Polyaniline Nanofibers-Embedded Gold Nanoparticles Obtained by Template-Free Procedure with Immobilization Prospects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Synthesis of the Conducting Polymer Nanofibers/Gold Nanoparticles (nCPs-AuNPs) Nanocomposite
2.4. Preparation Procedure of the Sonogel-Carbon Electrodes
2.5. Electrochemical Pre-Treatment of Sonogel-Carbon Electrodes
2.6. Modification of the Sonogel-Carbon Electrodes with the Nanocomposite Materials and Characterization by the Different Instrumental Techniques
2.7. Fabrication of the GOX-PANI-AuNPs Biosensor
2.8. Electrochemical Assessment of the GOX-PANI-AuNPs Biosensor
2.9. Determination of Glucose in Wines by Using the GOX-PANI-AuNPs Biosensor
3. Results and Discussion
3.1. Optimization of the Parameters of the Sonochemical Synthesis
3.1.1. Dopant Choice
3.1.2. Metal Salt and Monomer Concentration
3.1.3. Ultrasound Irradiation Time and Energy Applied to the Mixture
3.1.4. Time Stability of the nCPs-AuNPs Nanocomposites
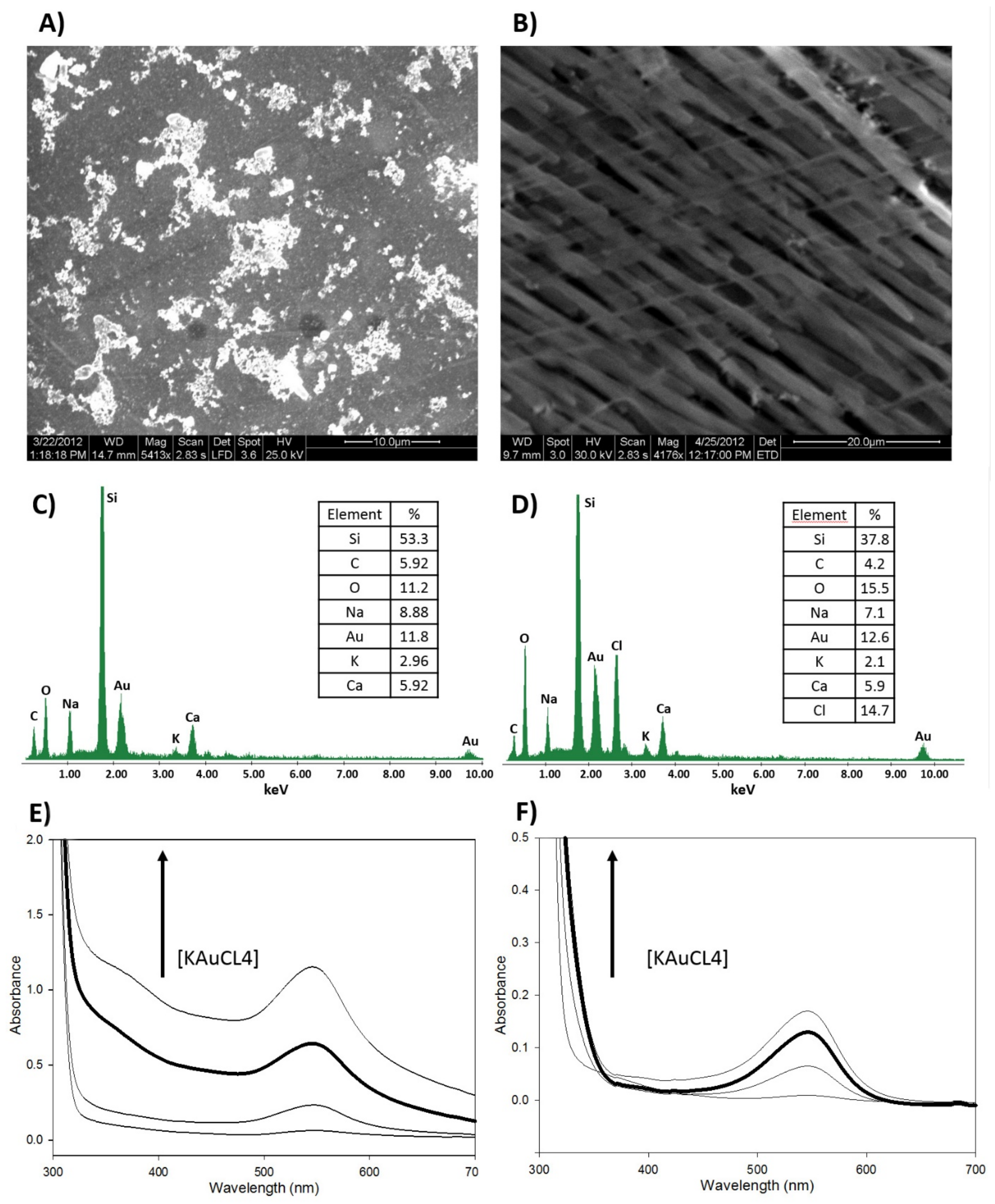
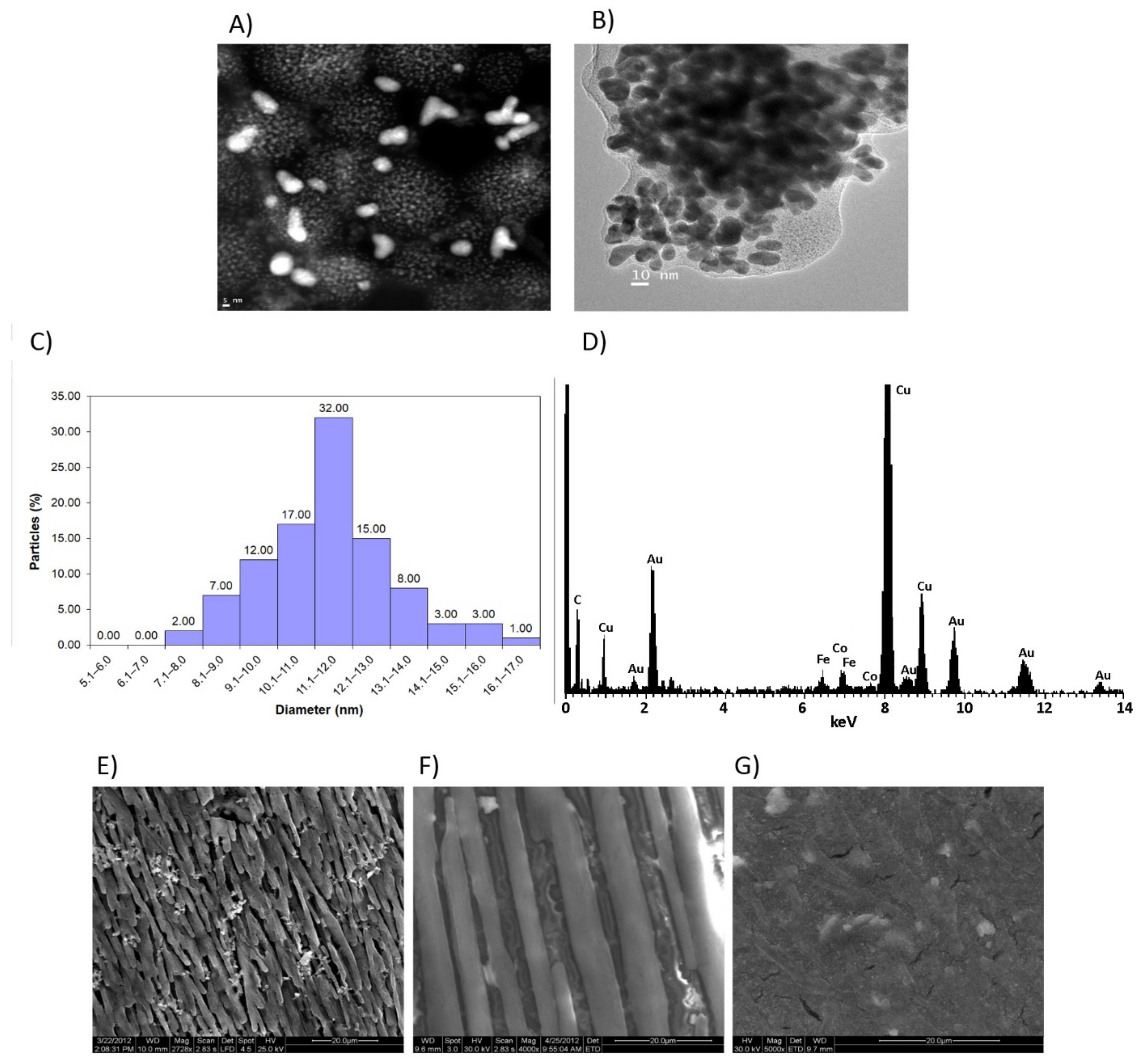
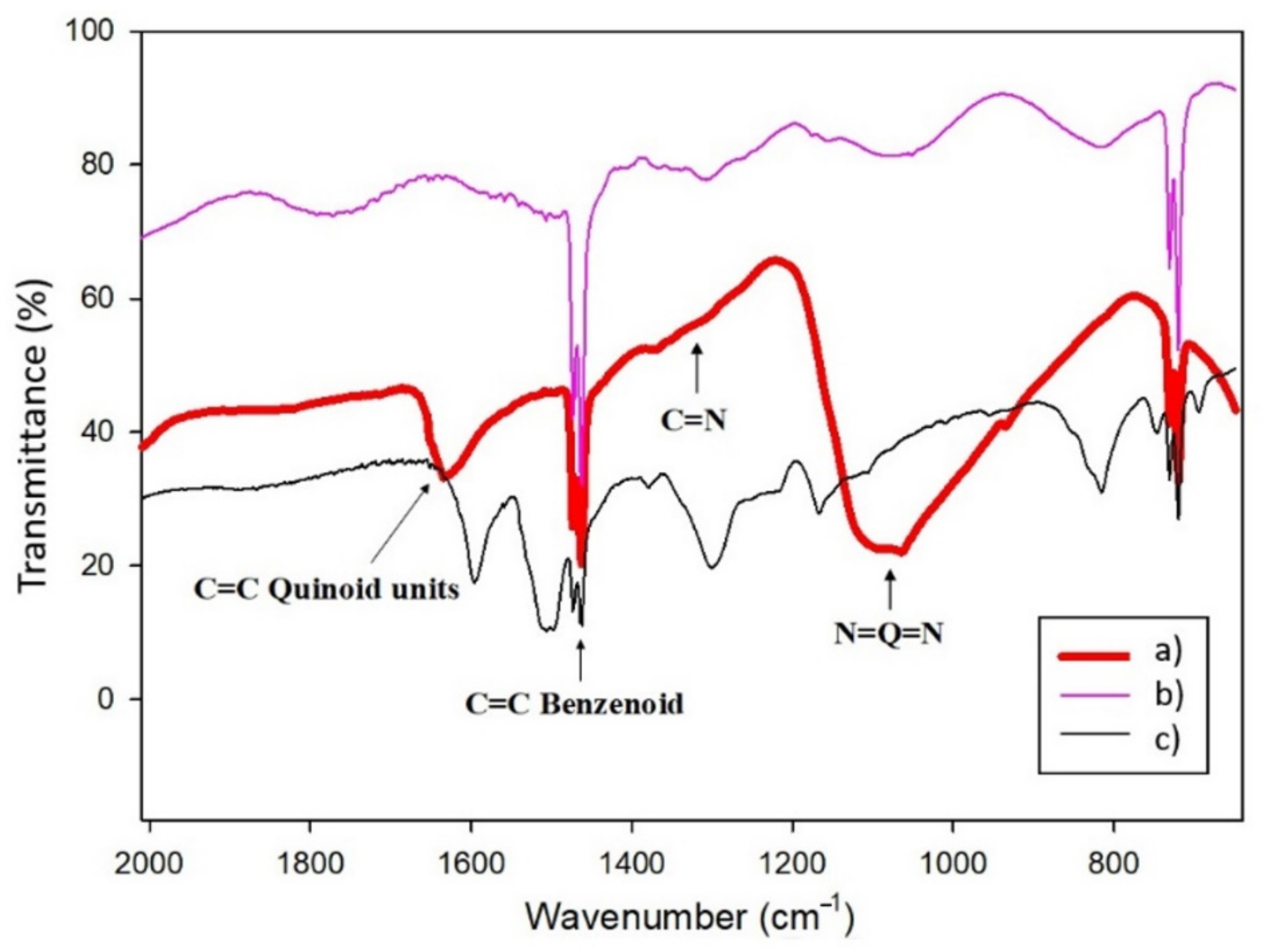
3.2. Structural Characterization of the nCPs-AuNPs Nanocomposites
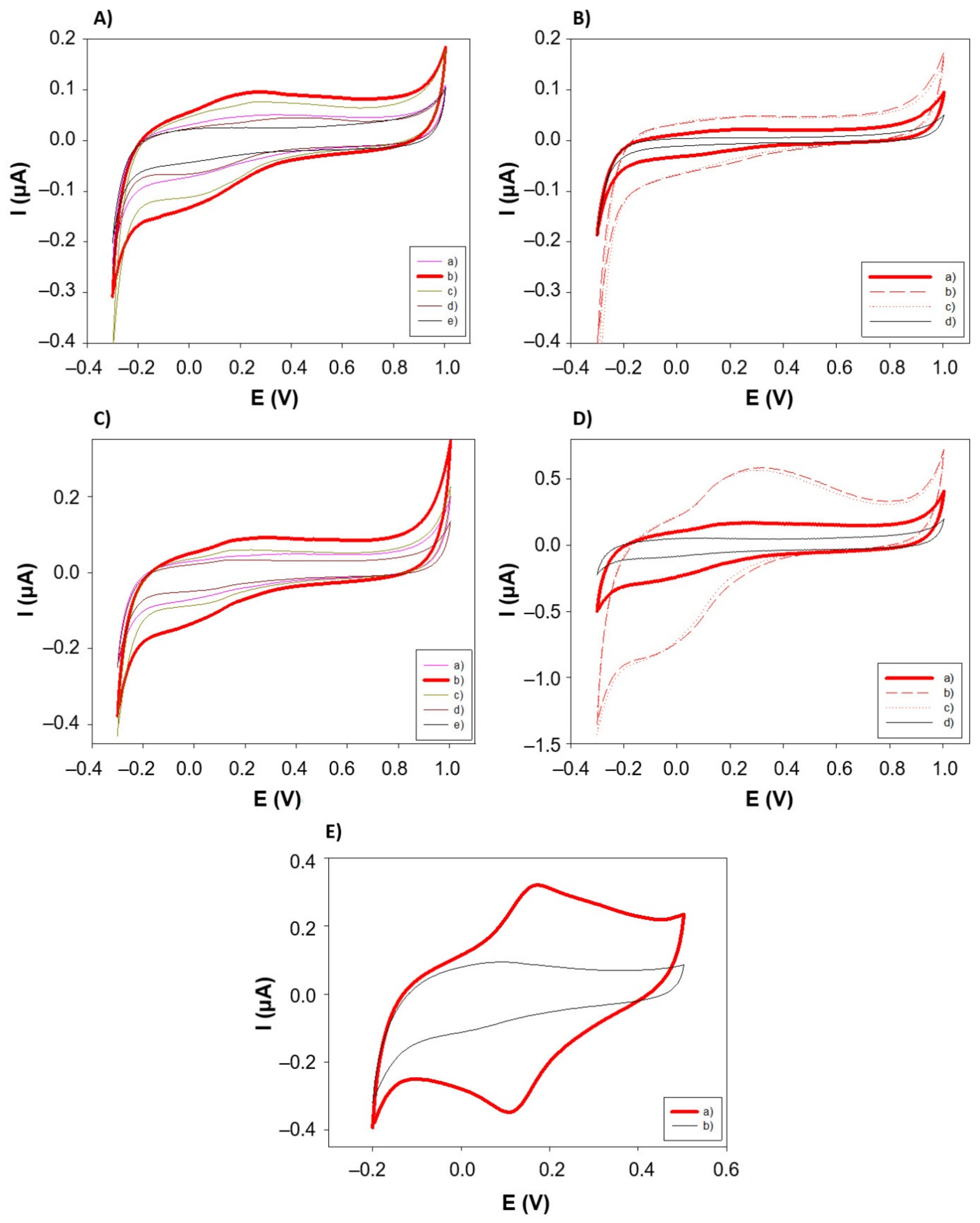
3.3. Electrochemical Characterization of the PANI-AuNPs Nanocomposite
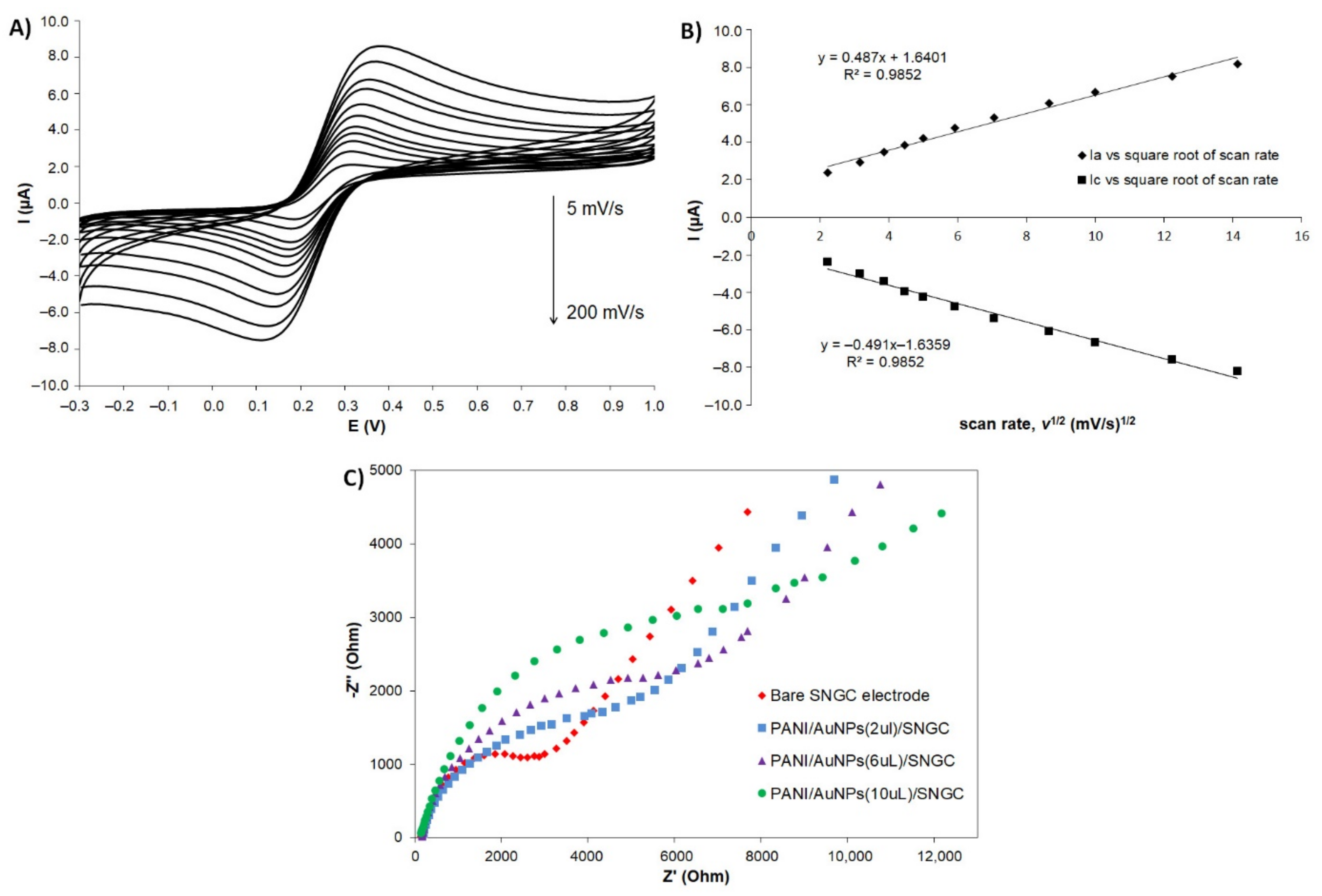
3.3.1. Electrode Capacity Assessment
3.3.2. Mechanism and Impedance Studies
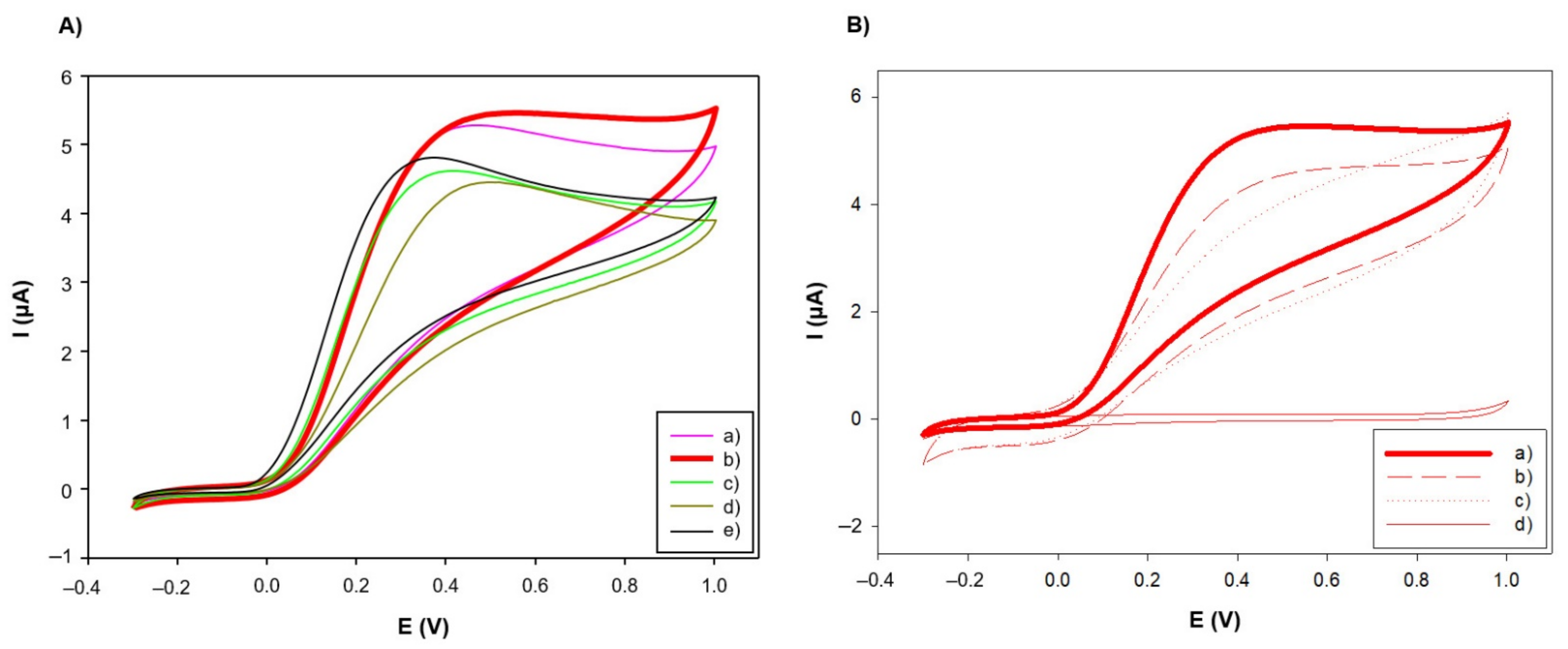
3.3.3. Benchmark Analyte Application
3.4. Application of GOX/PANI-AuNPs Biosensor for Glucose Detection
3.4.1. Effect of Enzyme Loading
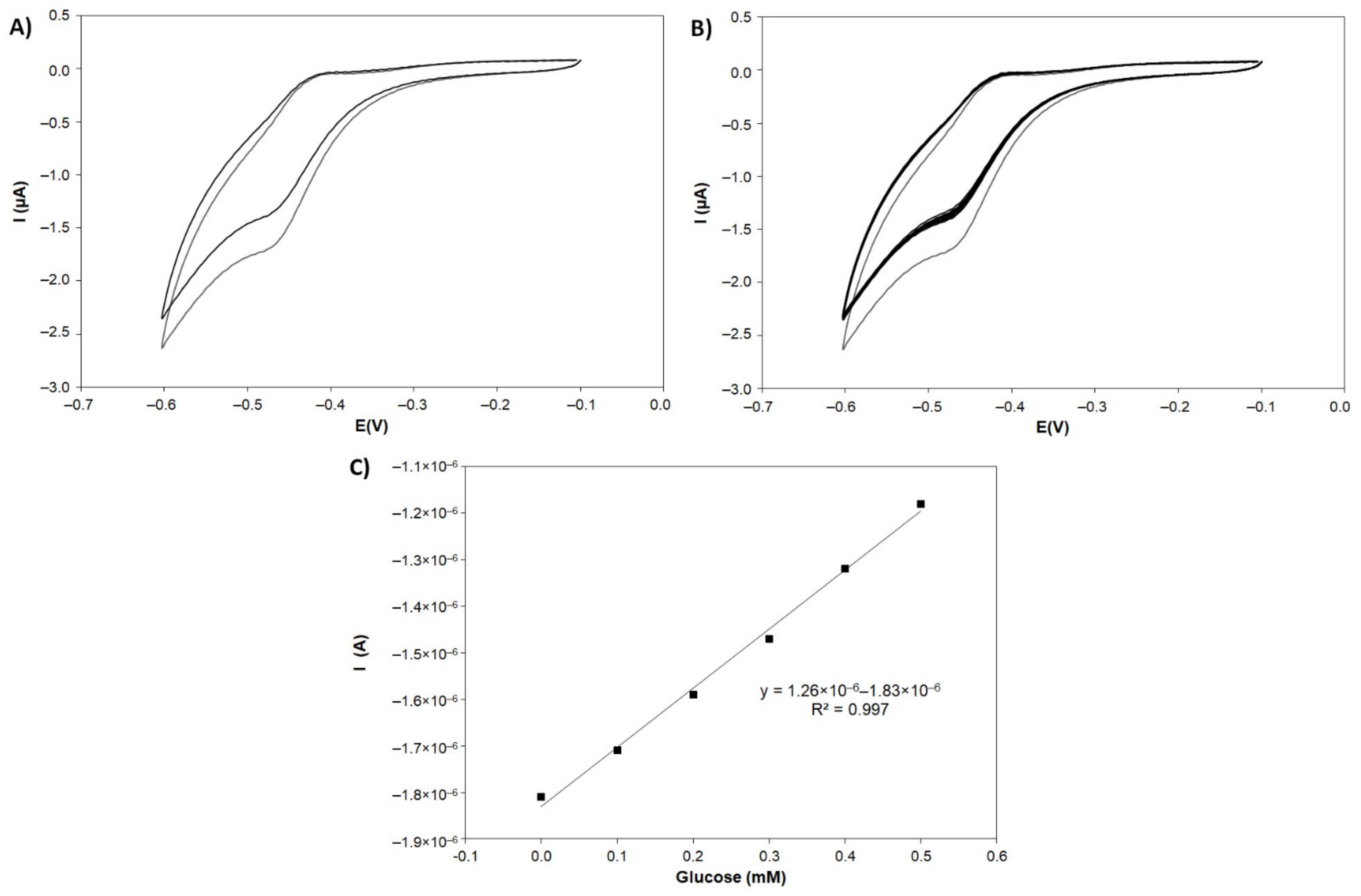
3.4.2. Electrochemical Assessment
3.4.3. Calibration of Glucose
3.4.4. Reproducibility and Stability Studies for Glucose Detection
3.5. Real Wines Samples Analysis Employing the GOX/PANI-AuNPs/SNGC Biosensor
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly (3,4-ethylenedioxythiophene): Poly (4-styrenesulfonate) (PEDOT: PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Seeber, R.; Pigani, L.; Terzi, F.; Zanardi, C. Amperometric sensing. A melting pot for material, electrochemical, and analytical sciences. Electrochim. Acta 2015, 179, 350–363. [Google Scholar] [CrossRef]
- Demir, F. Effect of aluminum reinforcement on the structural, physicochemical, and electrochemical properties of polyaniline-derived polymer/aluminum composites by in situ polymerization. Electrochim. Acta 2021, 385, 138444. [Google Scholar] [CrossRef]
- Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.-S.; Tae, H.-S. In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma. Polymers 2021, 13, 418. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Iwaki, L.E.O.; Scagion, V.P.; Oliveira, O.N., Jr.; Mattoso, L.H.C.; Correa, D.S. Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles. Electrochem 2021, 2, 41–49. [Google Scholar] [CrossRef]
- Jang, J. Conducting Polymer Nanomaterials and Their Applications. In Emissive Materials Nanomaterials. Advances in Polymer Science; Springer: Heidelberg, Germany, 2006; pp. 189–259. [Google Scholar] [CrossRef]
- Seeber, R.; Terzi, F.; Zanardi, C. Intrinsecally Conducting Polymers. In Functional Materials in Amperometric Sensing. Polymeric, Inorganic and Nanocomposite Materials for Modified Electrodes; Scholz, F., Ed.; Springer: Heidelberg, Germany, 2014; pp. 723–757. ISBN 978-3-662-45103-8. [Google Scholar]
- Pigani, L.; Foca, G.; Ionescu, K.; Martina, V.; Ulrici, A.; Terzi, F.; Vignali, M.; Zanardi, C. Amperometric sensors based on Poly (3,4-ethylenedioxythiophene)-modified electrodes. Discrimination of white wines. Anal. Chim. Acta 2008, 614, 213–222. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical sensors using conducting polymer/noble metal nanoparticle nanocomposites for the detection of various analytes: A review. J. Nanostruct. Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M. Composites of intrinsically conducting polymers as sensing nanomaterials. J. Am. Chem. Soc. 2007, 23, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jiang, J.; Jiang, Y.; Li, Y. Gold Nanoparticles Monolayer Doping PEDOT: PSS Janus Film for Hydrogen Sulfide Sensor. Electroanalysis 2021, 33, 1–7. [Google Scholar] [CrossRef]
- Radinović, K.; Milikić, J.; Stamenović, U.; Vodnik, V.; Otoničar, M.; Škapin, S.; Šljukić, B. Tailoring gold-conducting polymer nanocomposites for sensors applications: Proof of concept for As(III) sensing in aqueous media. Synth. Met. 2021, 278, 116834. [Google Scholar] [CrossRef]
- Selvam, S.P.; Kadam, A.N.; Maiyelvaganan, K.R.; Prakash, M.; Cho, S. Novel SeS2-loaded Co MOF with Au@PANI comprised electroanalytical molecularly imprinted polymer-based disposable sensor for patulin mycotoxin. Biosens. Bielectron. 2021, 187, 113302. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Sridevi, V. In situ electrodeposited gold nanoparticles on polyaniline-modified electrode surface for the detection of dopamine in presence of ascorbic acid and uric acid. Electrocataysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Terzi, F.; Zanfrognini, B.; Zanardi, C.; Pigani, L.; Seeber, R. Poly (3,4-ethylenedioxythiophene)/Au-nanoparticles composite as electrode coating suitable for electrocatalytic oxidation. Electrochim. Acta 2011, 56, 3575–3579. [Google Scholar] [CrossRef]
