Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
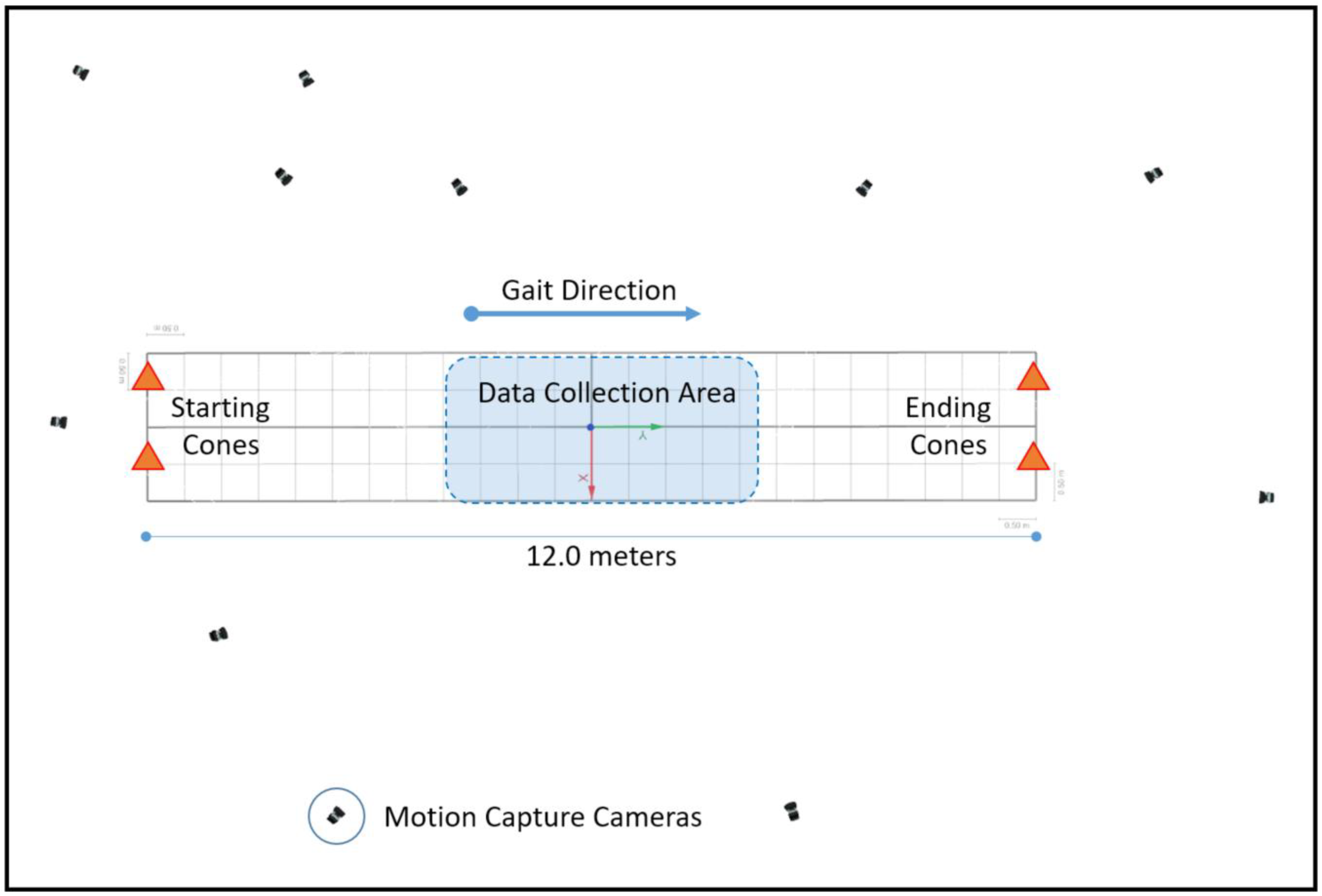
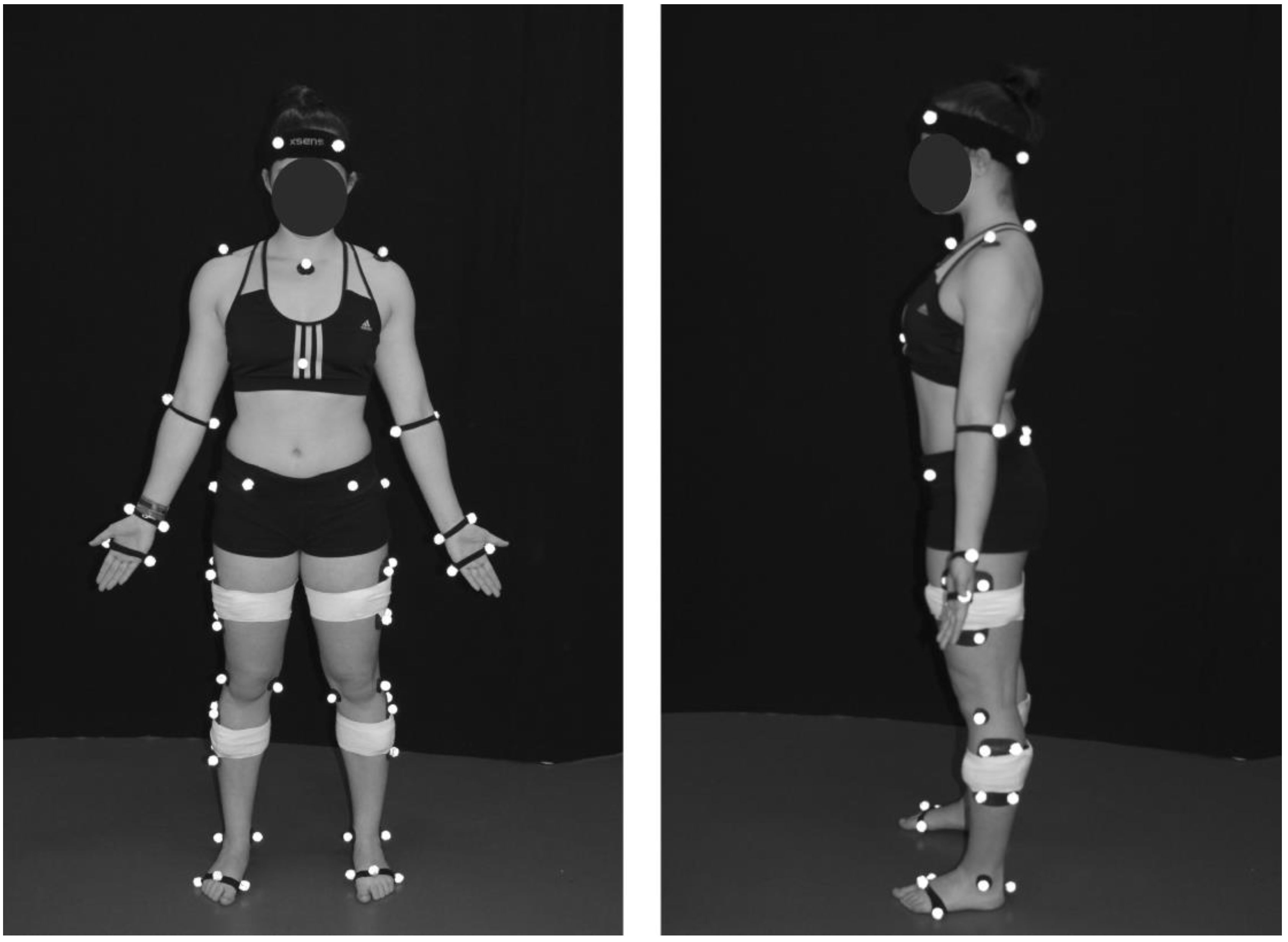
2.2. Experiment Protocol
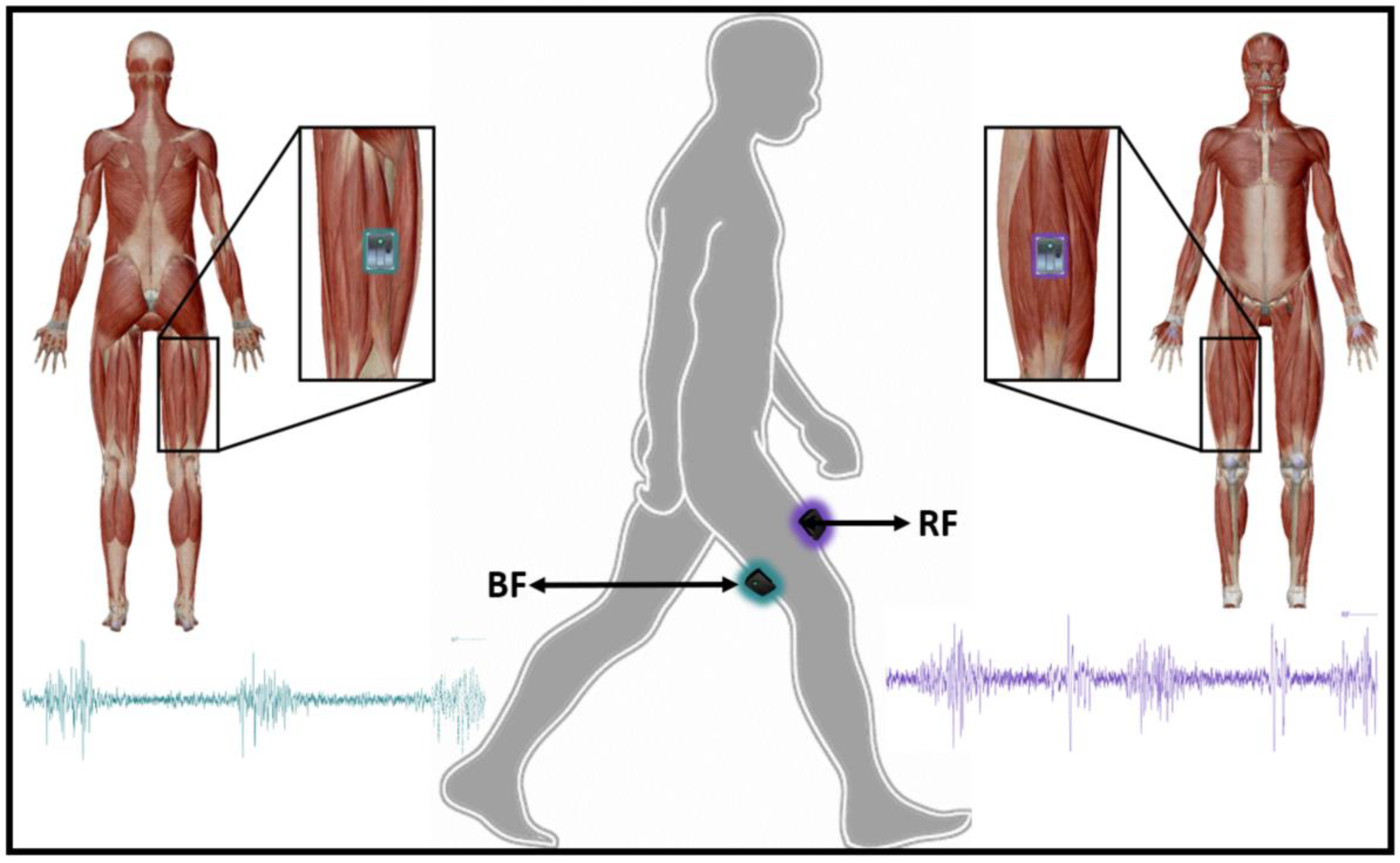
2.3. Data Processing
2.4. Muscle Co-Activation
2.5. Statistical Analysis
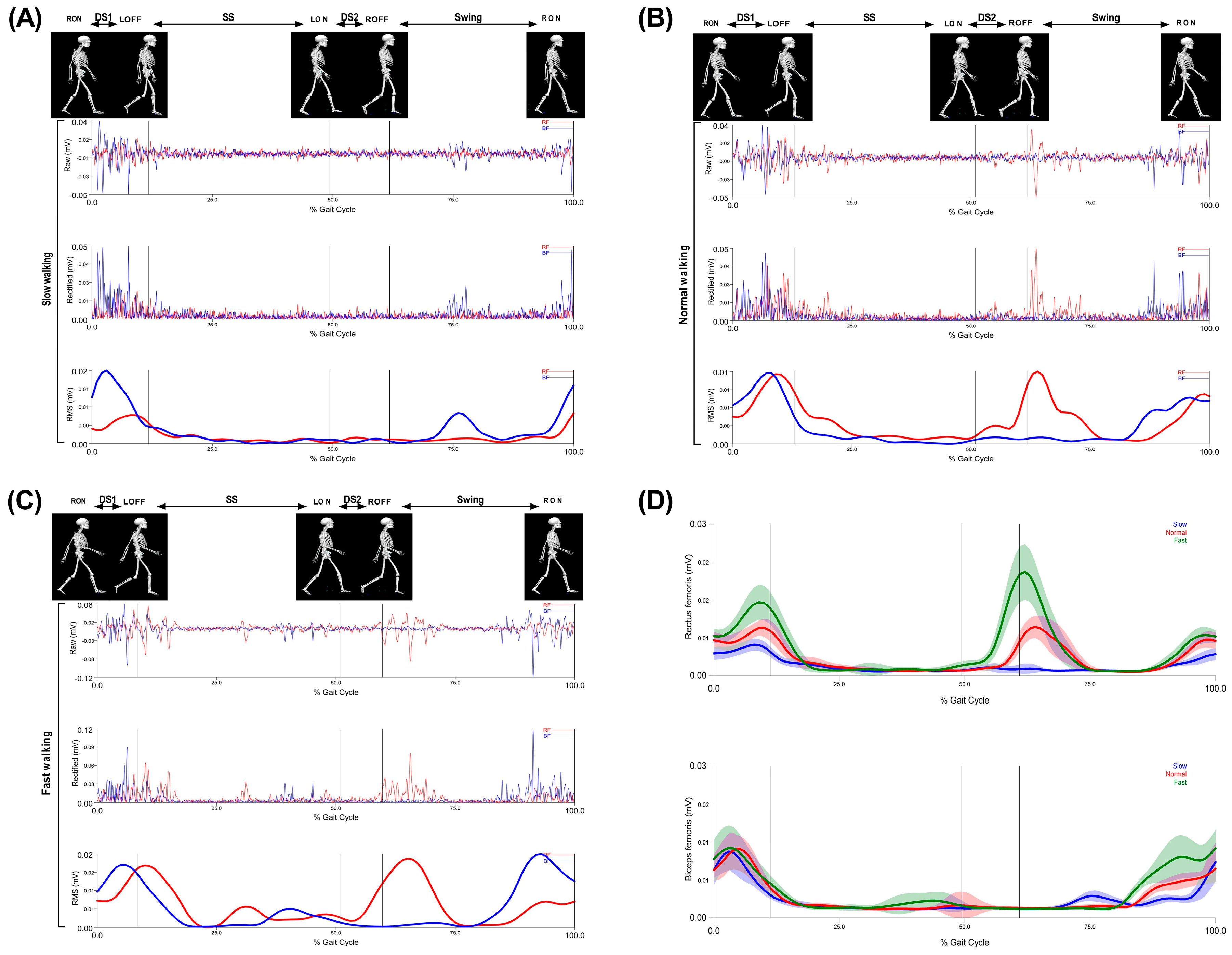
3. Results
3.1. Walking Characteristics
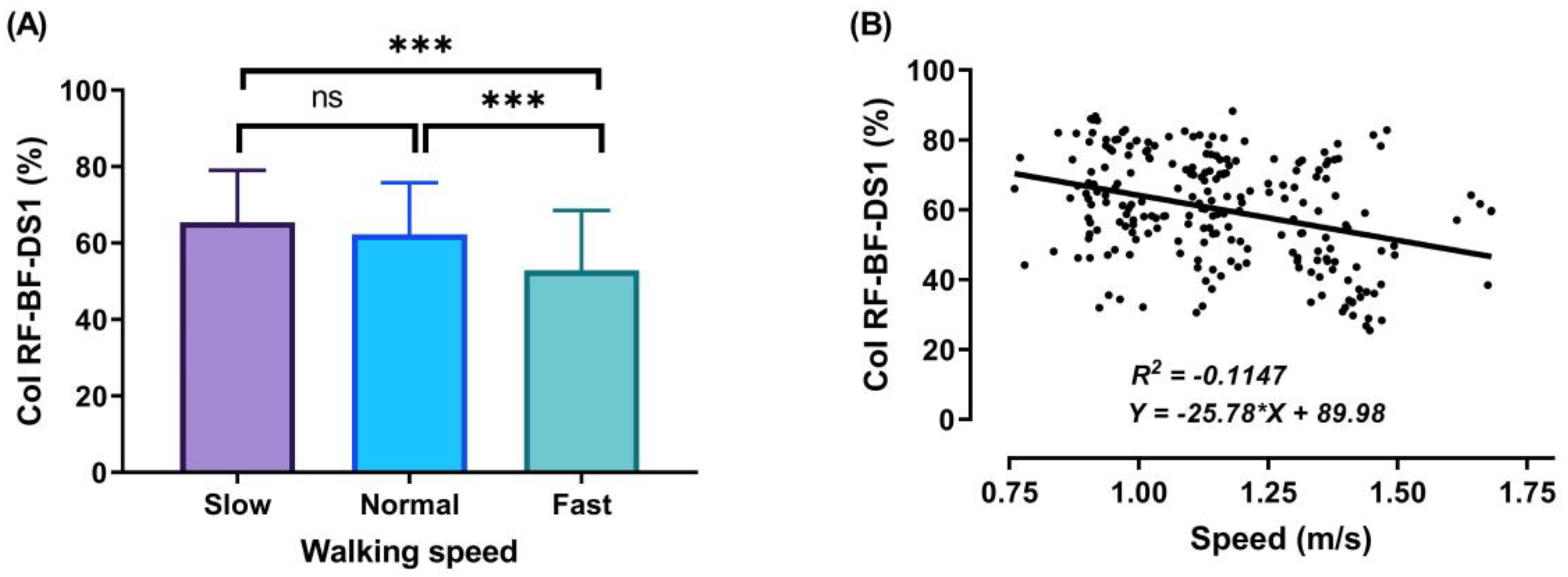
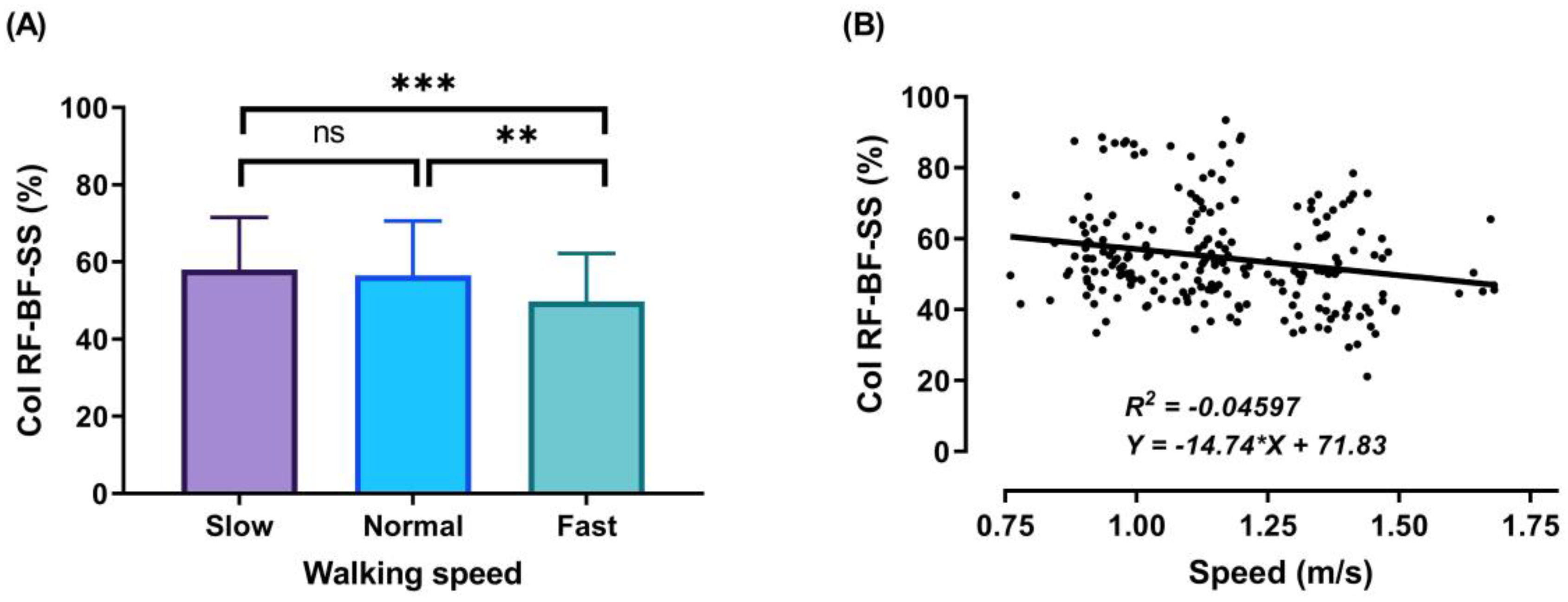
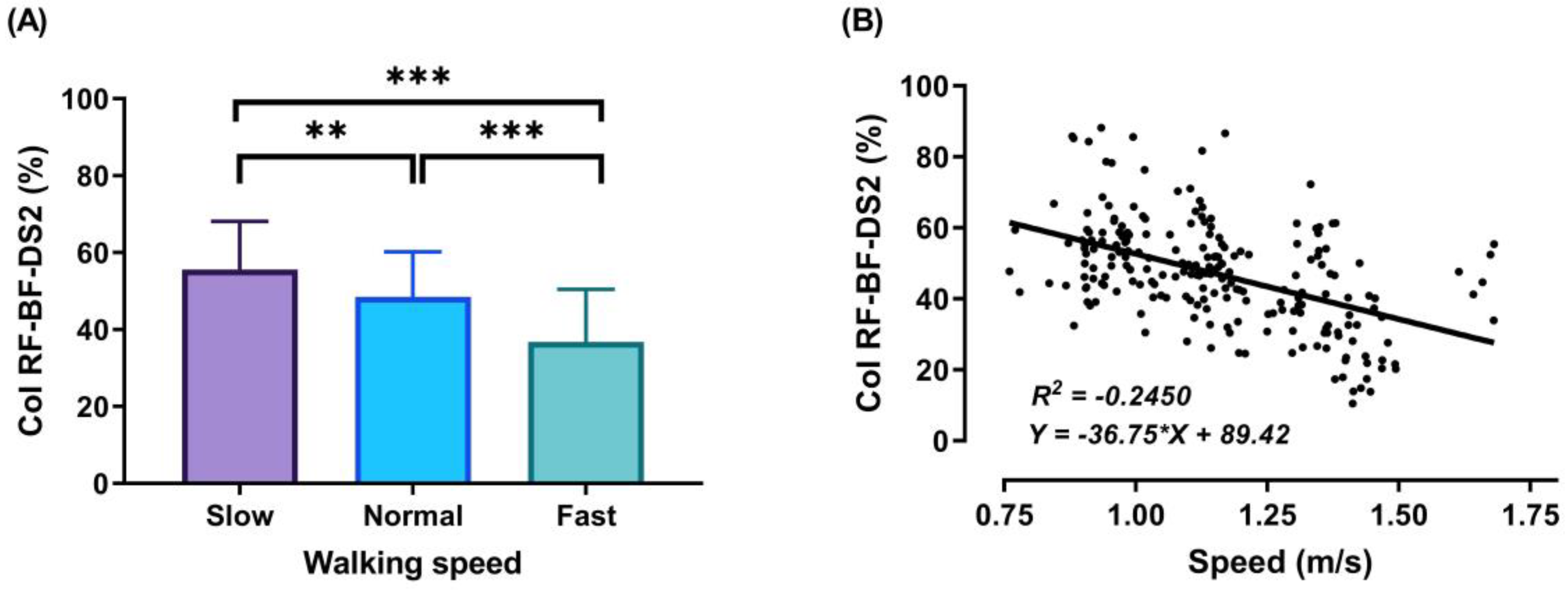
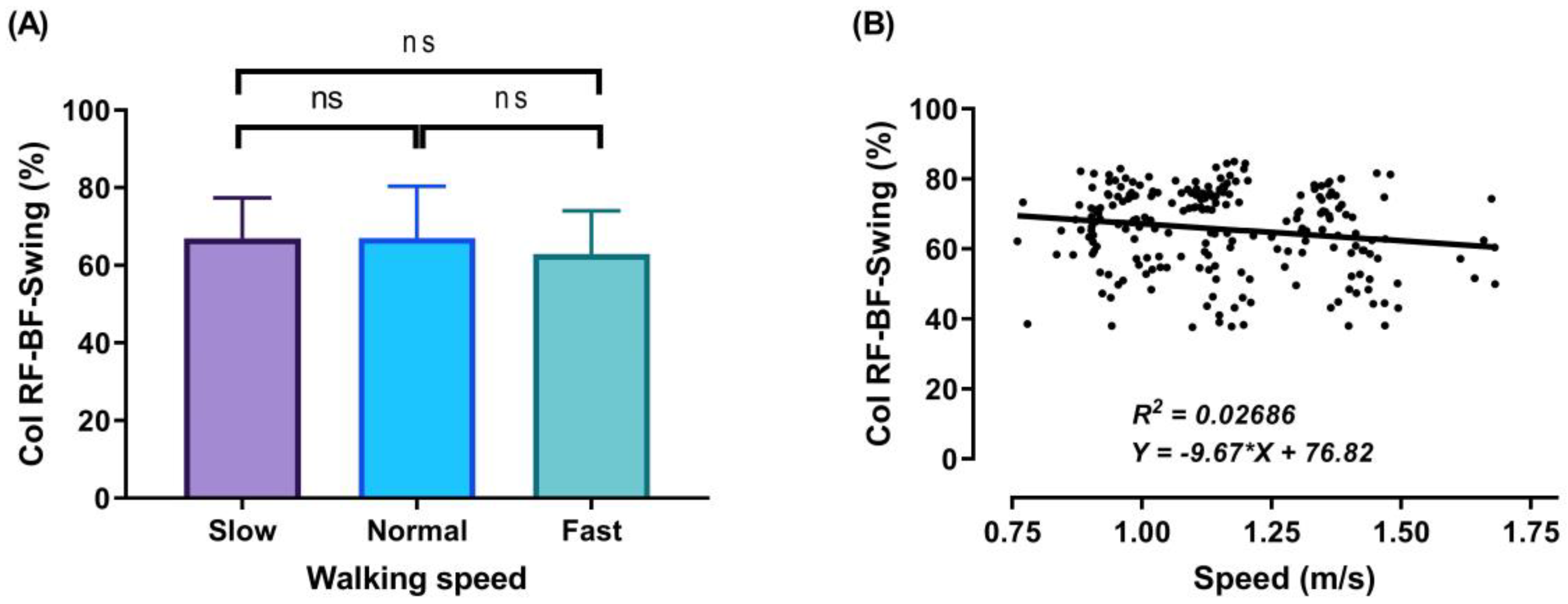
3.2. Thigh Muscles CoI at Different Walking Speeds
3.3. Relationship between Walking Speed and CoI of Thigh Muscles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akl, A.R.; Baca, A.; Richards, J.; Conceicao, F. Leg and lower limb dynamic joint stiffness during different walking speeds in healthy adults. Gait Posture 2020, 82, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.L.; Elzinga, H.; Grimmius, W.; Halbertsma, J.P.K. Speed dependence of averaged EMG profiles in walking. Gait Posture 2002, 16, 78–86. [Google Scholar] [CrossRef]
- Tirosh, O.; Sangeux, M.; Wong, M.; Thomason, P.; Graham, H.K. Walking speed effects on the lower limb electromyographic variability of healthy children aged 7–16 years. J. Electromyogr. Kinesiol. 2013, 23, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- den Otter, A.R.; Geurts, A.C.H.; Mulder, T.; Duysens, J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait Posture 2004, 19, 270–278. [Google Scholar] [CrossRef]
