Wearable Devices, Smartphones, and Interpretable Artificial Intelligence in Combating COVID-19
Abstract
:1. Introduction
2. Related Work
- Different prediction techniques were proposed, namely two independent prediction models: one for the HRV measures and the other is for analyzing the daily textual status of users as reported by them using NLP techniques.
- A model interpretation based on the LIME framework was introduced to better understand each feature’s contribution to the final decision.
- An accuracy of 83.34 ± 1.68% with 0.91, 0.88, and 0.89 precision, recall, and F-score, respectively, were obtained in predicting the infection two days before the onset of the symptoms.
- The decision fusion technique between the biometric model decision and the non-biometric model decision (i.e., feelings and reported status) improved the accuracy and the precision of the obtained results.
3. Participants, Dataset, and Methods
3.1. Participants
3.2. Dataset Description
3.3. Methods
- The data acquisition: The physiological signals (i.e., HRV) were collected from participants through the Welltory application over a continuous period. The collected data included textual logs as a part of ‘participants’ daily reports on the application. The text’s tags comprised short words like tired, fever, fatigue, back to active life, and other short messages.
- The preprocessing: This step included cleaning the data that do not conform with the required standards, such as having data before and after the onset, having correct onset dates, and having sufficient daily logs. The second step was to normalize the data due to the variable nature of HRV among participants by using the direct max-min normalization as appears in the following formula:
- Exploratory data analysis (EDA): This aims to visualize and test the data distributions and patterns before introducing them to the AI models.
- Feature extraction and selection: In this stage, domain knowledge and data-driven approaches are utilized. In the domain knowledge, we selected the HRV measures among other vital signs mentioned in Table 1 due to many reasons: (a) the well-established connections in the literature between HRV features and pathological changes including inflammatory onsets [40,41], (b) the timely manner response of some HRV features such as the standard deviation of NN intervals (SDNN) and the root mean square of successive differences between normal heartbeats (RMSSD) [42]. Nonetheless, HRV is still nonspecific to certain inflammatory infections like the COVID-19. Thus, we fused the model with non-physiological complementary data like the textual information tweeted by participants. From the initial screening of the textual tags posted on the application, we noticed a recurrent pattern of words expressed among those who started to feel unwell due to the COVID-19 infection (before the actual onset of symptoms). This additional source of information would be useless with asymptomatic patients as they would not report any significant feelings. Thus, HRV features remain the primary source in our work. Examples of HRV time-domain and frequency-domain are listed below:
- (a)
- The time-domain features:
- Beat per minute (BPM).
- Meanrr: The mean between two RR intervals.
- Mxdmnn: The difference between the maximum and minimum RR intervals.
- SDNN: The standard deviation of all the normal-to-normal RR intervals.
- RMSDD: The root mean square of successive differences between each heartbeat.
- pNN50: The mean number of times the changes in the normal-to-normal intervals exceed 50 ms.
- (b)
- The frequency-domain features:
- HF: The high frequency of the heart rate represents the activity in the 0.15–0.40 Hz range.
- LF: The low frequency of the heart rate represents the activity in the 0.04–0.15 Hz range.
- LF/HF: The ratio between the low and high frequencies.