- Wang, G.; Morrin, A.; Li, M.; Liu, N.; Luo, X. Nanomaterial-doped conducting polymers for electrochemical sensors and biosensors. J. Mater. Chem. B 2018, 6, 4173–4190. [Google Scholar] [CrossRef] [PubMed]
- Lete, C.; Lupu, S.; Lakard, B.; Hihn, J.Y.; Del Campo, F.J. Multi-analyte determination of dopamine and catechol at single-walled carbon nanotubes—Conducting polymer—Tyrosinase based electrochemical biosensors. J. Electroanal. Chem. 2015, 744, 53–61. [Google Scholar] [CrossRef]
- Chondath, S.K.; Menamparambath, M.M. Interface-assisted synthesis: A gateway to effective nanostructure tuning of conducting polymers. Nanoscale Adv. 2021, 3, 918–941. [Google Scholar] [CrossRef]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.-H.; Choi, J.-W. Application of conducting polymer nanostructures to electrochemical biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popov, A.; Aukstakojyte, R.; Gaidukevic, J.; Lisyte, V.; Kausaite-Minkstimiene, A.; Barkauskas, J.; Ramanaviciene, A. Reduced graphene oxide and polyaniline nanofibers nanocomposite for the development of an amperometric glucose biosensor. Sensors 2021, 21, 948. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Plast. Technol. Mater. 2021, 60, 504–521. [Google Scholar] [CrossRef]
- Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. E-Tongues/noses based on conducting polymers and composite materials: Expanding the possibilities in complex analytical sensing. Sensors 2021, 21, 4976. [Google Scholar] [CrossRef]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; Al-Anazy, M.M.; Ragupathy, D. Electrospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review. Synth. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Kailasa, S.; Rani, B.G.; Reddy, M.S.B.; Jayarambabu, N.; Munindra, P.; Sharma, S.; Rao, K.V. NiO nanoparticles-decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Mater. Chem. Phys. 2020, 242, 122524. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Chen, L.; Dong, Y.; Xie, P.; Li, Q. Preparation of CoB nanoparticles decorated PANI nanotubes as catalysts for hydrogen generation from NaBH4 hydrolysis. J. Taiwan Inst. Chem. Eng. 2021, 122, 148–156. [Google Scholar] [CrossRef]
- Spain, E.; Kojima, R.; Kaner, R.B.; Wallace, G.G.; O’Grady, J.; Lacey, K.; Barry, T.; Keyes, T.E.; Forster, R.J. High sensitivity DNA detection using gold nanoparticle functionalised polyaniline nanofibers. Biosens. Bioelectron. 2011, 26, 2613–2618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narouei, F.H.; Livernois, L.; Andreescu, D.; Andreescu, S. Highly sensitive mercury detection using electroactive gold-decorated polymer nanofibers. Sens. Actuators B Chem. 2021, 329, 129267. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Li, H. Preparation and characterization of pyrrole/aniline copolymer nanofibrils using the template-synthesis method. J. Appl. Polym. Sci. 2001, 81, 3002–3007. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Application of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Wang, Y.; Wu, D.; She, L.; Guo, Y. Polyaniline nanofibers prepared with ultrasonic irradiation. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1014–1019. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Y.; Qiao, M.; Zhang, H. Preparation Method of One-Dimensional Electromagnetic Nanofibers. Chinese Patent CN 104264272 A 20150107, 7 January 2015. [Google Scholar]
- Wang, Y.; Jing, X.; Kong, J. Polyaniline nanofibers prepared with hydrogen peroxide as oxidant. Synth. Met. 2007, 157, 269–275. [Google Scholar] [CrossRef]
- Sivakumar, M.; Gedanken, A. A sonochemical method for the synthesis of polyaniline and Au–polyaniline composites using H2O2 for enhancing rate and yield. Synth. Met. 2005, 148, 301–306. [Google Scholar] [CrossRef]
- Bellido-Milla, D.; Cubillana-Aguilera, L.M.; El Kaoutit, M.; Hernández-Artiga, M.P.; Hidalgo-Hidalgo de Cisneros, J.L.; Naranjo-Rodríguez, I.; Palacios-Santander, J.M. Recent advances in graphite powder-based electrodes. Anal. Bioanal. Chem. 2013, 405, 3525–3539. [Google Scholar] [CrossRef] [PubMed]
- Cubillana-Aguilera, L.M.; Palacios-Santander, J.M.; Naranjo-Rodriguez, I.; Hidalgo-Hidalgo de Cisneros, J.L. Study of the influence of graphite powder particle size on the structure of the Sonogel-Carbon materials. J. Sol-Gel Sci. Technol. 2006, 40, 55–64. [Google Scholar] [CrossRef]
- Huang, J.X.; Kaner, R.B. A General Chemical Route to Polyaniline Nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef]
- Zhang, X.; Manohar, S.K. Polyaniline nanofibers: Chemical synthesis using surfactants. Chem. Commun. 2004, 4, 2360–2361. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Wang, Z. Assembly of polyaniline nanostructures. Macromol. Rapid. Commun. 2007, 28, 84–87. [Google Scholar] [CrossRef]
- Rahy, A.; Sakrout, M.; Manohar, S.; Cho, S.J.; Ferraris, J.; Yang, D.J. Polyaniline nanofibers synthesis by Co-use of ammonium peroxydisulfate and sodium hypochlorite. Chem. Mater. 2008, 20, 4808–4814. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Liu, Z.; Robbinson, C. Inorganic/organic mesostructure directed synthesis of wire/ribbon-like polypyrrole nanostructures. Chem. Commun. 2004, 4, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Manohar, S.K. Bulk Synthesis of Polypyrrole Nanofibers by a Seeding Approach. J. Am. Chem. Soc. 2004, 126, 2714–12715. [Google Scholar] [CrossRef]