- Liu, M.Q.; Anderson, F.C.; Pandy, M.G.; Delp, S.L. Muscles that support the body also modulate forward progression during walking. J. Biomech. 2006, 39, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Neptune, R.R.; Zajac, F.E.; Kautz, S.A. Muscle force redistributes segmental power for body progression during walking. Gait Posture 2004, 19, 194–205. [Google Scholar] [CrossRef]
- Pandy, M.G. Computer modeling and simulation of human movement. Annu. Rev. Biomed. Eng. 2001, 3, 245–273. [Google Scholar] [CrossRef]
- Neptune, R.R.; Sasaki, K.; Kautz, S.A. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 2008, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Katsavelis, D.; Threlkeld, A.J. Quantifying thigh muscle co-activation during isometric knee extension contractions: Within- and between-session reliability. J. Electromyogr. Kinesiol. 2014, 24, 502–507. [Google Scholar] [CrossRef]
- Arias, P.; Espinosa, N.; Robles-Garcia, V.; Cao, R.; Cudeiro, J. Antagonist muscle co-activation during straight walking and its relation to kinematics: Insight from young, elderly and Parkinson’s disease. Brain Res. 2012, 1455, 124–131. [Google Scholar] [CrossRef]
- Peterson, D.S.; Martin, P.E. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture 2010, 31, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Hubley-Kozey, C.; Deluzio, K.; Dunbar, M. Muscle co-activation patterns during walking in those with severe knee osteoarthritis. Clin. Biomech. 