4. Results and Discussion
4.1. Features Interpretation
4.2. Heart Signals and Feelings Classification Results and Their Interpretations
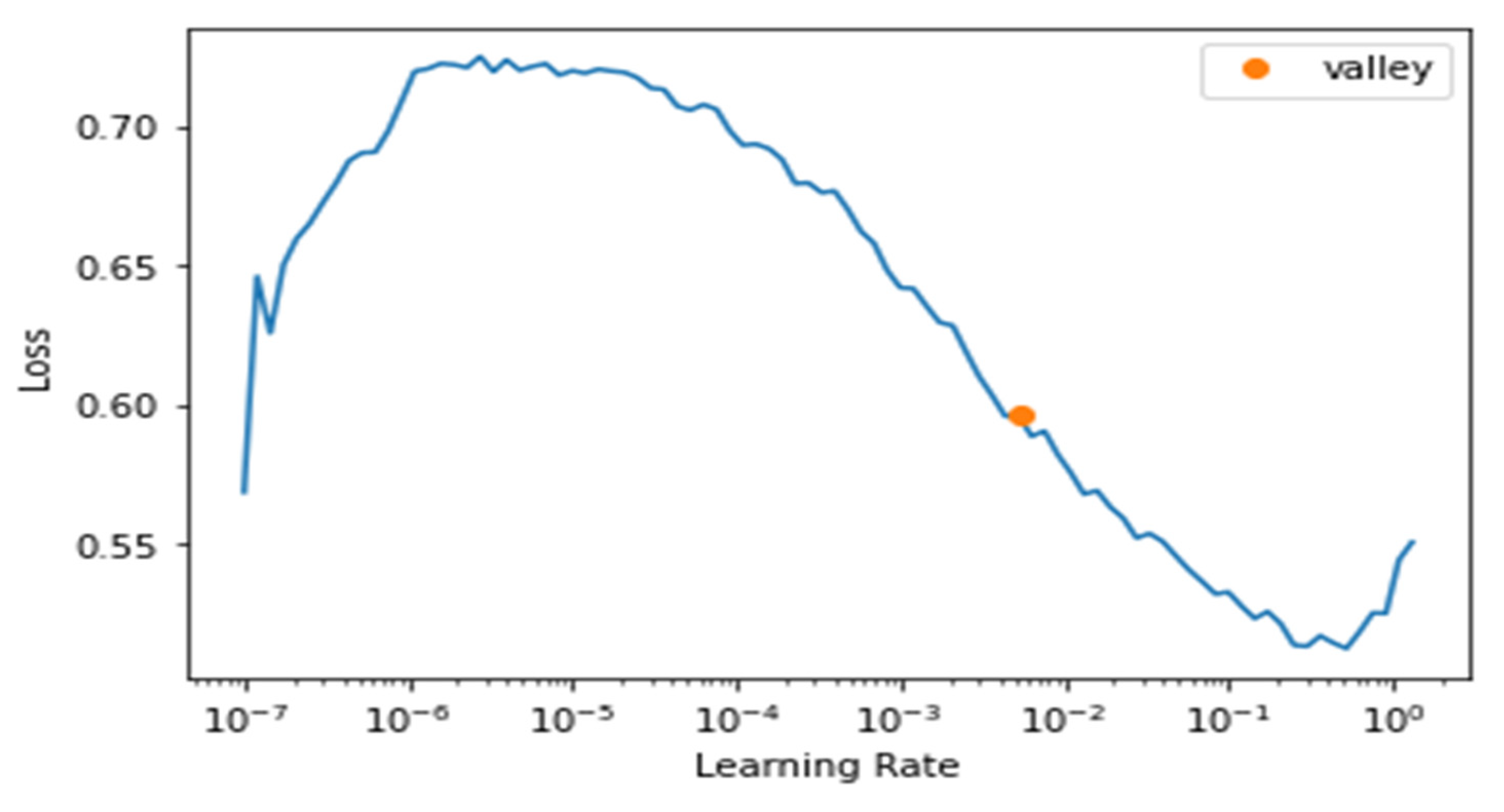
4.3. Daily Textual Logs Classification Results
5. Threats to Validity
6. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; Mchugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization: WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-sopening-remarks-at-the-media-briefing-on-covid-19-15-june-2020 (accessed on 19 June 2020).
- World Health Organization. COVID-19 Weekly Epidemiological Update. 30 November 2021. Available online: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19-30-november-2021 (accessed on 1 December 2021).
- World Health Organization. Laboratory Testing for Coronavirus Disease 2019 (COVID-19) in Suspected Human Cases: Interim Guidance. 2020. Available online: https://apps.who.int/iris/handle/10665/331329 (accessed on 23 November 2021).
