The Effect of Implanted Functional Electrical Stimulation on Gait Performance in Stroke Survivors: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
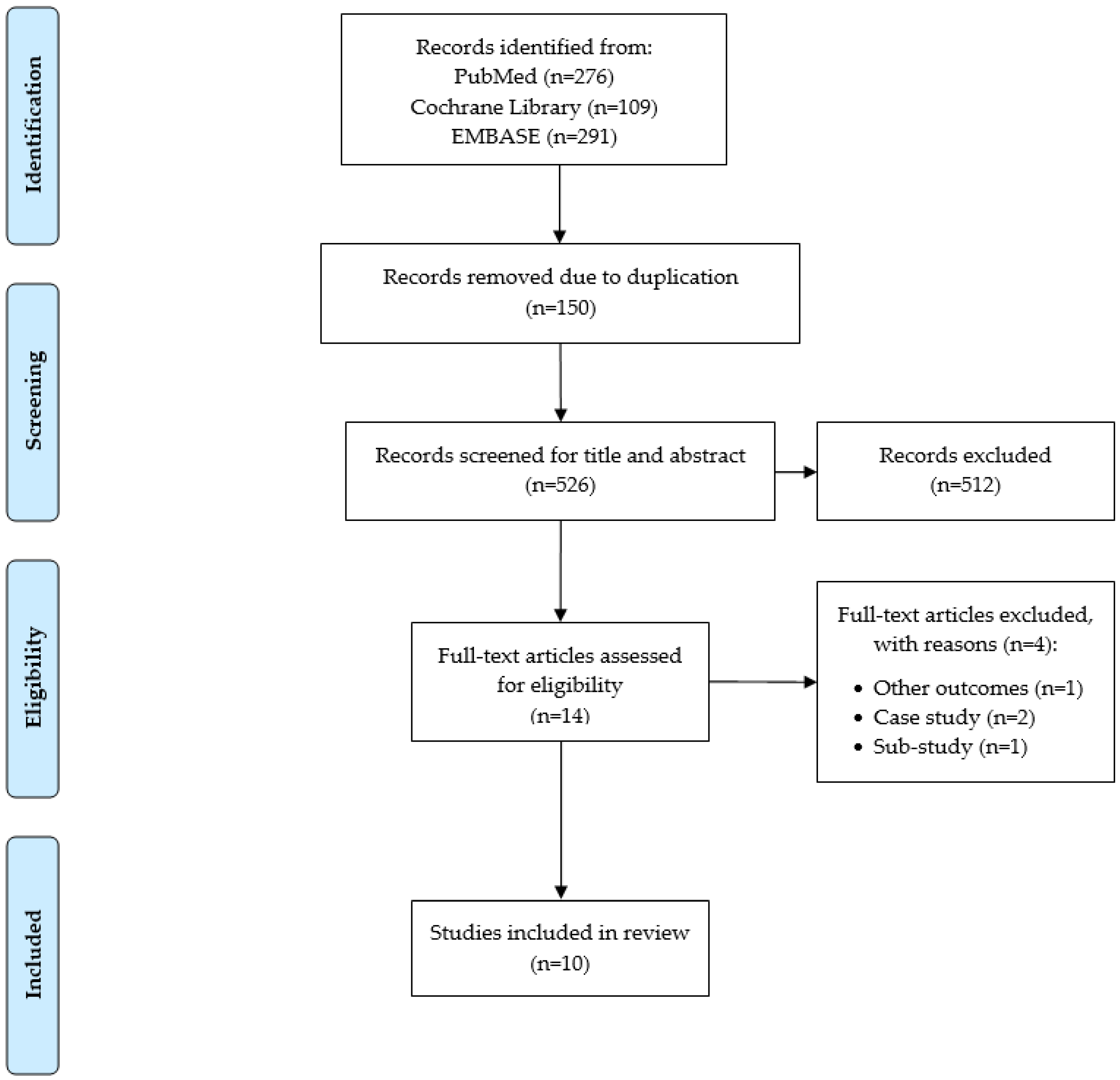
3.1. Search Results
3.2. Study Design and Countries
3.3. Characteristics of Stroke Survivors
3.4. Intervention
3.5. Gait Outcomes
3.6. Summary of Key Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. Available online: https://pubmed.ncbi.nlm.nih.gov/31992061/ (accessed on 9 December 2021). [CrossRef]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L. Forecasting the future of stroke in the United States: A policy statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. Available online: https://pubmed.ncbi.nlm.nih.gov/33501848/ (accessed on 9 December 2021). [CrossRef] [PubMed]
- Henriksson, K.M.; Farahmand, B.; Åsberg, S.; Edvardsson, N.; Terént, A. Comparison of cardiovascular risk factors and survival in patients with ischemic or hemorrhagic stroke. Int. J. Stroke 2012, 7, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E.S.; Coshall, C.; Dundas, R.; Stewart, J.; Rudd, A.G.; Howard, R.; Wolfe, C.D. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 2001, 32, 1279–1284. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H.; Bok, S.K.; Kim, Y.-J.; Hwang, S.L. Effect of lower limb strength on falls and balance of the elderly. Ann. Rehabil. Med. 2012, 36, 386. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Sonoda, S.; Misawa, K.; Saitoh, E.; Shimizu, Y.; Kotake, T. Incidence and consequence of falls in inpatient rehabilitation of stroke patients. Exp. Aging Res. 2005, 31, 457–469. [Google Scholar] [CrossRef]
- Mackintosh, S.F.; Hill, K.D.; Dodd, K.J.; Goldie, P.A.; Culham, E.G. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch. Phys. Med. Rehabil. 2006, 87, 1583–1589. [Google Scholar] [CrossRef]