2008, 23, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Kang, H.; Shin, G. Use of antagonist muscle EMG in the assessment of neuromuscular health of the low back. J. Physiol. Anthropol. 2015, 34, 18. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.H.; Puggaard, L.; Hämäläinen, U.; Aagaard, P. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J. Electromyogr. Kinesiol. 2008, 18, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Gagnat, Y.; Braendvik, S.M.; Roeleveld, K. Surface Electromyography Normalization Affects the Interpretation of Muscle Activity and Coactivation in Children With Cerebral Palsy During Walking. Front. Neurol. 2020, 11, 202. [Google Scholar] [CrossRef]
- Silva, A.; Sousa, A.S.; Silva, C.; Tavares, J.M.; Santos, R.; Sousa, F. Ankle antagonist coactivation in the double-support phase of walking: Stroke vs. healthy subjects. Somatosens. Mot. Res. 2015, 32, 153–157. [Google Scholar] [CrossRef]
- Silva, A.; Sousa, A.S.; Tavares, J.M.; Tinoco, A.; Santos, R.; Sousa, F. Ankle dynamic in stroke patients: Agonist vs. antagonist muscle relations. Somatosens. Mot. Res. 2012, 29, 111–116. [Google Scholar] [CrossRef][Green Version]
- Chow, J.W.; Yablon, S.A.; Stokic, D.S. Coactivation of ankle muscles during stance phase of gait in patients with lower limb hypertonia after acquired brain injury. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012, 123, 1599–1605. [Google Scholar] [CrossRef]
- Lamontagne, A.; Richards, C.L.; Malouin, F. Coactivation during gait as an adaptive behavior after stroke. J. Electromyogr. Kinesiol. 2000, 10, 407–415. [Google Scholar] [CrossRef]