- Wang, S.; Kang, B.; Ma, J.; Zeng, X.; Xiao, M.; Guo, J.; Cai, M.; Yang, J.; Li, Y.; Meng, X.; et al. A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19). Eur. Radiol. 2021, 31, 6096–6104. [Google Scholar] [CrossRef]
- Jin, C.; Chen, W.; Cao, Y.; Xu, Z.; Tan, Z.; Zhang, X.; Deng, L.; Zheng, C.; Zhou, J.; Shi, H.; et al. development and evaluation of an artificial intelligence system for COVID-19 diagnosis. Nat. Commun. 2020, 11, 5088. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Grassi, R.; Granata, V.; Setola, S.V.; Grassi, F.; Cozzi, D.; Pecori, B.; Izzo, F.; Petrillo, A. Artificial Intelligence and COVID-19 Using Chest CT Scan and Chest X-ray Images: Machine Learning and Deep Learning Approaches for Diagnosis and Treatment. J. Pers. Med. 2021, 11, 993. [Google Scholar] [CrossRef]
- Banaee, H.; Ahmed, M.U.; Loutfi, A. Data Mining for Wearable Sensors in Health Monitoring Systems: A Review of Recent Trends and Challenges. Sensors 2013, 13, 17472–17500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smarr, B.L.; Aschbacher, K.; Fisher, S.M.; Chowdhary, A.; Dilchert, S.; Puldon, K.; Rao, A.; Hecht, F.M.; Mason, A.E. Feasibility of continuous fever monitoring using wearable devices. Sci. Rep. 2020, 10, 21640. [Google Scholar] [CrossRef] [PubMed]
- Hirten, R.P.; Danieletto, M.; Tomalin, L.; Choi, K.H.; Zweig, M.; Golden, E.; Kaur, S.; Helmus, D.; Biello, A.; Pyzik, R.; et al. Use of physiological data from a wearable device to identify SARS-CoV-2 infection and symptoms and predict COVID-19 diagnosis: Observational study. J. Med. Internet Res. 2021, 23, e26107. [Google Scholar] [CrossRef] [PubMed]
- Hasty, F.; García, G.; Dávila, C.H.; Wittels, S.H.; Hendricks, S.; Chong, S. Heart Rate Variability as a Possible Predictive Marker for Acute Inflammatory Response in COVID-19 Patients. Mil. Med. 2020, 186, e34–e38. [Google Scholar] [CrossRef]
- Sadeghi-Haddad-Zavareh, M.; Bayani, M.; Shokri, M.; Ebrahimpour, S.; Babazadeh, A.; Mehraeen, R.; Moudi, E.; Rostami, A.; Barary, M.; Hosseini, A.; et al. C-Reactive Protein as a Prognostic Indicator in COVID-19 Patients. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 5557582. [Google Scholar] [CrossRef]
- Wang, L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020, 50, 332–334. [Google Scholar] [CrossRef]
- Matthias, A.T.; Indrakumar, J.; Gunatilake, S.B. Ventricular trigeminy in a patient with serologically confirmed dengue haemorrhagic fever. Int. Arch. Med. 2014, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, J.; Gugger, S. Fastai: A Layered API for Deep Learning. Information 2020, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Rajpurkar, P.; Irvin, J.; Zhu, K.; Yang, B.; Mehta, H.; Duan, T.; Ding, D.; Bagul, A.; Langlotz, C.; Shpanskaya, K.; et al. Chexnet: Radiologist-level pneumonia detection on chest x-rays with deep learning. arXiv 2017, arXiv:1711.05225. [Google Scholar]
- Channa, A.; Popescu, N.; Skibinska, J.; Burget, R. The Rise of Wearable Devices during the COVID-19 Pandemic: A Systematic Review. Sensors 2021, 21, 5787. [Google Scholar] [CrossRef]
- Grzesiak, E.; Bent, B.; McClain, M.T.; Woods, C.W.; Tsalik, E.L.; Nicholson, B.P.; Veldman, T.; Burke, T.W.; Gardener, Z.; Bergstrom, E.; et al. Assessment of the Feasibility of Using Noninvasive Wearable Biometric Monitoring Sensors to Detect Influenza and the Common Cold Before Symptom Onset. JAMA Netw. Open 2021, 4, e2128534. [Google Scholar] [CrossRef] [PubMed]
- Marinsek, N.; Shapiro, A.; Clay, I.; Bradshaw, B.; Ramirez, E.; Min, J.; Trister, A.; Wang, Y.; Althoff, T.; Foschini, L. Measuring COVID-19 and Influenza in the Real World via Person-Generated Health Data. medRxiv 2020, 1–23. [Google Scholar] [CrossRef]