- Moulton, S.E.; Innis, P.C.; Kane-Maguire, L.A.P.; Ngamma, O.; Wallace, G.G. Polymerisation and characterization of conducting polyaniline nanoparticle dispersions. Curr. Appl. Phys. 2004, 4, 402–406. [Google Scholar] [CrossRef]
- Cubillana-Aguilera, L.M.; Franco-Romano, M.; Gil, M.L.A.; Naranjo-Rodríguez, I.; de Cisneros, J.L.H.H.; Palacios-Santander, J.M. New, fast and green procedure for the synthesis of gold nanoparticles based on sonocatalysis. Ultrason. Sonochem. 2011, 18, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Breimer, M.A.; Yevgeny, G.; Sy, S.; Sadik, O.A. Incorporation of metal nanoparticles in photopolymerized organic conducting polymers: A Mechanism Insight. Nanoletters 2001, 1, 305–308. [Google Scholar] [CrossRef]
- Huang, J.X.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Xu, H.; Li, G. Preparation, modification, morphology tailor application of conjugated conductive polymer in chemical sensors. Second Int. Conf. Smart Mater. Nanotechnol. Eng. SPIE 2009, 7493, 1–8. [Google Scholar] [CrossRef]
- Virji, S.; Kojima, R.; Fowler, J.D.; Villanueva, J.G.; Kaner, R.B.; Weiller, B.H. Polyaniline nanofiber composites with amines: Novel materials for phosgene detection. Nano Res. 2009, 2, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Zhang, Z.; Wan, M. Formation Mechanism of Self-Assembled Polyaniline Micro/Nanotubes. Langmuir 2002, 18, 917–921. [Google Scholar] [CrossRef]
- Langer, J.J.; Framsk, G.; Golczak, S.; Gibinski, T. Fullerene-dopped polyaniline. Synth. Met. 2001, 119, 359–360. [Google Scholar] [CrossRef]
- Nogueira-Santos, A.; Werner-Soares, D.A.; Alencar de Queiroz, A.A. Low potential stable glucose detection at dendrimers modified polyaniline nanotubes. Mat. Res. 2010, 13, 5–10. [Google Scholar] [CrossRef]
- Liu, S.; Xua, H.; Ou, J.; Li, Z.; Yang, S.; Wang, J. A feasible approach to the fabrication of gold/polyaniline nanofiber composites and its application as electrocatalyst for oxygen reduction. Mater. Chem. Phys. 2012, 132, 500–504. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, C.; Jing, S.; Wang, Z. Morphology and conductivity of polyaniline nanofibers prepared by seeding polymerization. Polymer 2006, 47, 2305–2313. [Google Scholar] [CrossRef]
- Mondal, S.; Rana, U.; Bhattacharjee, R.; Malik, S. One pot green synthesis of polyaniline coated gold nanorods and its applications. RSC Adv. 2014, 4, 57282–57289. [Google Scholar] [CrossRef]