- Kitatani, R.; Ohata, K.; Sakuma, K.; Aga, Y.; Yamakami, N.; Hashiguchi, Y.; Yamada, S. Ankle muscle coactivation during gait is decreased immediately after anterior weight shift practice in adults after stroke. Gait Posture 2016, 45, 35–40. [Google Scholar] [CrossRef]
- Seidler, R.D.; He, J.; Stemach, G.E. Coactivation to reduce variability in the elderly. Mot. Control 1998, 2, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Ridge, S.T.; Henley, J.; Manal, K.; Miller, F.; Richards, J.G. Biomechanical analysis of gait termination in 11–17 year old youth at preferred and fast walking speeds. Hum. Mov. Sci. 2016, 49, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Maranesi, E.; Mengarelli, A.; Ghetti, G.; Burattini, L.; Fioretti, S. Assessment of the variability of vastii myoelectric activity in young healthy females during walking: A statistical gait analysis. J. Electromyogr. Kinesiol. 2015, 25, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Ait-Haddou, R.; Binding, P.; Herzog, W. Theoretical considerations on cocontraction of sets of agonistic and antagonistic muscles. J. Biomech. 2000, 33, 1105–1111. [Google Scholar] [CrossRef]
- Zoffoli, L.; Lucertini, F.; Federici, A.; Ditroilo, M. Trunk muscles activation during pole walking vs. walking performed at different speeds and grades. Gait Posture 2016, 46, 57–62. [Google Scholar] [CrossRef]