- Zhu, G.; Li, J.; Meng, Z.; Yu, Y.; Li, Y.; Tang, X.; Dong, Y.; Sun, G.; Zhou, R.; Wang, H.; et al. Learning from Large-Scale Wearable Device Data for Predicting the Epidemic Trend of COVID-19. Discret. Dyn. Nat. Soc. 2020, 2020, 6152041. [Google Scholar] [CrossRef]
- Mishra, T.; Wang, M.; Metwally, A.A.; Bogu, G.K.; Brooks, A.W.; Bahmani, A.; Alavi, A.; Celli, A.; Higgs, E.; Dagan-Rosenfeld, O.; et al. Early Detection Of COVID-19 Using A Smartwatch. medRxiv 2020. [Google Scholar] [CrossRef]
- Giovannini, G.; Haick, H.; Garoli, D. Detecting COVID-19 from Breath: A Game Changer for a Big Challenge. ACS Sens. 2021, 6, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Fedorin, I.; Slyusarenko, K.; Nastenko, M. Respiratory events screening using consumer smartwatches. In Proceedings of the 2020 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2020 ACM International Symposium on Wearable Computers, Virtual, Mexico, 12–17 September 2020; pp. 25–28. [Google Scholar]
- Miller, D.J.; Capodilupo, J.V.; Lastella, M.; Sargent, C.; Roach, G.D.; Lee, V.H.; Capodilupo, E.R. Analyzing changes in respiratory rate to predict the risk of COVID-19 infection. PLoS ONE 2020, 15, e0243693. [Google Scholar] [CrossRef] [PubMed]
- Quer, G.; Radin, J.M.; Gadaleta, M.; Baca-Motes, K.; Ariniello, L.; Ramos, E.; Kheterpal, V.; Topol, E.J.; Steinhubl, S.R. Wearable sensor data and self-reported symptoms for COVID-19 detection. Nat. Med. 2020, 27, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.C.; Yetisen, A.K.; Güder, F.; Dincer, C. Wearable devices for the detection of COVID-19. Nat. Electron. 2021, 4, 13–14. [Google Scholar] [CrossRef]
- Chung, Y.T.; Yeh, C.Y.; Shu, Y.C.; Chuang, K.T.; Chen, C.C.; Kao, H.Y.; Ko, W.-C.; Chen, P.-L.; Ko, N.Y. Continuous temperature monitoring by a wearable device for early detection of febrile events in the SARS-CoV-2 outbreak in Taiwan, 2020. J. Microbiol. Immunol. Infect. 2020, 53, 503. [Google Scholar] [CrossRef]
- Hassantabar, S.; Stefano, N.; Ghanakota, V.; Ferrari, A.; Nicola, G.N.; Bruno, R.; Marino, I.R.; Hamidouche, K.; Jha, N.K. CovidDeep: SARS-CoV-2/COVID-19 Test Based on Wearable Medical Sensors and Efficient Neural Networks. arXiv 2020, arXiv:2007.10497. [Google Scholar] [CrossRef]
- Ashraf, M.U.; Hannan, A.; Cheema, S.M.; Ali, Z.; Alofi, A. Detection and tracking contagion using IoT-edge technologies: Confronting COVID-19 pandemic. In Proceedings of the 2020 International Conference on Electrical, Communication, and Computer Engineering (ICECCE), Istanbul, Turkey, 12–13 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–6. [Google Scholar]
- Lonini, L.; Shawen, N.; Botonis, O.; Fanton, M.; Jayaraman, C.; Mummidisetty, C.K.; Shin, S.Y.; Rushin, C.; Jenz, S.; Xu, S.; et al. Rapid Screening of Physiological Changes Associated With COVID-19 Using Soft-Wearables and Structured Activities: A Pilot Study. IEEE J. Transl. Eng. Health Med. 2021, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al Bassam, N.; Hussain, S.A.; Al Qaraghuli, A.; Khan, J.; Sumesh, E.; Lavanya, V. IoT based wearable device to monitor the signs of quarantined remote patients of COVID-19. Informatics Med. Unlocked 2021, 24, 100588. [Google Scholar] [CrossRef] [PubMed]
- Khalpey, Z.I.; Khalpey, A.H.; Modi, B.; Deckwa, J. Autonomic Dysfunction in COVID-19: Early Detection and Prediction Using Heart Rate Variability. J. Am. Coll. Surg. 2021, 233, e20–e21. [Google Scholar] [CrossRef]
- Ponomarev, A.; Tyapochkin, K.; Surkova, E.; Smorodnikova, E.; Pravdin, P. Heart Rate Variability as a Prospective Predictor of Early COVID-19 Symptoms. medRxiv 2021. [Google Scholar] [CrossRef]
- COVID-19 and Wearables Open Data Research. [Data Set]. 2020. Available online: https://github.com/Welltory/hrv-covid19 (accessed on 10 November 2021).