- Teasell, R.; McRae, M.; Foley, N.; Bhardwaj, A. The incidence and consequences of falls in stroke patients during inpatient rehabilitation: Factors associated with high risk. Arch. Phys. Med. Rehabil. 2002, 83, 329–333. [Google Scholar] [CrossRef]
- Schmid, A.A.; Rittman, M. Fear of falling: An emerging issue after stroke. Top. Stroke Rehabil. 2007, 14, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Murphy, T.K.; Kunik, M.E.; Badr, H.J.; Workeneh, B.T.; Yellapragada, S.V.; Sada, Y.H.; Najafi, B. The detrimental association between fear of falling and motor performance in older cancer patients with chemotherapy-induced peripheral neuropathy. Gait Posture 2021, 88, 161–166. [Google Scholar] [CrossRef]
- Kang, G.E.; Najafi, B. Sensor-based daily physical activity: Towards prediction of the level of concern about falling in peripheral neuropathy. Sensors 2020, 20, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, L.T.; Camargo, L.B.A.; Estarlino, L.D.; Teixeira-Salmela, L.F.; de Morais Faria, C.D.C. Strength of the lower limb and trunk muscles is associated with gait speed in individuals with sub-acute stroke: A cross-sectional study. Braz. J. Phys. Ther. 2018, 22, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kluding, P.M.; Dunning, K.; O’Dell, M.W.; Wu, S.S.; Ginosian, J.; Feld, J.; McBride, K. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke 2013, 44, 1660–1669. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.; Adcock, L. Foot Drop Stimulators for Foot Drop: A Review of Clinical, Cost-Effectiveness, and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537874/ (accessed on 9 December 2021).