- Wang, A.-J.; Feng, J.-J.; Li, Y.-F.; Xi, J.-L.; Dong, W.-J. In-situ decorated gold nanoparticles on polyaniline with enhanced electrocatalysis toward dopamine. Microchim. Acta 2010, 171, 431–436. [Google Scholar] [CrossRef]
- Ajaero, C.; Abdelrahim, M.Y.M.; Palacios-Santander, J.M.; Gil, M.L.A.; Naranjo-Rodríguez, I.; Hidalgo-Hidalgo de Cisneros, J.L.H.H.; Cubillana-Aguilera, L.C. Comparative study of the electrocatalytic activity of different types of gold nanoparticles using Sonogel-Carbon material as supporting electrode. Sens. Actuators B Chem. 2012, 171–172, 1244–1256. [Google Scholar] [CrossRef]
- Gun, G.; Tsionsky, M.; Lev, O. Voltammetric studies of composite ceramic carbon working electrodes. Anal. Chim. Acta 1994, 294, 261–270. [Google Scholar] [CrossRef]
- Adams, R.N. Electrochemistry at Solid Electrodes; Marcel Dekker: New York, NY, USA, 1969. [Google Scholar] [CrossRef]
- Wang, X.; Yang, T.; Feng, Y.; Jiao, K.; Li, G. A novel hydrogen peroxide biosensor based on the synergistic effect of gold-platinum alloy nanoparticles/polyaniline nanotube/chitosan nanocomposite membrane. Electroanalysis 2009, 21, 819–825. [Google Scholar] [CrossRef]
- Abdelrahim, M.Y.M.; Benjamin, S.R.; Cubillana-Aguilera, L.M.; Naranjo-Rodríguez, I.; Hidalgo-Hidalgo de Cisneros, J.L.; Delgado, J.J.; Palacios-Santander, J.M. Study of the Electrocatalytic Activity of Cerium Oxide and Gold-Studded Cerium Oxide Nanoparticles Using a Sonogel-Carbon Material as Supporting Electrode: Electroanalytical Study in Apple Juice for Babies. Sensors 2013, 13, 4979–5007. [Google Scholar] [CrossRef]
- Ding, S.; Chao, D.; Zhang, M.; Zhang, W. Structure and electrochemical properties of polyamide and polyaniline. J. Appl. Polym. Sci. 2008, 107, 3408–3412. [Google Scholar] [CrossRef]
- Shamsipur, M.; Kazemib, S.H.; Mousavi, M.F. Impedance studies of a nano-structured conducting polymer and its application to the design of reliable scaffolds for impedimetric biosensors. Biosens. Bioelectron. 2008, 24, 104–110. [Google Scholar] [CrossRef]
- Attaallah, R.; Amine, A. The kinetic and analytical aspects of enzyme competitive inhibition: Sensing of tyrosinase inhibitors. Biosensors 2021, 11, 322. [Google Scholar] [CrossRef]
- Zheng, B.; Xie, S.; Qian, L.; Yuan, H.; Xiao, D.; Choi, M.M.F. Gold nanoparticles-coated eggshell membrane with immobilized glucose oxidase for fabrication of glucose biosensor. Sens. Actuators B Chem. 2011, 152, 49–55. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education/Prentice Hall: Gosport, UK, 2010; ISBN 978-0273730422. [Google Scholar]