- Pinheiro, N.; Couceiro, R.; Henriques, J.; Muehlsteff, J.; Quintal, I.; Goncalves, L.; Carvalho, P. Can PPG be used for HRV analysis? In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 2945–2949. [Google Scholar]
- Bhowmik, T.; Dey, J.; Tiwari, V.N. A novel method for accurate estimation of HRV from smartwatch PPG signals. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 109–112. [Google Scholar]
- Minaee, S.; Kafieh, R.; Sonka, M.; Yazdani, S.; Soufi, G.J. Deep-COVID: Predicting COVID-19 from chest X-ray images using deep transfer learning. Med. Image Anal. 2020, 65, 101794. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.P.; Dao, L.; Roth, K.; Morrison, P.; Bengio, Y.; Abbasi, A.F.; Shen, B.; Mahsa, H.K.; Ghassemi, M.; Li, H.; et al. Predicting COVID-19 Pneumonia Severity on Chest X-ray With Deep Learning. Cureus 2020, 12, e9448. [Google Scholar] [CrossRef] [PubMed]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagmair, S.; Bachler, M.; Wassertheurer, S.; Mayer, C. Nonlinear Methods in Heart Rate Variability: Can they Distinguish between Nonpathological and Pathological Subjects? SNE Simul. Notes Eur. 2015, 25, 145–150. [Google Scholar] [CrossRef]
- Williams, D.P.; Koenig, J.; Carnevali, L.; Sgoifo, A.; Jarczok, M.N.; Sternberg, E.M.; Thayer, J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain, Behav. Immun. 2019, 80, 219–226. [Google Scholar] [CrossRef]
- Porto, L.G.G.; Junqueira, L.F., Jr. Comparison of time-domain short-term heart interval variability analysis using a wrist-worn heart rate monitor and the conventional electrocardiogram. Pacing Clin. Electrophysiol. 2009, 32, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.A. Statistical Models: Theory and Practice; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Vergara, J.R.; Estévez, P.A. A review of feature selection methods based on mutual information. Neural Comput. Appl. 2013, 24, 175–186. [Google Scholar] [CrossRef]
- Merity, S.; Keskar, N.S.; Socher, R. Regularizing and optimizing LSTM language models. arXiv 2017, arXiv:1708.02182. [Google Scholar]
- Tanwar, G.; Chauhan, R.; Singh, M.; Singh, D. Pre-Emption of Affliction Severity Using HRV Measurements from a Smart Wearable; Case-Study on SARS-Cov-2 Symptoms. Sensors 2020, 20, 7068. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. Model-agnostic interpretability of machine learning. arXiv 2016, arXiv:1606.05386. [Google Scholar]
- Pan, Y.; Yu, Z.; Yuan, Y.; Han, J.; Wang, Z.; Chen, H.; Jiang, H. Alteration of autonomic nervous system is associated with severity and outcomes in patients with COVID-19. Front. Physiol. 2021, 12, 630038. [Google Scholar] [CrossRef] [PubMed]










| Source | Data | Details |
|---|---|---|