- Liberson, W. Functional electrotherapy: Stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch. Phys. Med. 1961, 42, 101–105. [Google Scholar]
- Melo, P.; Silva, M.; Martins, J.; Newman, D. Technical developments of functional electrical stimulation to correct drop foot: Sensing, actuation and control strategies. Clin. Biomech. 2015, 30, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulley, C.; Mercer, T.H.; Hooper, J.E.; Cowan, P.; Scott, S.; van der Linden, M.L. Experiences of functional electrical stimulation (FES) and ankle foot orthoses (AFOs) for foot-drop in people with multiple sclerosis. Disabil. Rehabil. Assist. Technol. 2015, 10, 458–467. [Google Scholar] [CrossRef]
- Yao, D.; Jakubowitz, E.; Tecante, K.; Lahner, M.; Ettinger, S.; Claassen, L.; Plaass, C.; Stukenborg-Colsman, C.; Daniilidis, K. Restoring mobility after stroke: First kinematic results from a pilot study with a hybrid drop foot stimulator. Musculoskelet. Surg. 2016, 100, 223–229. [Google Scholar] [CrossRef]
- van Swigchem, R.; van Duijnhoven, H.J.; den Boer, J.; Geurts, A.C.; Weerdesteyn, V. Effect of peroneal electrical stimulation versus an ankle-foot orthosis on obstacle avoidance ability in people with stroke-related foot drop. Phys. Ther. 2012, 92, 398–406. [Google Scholar] [CrossRef]
- Kenney, L.; Bultstra, G.; Buschman, R.; Taylor, P.; Mann, G.; Hermens, H.; Holsheimer, J.; Nene, A.; Tenniglo, M.; Van der Aa, H. An implantable two channel drop foot stimulator: Initial clinical results. Artif. Organs 2002, 26, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.; Haugland, M.; Larsen, B.; Pickering, R.M.; Svaneborg, N.; Iversen, H.K.; Christensen, P.B.; Haase, J.; Brennum, J.; Sinkjaer, T. Phase II trial to evaluate the ActiGait implanted drop-foot stimulator in established hemiplegia. J. Rehabil. Med. 2007, 39, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottink, A.I.; Hermens, H.J.; Nene, A.V.; Tenniglo, M.J.; van der Aa, H.E.; Buschman, H.P.; IJzerman, M.J. A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch. Phys. Med. Rehabil. 2007, 88, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Kottink, A.I.; Tenniglo, M.J.; de Vries, W.H.; Hermens, H.J.; Buurke, J.H. Effects of an implantable two-channel peroneal nerve stimulator versus conventional walking device on spatiotemporal parameters and kinematics of hemiparetic gait. J. Rehabil. Med. 2012, 44, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Ernst, J.; Grundey, J.; Hewitt, M.; von Lewinski, F.; Kaus, J.; Schmalz, T.; Rohde, V.; Liebetanz, D. Towards physiological ankle movements with the ActiGait implantable drop foot stimulator in chronic stroke. Restor. Neurol. Neurosci. 2013, 31, 557–569. [Google Scholar]
- Schiemanck, S.; Berenpas, F.; van Swigchem, R.; van den Munckhof, P.; de Vries, J.; Beelen, A.; Nollet, F.; Geurts, A.C. Effects of implantable peroneal nerve stimulation on gait quality, energy expenditure, participation and user satisfaction in patients with post-stroke drop foot using an ankle-foot orthosis. Restor. Neurol. Neurosci. 2015, 33, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.D.; Polanski, W.H.; Schulz, A.-K.; Jöbges, M.; Hoff, H.; Schackert, G.; Pinzer, T.; Sobottka, S.B. Restoration of ankle movements with the ActiGait implantable drop foot stimulator: A safe and reliable treatment option for permanent central leg palsy. J. Neurosurg. 2016, 124, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniilidis, K.; Jakubowitz, E.; Thomann, A.; Ettinger, S.; Stukenborg-Colsman, C.; Yao, D. Does a foot-drop implant improve kinetic and kinematic parameters in the foot and ankle? Arch. Orthop. Trauma Surg. 2017, 137, 499–506. [Google Scholar] [CrossRef]
