 21(3):698. https://doi.org/10.3390/s21030698
Chicago/Turabian StyleChen, Pin-Chuan, Ya-Ting Lin, Chi-Minh Truong, Pai-Shan Chen, and Huihua-Kenny Chiang. 2021. "Development of an Automated Optical Inspection System for Rapidly and Precisely Measuring Dimensions of Embedded Microchannel Structures in Transparent Bonded Chips" Sensors 21, no. 3: 698. https://doi.org/10.3390/s21030698
APA StyleChen, P.-C., Lin, Y.-T., Truong, C.-M., Chen, P.-S., & Chiang, H.-K. (2021). Development of an Automated Optical Inspection System for Rapidly and Precisely Measuring Dimensions of Embedded Microchannel Structures in Transparent Bonded Chips. Sensors, 21(3), 698. https://doi.org/10.3390/s21030698