- Guo, C.X.; Li, C.M. Direct electron transfer of glucose oxidase and biosensing of glucose on hollow sphere-nanostructured conducting polymer/metal oxide composite. Phys. Chem. Chem. Phys. 2019, 12, 12153–12159. [Google Scholar] [CrossRef]
- Li, J.; Tang, Y.; Yang, J.; Yang, Z.; Zhang, Y.; Hu, X. Cage-like PbS nanostructure for the construction of novel glucose electrochemical biosensor. Sens. Actuators B Chem. 2014, 190, 549–554. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Song, J.; Han, D.; Ivaska, A.; Niu, L. Direct Electrochemistry of Glucose Oxidase and Biosensing for Glucose Based on Graphene. Anal. Chem. 2009, 81, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, X.; Zhang, J.; Zhu, J.; Wong, D.K.Y. Enhancing direct electron transfer of glucose oxidase using a gold nanoparticle/titanate nanotube nanocomposite on a biosensor. Electrochim. Acta 2015, 163, 64–70. [Google Scholar] [CrossRef]
- Velmurugan, M.; Sakthinathan, S.; Chen, S.M.; Karuppiah, C. Direct electron transfer of glucose oxidase and electrocatalysis of glucose based on gold nanoparticles/electroactivated graphite nanocomposite. Int. J. Electrochem. Sci. 2015, 10, 6663–6671. [Google Scholar]
- Qiu, C.; Wang, X.; Liu, X.; Hou, S.; Ma, H. Direct electrochemistry of glucose oxidase immobilized on nanostructured gold thin films and its application to bioelectrochemical glucose sensor. Electrochim. Acta 2012, 67, 140–146. [Google Scholar] [CrossRef]
- Yue, X.; Feng, J.; Li, H.; Xiao, Z.; Qiu, Y.; Yu, X.; Xiang, J. Novel synthesis of carbon nanofiber aerogels from coconut matrix for the electrochemical detection of glucose. Diam. Relat. Mater. 2021, 111, 108180. [Google Scholar] [CrossRef]
- Beaucamp, A.; Culebras, M.; Collins, M.N. Sustainable mesoporous carbon nanostructures derived from lignin for early detection of glucose. Green Chem. 2021, 23, 5696. [Google Scholar] [CrossRef]
- Dinesh, B.; Shalini Devi, K.S.; Krishnan, U.M. Achieving a stable high surface excess of glucose oxidase on pristine multiwalled carbon nanotubes for glucose quantification. ACS Appl. Bio Mater. 2019, 2, 1740–1750. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, X.; Li, Y.; Zheng, H.; Liu, Y.; Suye, S.-I. A glucose biosensor based on direct electron transfer of glucose oxidase on PEDOT modified microelectrode. J. Electrochem. Soc. 2020, 167, 067502. [Google Scholar] [CrossRef]
- Goran, J.M.; Mantilla, S.M.; Stevenson, K.J. Influence of surface adsorption on the interfacial electron transfer of flavin adenine dinucleotide and glucose oxidase at carbon nanotube and nitrogen-doped carbon nanotube electrodes. Anal. Chem. 2013, 85, 1571–1581. [Google Scholar] [CrossRef] [PubMed]

| PANI Configurations | 0.1 M KCl | 0.1 M PBS (pH 7.2) | ||