- Holt, K.G.; Wagenaar, R.C.; LaFiandra, M.E.; Kubo, M.; Obusek, J.P. Increased musculoskeletal stiffness during load carriage at increasing walking speeds maintains constant vertical excursion of the body center of mass. J Biomech. 2003, 36, 465–471. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chang, W.H.; Choi, B.-O.; Ryu, G.-H.; Kim, Y.-H. Age-related differences in muscle co-activation during locomotion and their relationship with gait speed: A pilot study. BMC Geriatr. 2017, 17, 44. [Google Scholar] [CrossRef]
- Khan, S.S.; Khan, S.J.; Usman, J. Effects of toe-out and toe-in gait with varying walking speeds on knee joint mechanics and lower limb energetics. Gait Posture 2017, 53, 185–192. [Google Scholar] [CrossRef]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. Electromyographic patterns of tibialis posterior and related muscles when walking at different speeds. Gait Posture 2014, 39, 1080–1085. [Google Scholar] [CrossRef]
- Kodesh, E.; Kafri, M.; Dar, G.; Dickstein, R. Walking speed, unilateral leg loading, and step symmetry in young adults. Gait Posture 2012, 35, 66–69. [Google Scholar] [CrossRef]
- Quittmann, O.J.; Meskemper, J.; Albracht, K.; Abel, T.; Foitschik, T.; Struder, H.K. Normalising surface EMG of ten upper-extremity muscles in handcycling: Manual resistance vs. sport-specific MVICs. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2020, 51, 102402. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; Nordez, A.; Hug, F.; Ates, F.; Coppieters, M.W.; Pezarat-Correia, P.; Freitas, S.R. Non-invasive assessment of sciatic nerve stiffness during human ankle motion using ultrasound shear wave elastography. J. Biomech. 