- Berenpas, F.; Schiemanck, S.; Beelen, A.; Nollet, F.; Weerdesteyn, V.; Geurts, A. Kinematic and kinetic benefits of implantable peroneal nerve stimulation in people with post-stroke drop foot using an ankle-foot orthosis. Restor. Neurol. Neurosci. 2018, 36, 547–558. [Google Scholar] [CrossRef]
- Bucklitsch, J.; Müller, A.; Weitner, A.; Filmann, N.; Patriciu, A.; Behmanesh, B.; Seifert, V.; Marquardt, G.; Quick-Weller, J. Significant impact of implantable functional electrical stimulation on gait parameters: A kinetic analysis in foot drop patients. World Neurosurg. 2019, 127, e236–e241. [Google Scholar] [CrossRef]
- Buentjen, L.; Kupsch, A.; Galazky, I.; Frantsev, R.; Heinze, H.-J.; Voges, J.; Hausmann, J.; Sweeney-Reed, C.M. Long-term outcomes of semi-implantable functional electrical stimulation for central drop foot. J. Neuroeng. Rehabil. 2019, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.E.; Yang, J.; Najafi, B. Does the presence of cognitive impairment exacerbate the risk of falls in people with peripheral neuropathy? An application of body-worn inertial sensors to measure gait variability. Sensors 2020, 20, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.E.; Naik, A.D.; Ghanta, R.K.; Rosengart, T.K.; Najafi, B. A Wrist-Worn Sensor-Derived Frailty Index Based on an Upper-Extremity Functional Test in Predicting Functional Mobility in Older Adults. Gerontology 2021, 67, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Perna, R.; Temple, J. Rehabilitation outcomes: Ischemic versus hemorrhagic strokes. Behav. Neurol. 2015, 2015, 891651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katrak, P.H.; Black, D.; Peeva, V. Do stroke patients with intracerebral hemorrhage have a better functional outcome than patients with cerebral infarction? PMR 2009, 1, 427–433. [Google Scholar] [CrossRef]
- Cheng, D.K.; Nelson, M.; Brooks, D.; Salbach, N.M. Validation of stroke-specific protocols for the 10-meter walk test and 6-minute walk test conducted using 15-meter and 30-meter walkways. Top. Stroke Rehabil. 2020, 27, 251–261. [Google Scholar] [CrossRef] [PubMed]
| PubMed Search | |
|---|---|
| “Electric Stimulation” [MeSH Terms] OR “Electric Stimulation Therapy” [MeSH Terms] OR “Electrical Stimulation” [Text Word] OR “Foot Drop Stimulator” [Text Word] OR “Peroneal Nerve Stimulator” [Text Word] | |
| AND | “Walking” [MeSH Terms] OR “Gait” [MeSH Terms] OR “Locomotion” [MeSH Terms] OR “Gait Parameters” [Text Word] OR “Gait Speed” [Text Word] OR “Gait Stability” [Text Word] OR “Gait Initiation” [Text Word] OR “Gait Kinematics” [Text Word] OR “Gait Kinetics” [Text Word] OR “Ankle Power” [Text Word] OR “Mobility” [Text Word] |
| AND | “Stroke” [MeSH Terms] OR “Stroke Rehabilitation” [MeSH Terms] OR “Stroke Rehabilitation” [Text Word] OR “Stroke Survivors” [Text Word] OR “Post Stroke” [Text Word] OR “Poststroke” [Text Word] OR “Post-stroke” [Text Word] |
| Cochrane Library Search | |
| MeSH descriptor: [Electric Stimulation] explode all trees OR MeSH descriptor: [Electric Stimulation Therapy] explode all trees OR (Foot Drop Stimulator):ti,ab,kw OR (Peroneal Nerve Stimulator):ti,ab,kw | |
| AND | MeSH descriptor: [Gait] explode all trees OR MeSH descriptor: [Gait] explode all trees OR MeSH descriptor: [Locomotion] explode all trees OR (Gait Parameters):ti,ab,kw OR (Gait Speed):ti,ab,kw OR (Gait Stability):ti,ab,kw OR (Gait Initiation):ti,ab,kw OR (Gait Kinematics):ti,ab,kw OR (Ankle Power):ti,ab,kw OR (Gait Kinetics):ti,ab,kw OR (Mobility):ti,ab,kw OR |