|---|---|---|---|---|
| Cobs (µF/cm2) | Cdl (µF/cm2) | Cobs (µF/cm2) | Cdl (µF/cm2) | |
| PANI-Au | 41.77 | 40.40 | 22.45 | 21.02 |
| PANI-Au-Li | 107.56 | 103.97 | 39.92 | 36.61 |
| PANI-Au-SDS | 57.91 | 55.04 | 26.46 | 25.09 |
| PANI-Au-Li+SDS | 33.52 | 32.48 | 15.46 | 14.71 |
| Bare SNGC | 44.11 | 43.18 | 14.78 | 14.21 |
| Glucose Biosensor 1 | Linear Range (μM) | Detection Limit (μM) | Sensitivity (μA mM−1·cm−2) | Ref. |
|---|---|---|---|---|
| GOX/PANI-AuNPs/SNGC | 100–500 | 56 | 30.36 | This work |
| TNT/AuNPs|[Demim]Br|Nafion|GOX|GCE | 10–1200 | - | 72.15 | [69] |
| GOX/AuNPs-EGr/SPCE | 50–1600 | 2.5 | 255 | [70] |
| GOX/Au/GCE | 60–130 | 0.32 | - | [71] |
| CMCNFs-1000/GOX/GCE | 100–2000 | 17.2 | 2.34 | [72] |
| Nafion/GOX/CNF/AuE | 150–2700 | 89 | 50 | [73] |
| PMWCNT/GOX/GCE | 200–5800 | 45 | 6.6 | [74] |
| GOX-PEDOT/CFμE | 500–15,000 | - | 8.5 | [75] |
| GOX/cage-like-PbS/Nafion/GCE | 50–1450 | 10 | 11.02 | [68] |
| GOX/N-CNTE | Up to 6500 | 24 | 11 | [76] |
| Glucose Content (g/L) | ||
|---|---|---|
| Wine sample | White Wine | Red Wine |
| Reference method (HPLC) | 7.48 | 4.55 |
| GOX/PANI-AuNPs/SNGC biosensor | 7.51 | 4.49 |
| RSD (%) | 1.20 | 2.24 |
| Recovery (%) | 100.4 | 98.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Rosa, J.R.; Sierra-Padilla, A.; García-Guzmán, J.J.; López-Iglesias, D.; Bellido-Milla, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Polyaniline Nanofibers-Embedded Gold Nanoparticles Obtained by Template-Free Procedure with Immobilization Prospects. Sensors 2021, 21, 8470. https://doi.org/10.3390/s21248470
Crespo-Rosa JR, Sierra-Padilla A, García-Guzmán JJ, López-Iglesias D, Bellido-Milla D, Palacios-Santander JM, Cubillana-Aguilera L. Polyaniline Nanofibers-Embedded Gold Nanoparticles Obtained by Template-Free Procedure with Immobilization Prospects. Sensors. 2021; 21(24):8470. https://doi.org/10.3390/s21248470
Chicago/Turabian StyleCrespo-Rosa, Joaquín Rafael, Alfonso Sierra-Padilla, Juan José García-Guzmán, David López-Iglesias, Dolores Bellido-Milla, José María Palacios-Santander, and Laura Cubillana-Aguilera. 2021. "Polyaniline Nanofibers-Embedded Gold Nanoparticles Obtained by Template-Free Procedure with Immobilization Prospects" Sensors 21, no. 24: 8470. https://doi.org/10.3390/s21248470
APA StyleCrespo-Rosa, J. R., Sierra-Padilla, A., García-Guzmán, J. J., López-Iglesias, D., Bellido-Milla, D., Palacios-Santander, J. M., & Cubillana-Aguilera, L. (2021). Polyaniline Nanofibers-Embedded Gold Nanoparticles Obtained by Template-Free Procedure with Immobilization Prospects. Sensors, 21(24), 8470. https://doi.org/10.3390/s21248470