2015, 49, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.; Daud, O.; Ruiz-del-Solar, J. EMG Signal Filtering Based on Independent Component Analysis and Empirical Mode Decomposition for Estimation of Motor Activation Patterns. J. Med. Biol. Eng. 2017, 37, 140–155. [Google Scholar] [CrossRef]
- Oliveira, C.F.d.; Soares, D.P.; Bertani, M.C.; Machado, L.J.R.; Vila-Boas, J.P. Effects of Fast-Walking on Muscle Activation in Young Adults and Elderly Persons. J. Nov. Physiother. Rehabil. 2017, 1, 12–19. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Di Nardo, F.; Strazza, A.; Mengarelli, A.; Ercolani, S.; Morgoni, N.; Burattini, L.; Agostini, V.; Knaflitz, M.; Fioretti, S. Surface EMG patterns for quantification of thigh muscle co-contraction in school-age children: Normative data during walking. Gait Posture 2018, 61, 25–33. [Google Scholar] [CrossRef]
- Markowitz, J.; Herr, H. Human Leg Model Predicts Muscle Forces, States, and Energetics during Walking. PLoS Comput. Biol. 2016, 12, e1004912. [Google Scholar] [CrossRef]
- Mari, S.; Serrao, M.; Casali, C.; Conte, C.; Martino, G.; Ranavolo, A.; Coppola, G.; Draicchio, F.; Padua, L.; Sandrini, G.; et al. Lower Limb Antagonist Muscle Co-Activation and its Relationship with Gait Parameters in Cerebellar Ataxia. Cerebellum 2014, 13, 226–236. [Google Scholar] [CrossRef]