| AND | MeSH descriptor: [Stroke] explode all trees OR MeSH descriptor: [Stroke Rehabilitation] explode all trees OR (Stroke Survivor):ti,ab,kw OR (Post Stroke):ti,ab,kw OR (Post-stroke):ti,ab,kw OR (Poststroke):ti,ab,kw |
| EMBASE Search | |
| ‘Electrostimulation’/exp OR Electrostimulation | |
| AND | ‘Gait’/exp OR Gait OR ‘Walking’/exp OR Walking OR ‘Locomotion’/exp OR Locomotion) |
| AND | ‘Stroke’/exp OR Stroke |
| Study Country Study Design | Participant Characteristics Follow-Up | Electrical Stimulator Stimulation Specification (Voltage, Current, Phase Duration, Frequency) | Surgical Procedure Surgery-Related Adverse Events | Gait Outcomes | Key Results |
|---|---|---|---|---|---|
| Burridge et al. 2007 Denmark Single arm trial | Baseline n = 15 (4 men; 11 women) Mean age (years) = 56.8 ± 7.6 Side of hemiplegia (7 right; 8 left) Type of stroke (8 ischemic; 5 hemorrhage; 1 unknown) Time since stroke (years) = 4.9 ± 1.9 90-day (n = 13) 15-month (n = 13) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation specification not reported | Spinal anesthesia (n = 8) General anesthesia (n = 7) Implant location (the electrode cuff): Just proximal to the common peroneal nerve’s bifurcation into the deep and superficial branches to the tibialis anterior and the peronei muscles Stimulator location: 1 or 2 sutures to the lateral femoral fascia A longitudinal incision along the tendon of the biceps femoris Second incision: The lateral side of the femur, posterior to the location for the simulator body Minor wound infections (n = 2; treated with antibiotics) Delayed wound healing (n = 1) | 4-min walk distance Walking speed (20-m) | Compared to baseline: At 90-day 4-min walk distance ↑ (p ≥ 0.05) Walking speed ↑ (p < 0.05) At 15-month 4-min walk distance ↑ (p < 0.05) Walking speed ↑ (p < 0.05) |
| Kottink et al. 2007 The Netherlands Randomized controlled trial | Intervention group (IG) Baseline n = 14 (10 men; 4 women) Mean age (years) = 55.2 ± 11.4 Side of hemiplegia (7 right; 7 left) Type of stroke (not reported) Time since stroke (years) = 9.1 ± 9.3 Control group (CG) Baseline n = 15 (10 men; 5 women) Mean age (years) = 52.9 ± 9.9 Side of hemiplegia (9 right; 6 left) Type of stroke (not reported) Time since stroke (years) = 9.1 ± 9.3 4-week (n = 14 in IG; n = 12 in CG) 8-week (n = 14 in IG; n = 12 in CG) 12-week (n = 14 in IG; n = 11 in CG) 26-week (n = 14 in IG; n = 11 in CG) | IG 2-channel peroneal nerve stimulator; an external transmitter; a foot switch Stimulation pulse: Asymmetric biphasic charge-balanced-waveform (30 Hz); No other information reported CG Ankle–foot orthosis, orthopedic shoes, or no device | Spinal or general anesthesia (number of participants not reported) Implant location: One electrode was placed under the epineurium of the superficial peroneal nerve The other electrode was placed under the epineurium of the deep peroneal nerve The receiver body was placed ina subcutaneous pocket Incision: Approximately 50 mm along the common peroneal nerve Adverse events not reported | 6-min walk distance Walking speed (10-m) Other outcomes: 5-day activity level | Compared to baseline (IG): At 4-week No 6-min walk distance results Walking speed ↓ (p-value not reported) No activity level results At 8-week No 6-min walk distance results Walking speed ↑ (p-value not reported) No activity level results At 12-week 6-min walk distance ↑ (p-value not reported) Walking speed ↑ (p-value not reported) No activity level results At 26-week 6-min walk distance ↑ (p-value not reported) Walking speed ↑ (p < 0.05) % Duration of walking ↓ (p < 0.05) % Duration of standing ↓ (p ≥ 0.05) % Duration of sitting/lying ↑ (p < 0.05) Note: At every follow-up, IG had greater 6-min walk distance and walking speed than CG. |