| Welltory Mobile Application | HRV | Daily readings of beats per minute and HRV features such as SDNN, RMSSD, pNN50, COVID-19 onset date. Moreover, textual tags were provided by patients about their status daily. |
| Blood pressure | Diastolic and systolic readings, functional change index. | |
| Heart Rate | Beats per minute readings, and a binary answer (whether heart rate was measured at rest). | |
| Surveys | COVID symptoms such as cough assessment, fever, breath shortness, fatigue, etc. | |
| Wearables | Physiological metrics and fitness data | Resting heart rate, heart rate, oxygen saturation, steps count, walking distances. |
| Sleep data | Sleep begins and ends, sleep duration, light, and deep sleep information. |
| Modality | Model | Accuracy | Precision | Recall | F1-Score |
|---|---|---|---|---|---|
| HRV features + feeling assessment | SVM | 83.34 ± 1.68% | 0.91 | 0.88 | 0.89 |
| KNN | 83.06 ± 1.99% | 0.80 | 0.80 | 0.80 | |
| Decision Tree | 74.28 ± 0.613% | 0.80 | 0.79 | 0.79 | |
| Logistic Regression | 78.93 ± 2.34% | 0.80 | 0.80 | 0.79 | |
| HRV features only | SVM | 78.85 ± 3.04% | 0.79 | 0.81 | 0.78 |
| KNN | 80.17 ± 0.28% | 0.75 | 0.75 | 0.75 | |
| Decision Tree | 76.30 ± 0.98% | 0.71 | 0.71 | 0.71 | |
| Logistic Regression | 79.75 ± 4.31% | 0.79 | 0.79 | 0.79 | |
| Feeling assessment only | SVM | 65.38 ± 8.21% | 0.66 | 0.67 | 0.65 |
| KNN | 41.74 ± 9.68% | 0.46 | 0.47 | 0.41 | |
| Decision Tree | 50.58 ± 7.03% | 0.57 | 0.55 | 0.53 | |
| Logistic Regression | 58.68 ± 11.28% | 0.27 | 0.50 | 0.35 |
| Epoch | Train Loss | Valid Loss | Accuracy |
|---|---|---|---|
| 0 | 0.362183 | 0.264590 | 0.925373 |
| 1 | 0.355476 | 1.288716 | 0.686567 |
| 2 | 0.461005 | 0.675994 | 0.791045 |
| 3 | 0.447026 | 0.774474 | 0.805970 |
| 4 | 0.420781 | 0.499611 | 0.805970 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijazi, H.; Abu Talib, M.; Hasasneh, A.; Bou Nassif, A.; Ahmed, N.; Nasir, Q. Wearable Devices, Smartphones, and Interpretable Artificial Intelligence in Combating COVID-19. Sensors 2021, 21, 8424. https://doi.org/10.3390/s21248424
Hijazi H, Abu Talib M, Hasasneh A, Bou Nassif A, Ahmed N, Nasir Q. Wearable Devices, Smartphones, and Interpretable Artificial Intelligence in Combating COVID-19. Sensors. 2021; 21(24):8424. https://doi.org/10.3390/s21248424
Chicago/Turabian StyleHijazi, Haytham, Manar Abu Talib, Ahmad Hasasneh, Ali Bou Nassif, Nafisa Ahmed, and Qassim Nasir. 2021. "Wearable Devices, Smartphones, and Interpretable Artificial Intelligence in Combating COVID-19" Sensors 21, no. 24: 8424. https://doi.org/10.3390/s21248424
APA StyleHijazi, H., Abu Talib, M., Hasasneh, A., Bou Nassif, A., Ahmed, N., & Nasir, Q. (2021). Wearable Devices, Smartphones, and Interpretable Artificial Intelligence in Combating COVID-19. Sensors, 21(24), 8424. https://doi.org/10.3390/s21248424