- Kellis, E.; Arabatzi, F.; Papadopoulos, C. Muscle co-activation around the knee in drop jumping using the co-contraction index. J. Electromyogr. Kinesiol. 2003, 13, 229–238. [Google Scholar] [CrossRef]
- Martino, G.; Ivanenko, Y.P.; Serrao, M.; Ranavolo, A.; d’Avella, A.; Draicchio, F.; Conte, C.; Casali, C.; Lacquaniti, F. Locomotor patterns in cerebellar ataxia. J. Neurophysiol. 2014, 112, 2810–2821. [Google Scholar] [CrossRef]
- Ko, S.U.; Hausdorff, J.M.; Ferrucci, L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: Results from the Baltimore longitudinal study of ageing. Age Ageing 2010, 39, 688–694. [Google Scholar] [CrossRef]
- Fox, M.D.; Delp, S.L. Contributions of muscles and passive dynamics to swing initiation over a range of walking speeds. J. Biomech. 2010, 43, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Holt, K.G.; Saltzman, E.; Wagenaar, R.C. Changes in axial stiffness of the trunk as a function of walking speed. J. Biomech. 2006, 39, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Yack, H.J. EMG profiles during normal human walking: Stride-to-stride and inter-subject variability. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 402–411. [Google Scholar] [CrossRef]
- Nagai, K.; Yamada, M.; Uemura, K.; Yamada, Y.; Ichihashi, N.; Tsuboyama, T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch. Gerontol. Geriatr. 2011, 53, 338–343. [Google Scholar] [CrossRef]
- Hortoba’gyi, T.; DeVita, P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J. Electromyogr. Kinesiol. 2000, 10, 117–126. [Google Scholar] [CrossRef]
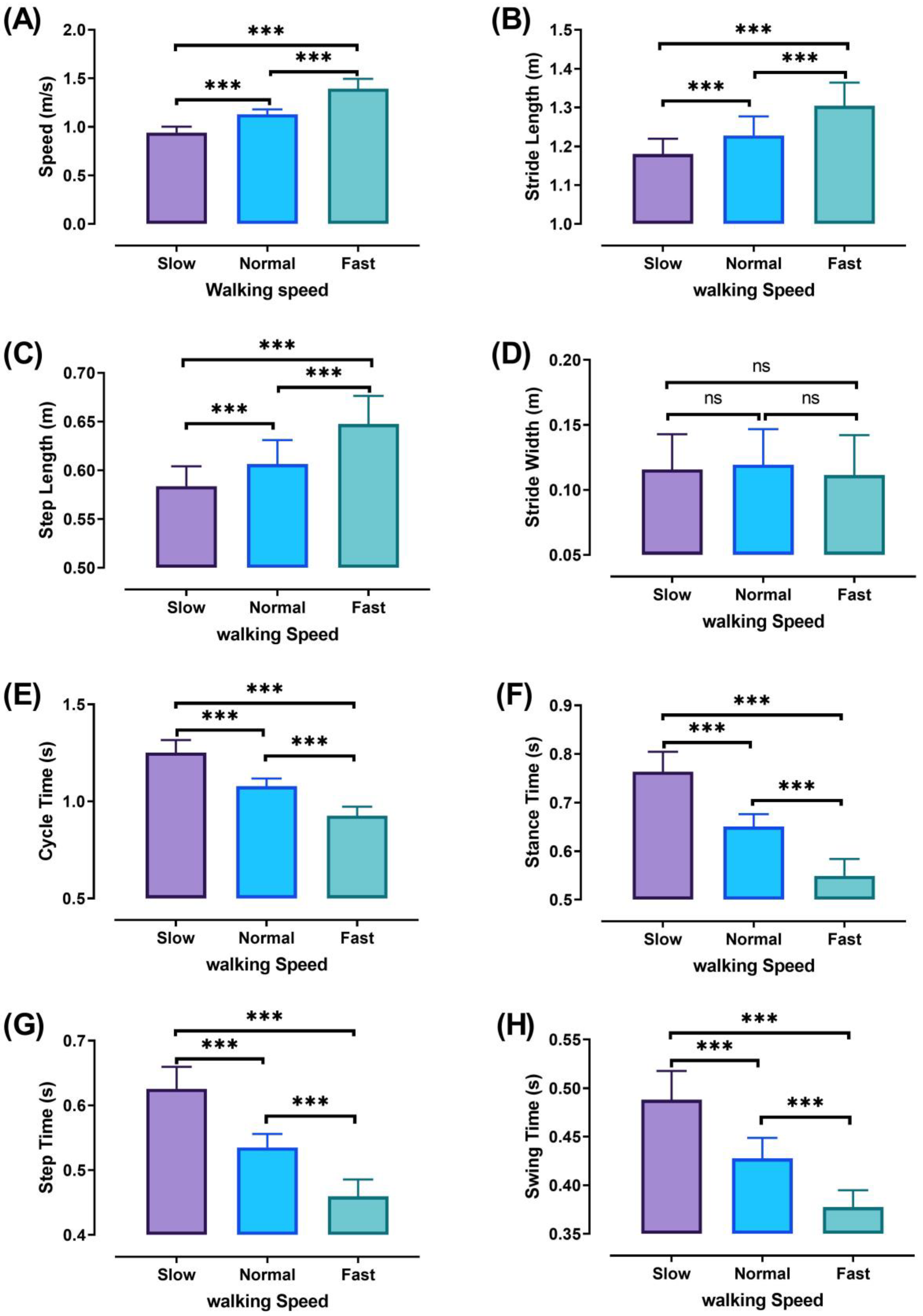