| Kottink et al. 2012 The Netherlands Randomized controlled trial | IG Baseline n = 10 (7 men; 3 women) Mean age (years) = 55.6 ± 13.2 Side of hemiplegia (4 right; 6 left) Type of stroke (not reported) Time since stroke (years) = 9.0 ± 10.0 CG Baseline n = 13 (8 men; 5 women) Mean age (years) = 53.3 ± 10.6 Side of hemiplegia (5 right; 8 left) Type of stroke (not reported) Time since stroke (years) = 6.2 ± 4.8 26-week (n = 9 in IG; n = 12 in CG) Note: Participants are a subset of Kottink et al. (2007). | Same as Kottink et al. (2007) | Surgical procedure is same as Kottink et al. (2007) | Walking speed (10-m) Stride time Stride width Step length (paretic side and non-paretic side) Stance phase (paretic side and non-paretic side) Double support phase (paretic side and non-paretic side) Single support phase (paretic side and non-paretic side) Sagittal hip range of motion Sagittal knee range of motion Sagittal ankle range of motion | Compared to baseline (IG): At 26-week Walking speed ↑ (p ≥ 0.05) Stride time ↓ (p < 0.05) Stride width no change Step length (paretic side) ↓ (p ≥ 0.05) Step length (non-paretic side) ↑ (p ≥ 0.05) Stance phase (paretic side) ↓ (p ≥ 0.05) Stance phase (non-paretic side) ↓ (p ≥ 0.05) Double support phase (paretic side) ↓ (p ≥ 0.05) Double support phase (non-paretic side) ↓ (p ≥ 0.05) Single support phase (paretic side) ↑ (p ≥ 0.05) Single support phase (non-paretic side) ↑ (p ≥ 0.05) Sagittal hip range of motion ↑ (p ≥ 0.05) Sagittal knee range of motion ↓ (p ≥ 0.05) Sagittal ankle range of motion ↓ (p ≥ 0.05) |
| Ernst et al. 2013 Germany Single arm trial | Baseline n = 5 (3 men; 2 women) Mean age (years) = 47.2 (standard deviation not reported) Side of hemiplegia (3 right; 2 left) Type of stroke (not reported) Time since stroke (years) = 5.6 (standard deviation not reported) 6-week (n = 5) 12-week (n = 5) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation specification not reported | General anesthesia (n = 5) Surgical procedure is same as Burridge et al. (2007) Serious adverse event (n = 1; a hematoma around the distal incision and bleeding after removing stitches) Post-surgical lymphoedema around the proximal incision (n = 1); Both participants were included in the study | 6-min walk distance Walking speed (10-m) Sagittal ankle angle | Compared to baseline: At 6-week 6-min walk distance ↑ (p-value not reported) Walking speed ↑ (p-value not reported) Sagittal ankle angle not assessed At 12-week 6-min walk distance ↑ (p-value not reported) Walking speed ↑ (p-value not reported) Sagittal ankle angle at heel strike and during loading-phase ↓ (p < 0.05) Sagittal ankle angle at mid-stance and during pre-swing ↓ (p ≥ 0.05) |
| Schiemanck et al. 2015 The Netherlands Single arm trial | Baseline n = 10 (5 men; 5 women) Mean age (years) = 47.4 ± 14.5 Side of hemiplegia (4 right; 6 left) Type of stroke (8 ischemic; 2 hemorrhage) Time since stroke (years) = 5.6 ± 2.4 2-week (n = 8) 8-week (n = 8) 26-week (n = 8) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation specification not reported Note: Participants used ankle–foot orthosis along with the electrical stimulation. | Surgical procedure not reported Adverse events not reported | Ankle plantarflexion power (15-m) | Compared to baseline: Ankle plantarflexion power ↑ at all follow-ups (p-value not reported) |