| Walking Characteristics/Speed | Slow/Normal (%) | Slow/Fast (%) | Normal/Fast (%) |
|---|---|---|---|
| Speed | S < N (16.67) | S < F (32.38) | N < F (18.85) |
| Cycle time | S > N (16.09) | S > F (35.04) | N > F (16.33) |
| Stance time | S > N (17.37) | S > F (39.00) | N > F (18.43) |
| Step time | S > N (16.89) | S > F (36.12) | N > F (16.45) |
| Swing time | S > N (14.14) | S > F (29.29) | N > F (13.27) |
| Stride Length | S < N (3.87) | S < F (9.50) | N < F (5.86) |
| Step Length | S < N (3.72) | S < F (9.82) | N < F (6.34) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akl, A.-R.; Gonçalves, P.; Fonseca, P.; Hassan, A.; Vilas-Boas, J.P.; Conceição, F. Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females. Sensors 2021, 21, 677. https://doi.org/10.3390/s21030677
Akl A-R, Gonçalves P, Fonseca P, Hassan A, Vilas-Boas JP, Conceição F. Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females. Sensors. 2021; 21(3):677. https://doi.org/10.3390/s21030677
Chicago/Turabian StyleAkl, Abdel-Rahman, Pedro Gonçalves, Pedro Fonseca, Amr Hassan, João Paulo Vilas-Boas, and Filipe Conceição. 2021. "Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females" Sensors 21, no. 3: 677. https://doi.org/10.3390/s21030677
APA StyleAkl, A.-R., Gonçalves, P., Fonseca, P., Hassan, A., Vilas-Boas, J. P., & Conceição, F. (2021). Muscle Co-Activation around the Knee during Different Walking Speeds in Healthy Females. Sensors, 21(3), 677. https://doi.org/10.3390/s21030677