| Martin et al. 2016 Germany Single arm trial | Baseline n = 27 (14 men; 13 women) Mean age (years) = 51.0 ± 11.6 Side of hemiplegia (15 right; 12 left) Type of stroke (21 ischemic; 6 hemorrhage) Time since foot drop (years) = 5.2 ± 4.8 6-week (n = 27) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation pulse: 1 mA, 20–35 Hz, 45–330 µs pulse width Ankle dorsiflexion tested with maximum 6 volts, 10 mA, 2.5 Hz for electrode placement | General anesthesia (n = 27) Other surgical procedure is same as Burridge et al. (2007) Nerve injury (n = 2; reoperation was performed) Wound healing disorder (n = 8) Neurodermatitis and infection (n = 1) | 6-min walk distance Walking speed (20-m) Other outcomes: Duration of timed up and go | Compared to baseline: At 6-week 6-min walk distance ↑ (p < 0.05) Walking speed ↑ (p < 0.05) Duration of timed up and go ↓ (p < 0.05) |
| Daniilidis et al. 2017 Germany Single arm trial | Baseline n = 18 (12 men; 6 women) Mean age (years) = 51.3 ± 8.4 Side of hemiplegia (9 right; 9 left) Type of stroke (13 ischemic; 5 hemorrhage) Time since stroke (years) = 7.2 ± 5.2 12-month (n = 18) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation pulse: 1.1 mA, 20–30 Hz, 70 μs pulse width (initially; pusle width and timing were adjusted for each patient throughout the study) Ankle dorsiflexion tested with maximum 6 volts, 10 mA, 2.5 Hz for electrode placement | General anesthesia (n = 18) Other surgical procedure is same as Burridge et al. (2007) Adverse events not reported | Walking speed (walking distance not reported) Stride length Cadence Double support phase Ankle dorsiflexion angle Vertical ground reaction force Anterior-posterior ground reaction force | Compared to baseline: At 12-month Walking speed ↑ (p ≥ 0.05) Stride length ↑ (p ≥ 0.05) Cadence ↑ (p ≥ 0.05) Double support phase ↑ (p ≥ 0.05) Ankle dorsiflexion angle ↑ (p < 0.05) Peak vertical ground reaction force ↑ (p < 0.05) Peak anterior-posterior ground reaction force ↑ (p ≥ 0.05) |
| Berenpas et al. 2018 The Netherlands Single arm trial | Baseline n = 19 (14 men; 5 women) Mean age (years) = 54.4 ± 12.3 Side of hemiplegia (8 right; 11 left) Type of stroke (14 ischemic; 5 hemorrhage) Time since stroke (years) = 5.0 ± 3.7 2-week (n = 19) 8-week (n = 19) 26-week (n = 19) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation specification not reported Note: Participants use ankle–foot orthosis along with the electrical stimulation. | Surgical procedure is same as Burridge et al. (2007) Anesthesia information not reported Peroneal nerve damage (n = 1) Death (n = 1; Not related to surgery) Severe calf muscle clonus in reaction to electrical stimulation (n = 1) | Walking speed (10-m) Step length asymmetry Maximum hip flexion angle and velocity Maximum knee flexion angle and velocity Maximum knee extension velocity Maximum ankle plantarflexion angle, velocity, and power | Compared to 2-week (no baseline data were provided except walking speed): At 8-week Walking speed ↑ (p ≥ 0.05) Step length asymmetry ↓ (p ≥ 0.05) Maximum hip flexion angle ↑ (p < 0.05) Maximum hip flexion velocity ↓ (p ≥ 0.05) Maximum knee flexion angle ↑ (p < 0.05) Maximum knee flexion velocity ↑ (p ≥ 0.05) Maximum knee extension velocity ↑ (p < 0.05) Maximum ankle plantarflexion angle ↑ (p ≥ 0.05) Maximum ankle plantarflexion velocity ↑ (p ≥ 0.05) Maximum ankle plantarflexion power ↑ (p ≥ 0.05) At 26-week Walking speed ↑ (p ≥ 0.05) Step length asymmetry ↑ (p ≥ 0.05) Maximum hip flexion angle ↑ (p < 0.05) Maximum hip flexion velocity ↑ (p ≥ 0.05) Maximum knee flexion angle ↑ (p < 0.05) Maximum knee flexion velocity ↑ (p ≥ 0.05) Maximum knee extension velocity ↑ (p < 0.05) Maximum ankle plantarflexion angle ↓ (p ≥ 0.05) Maximum ankle plantarflexion velocity ↑ (p ≥ 0.05) Maximum ankle plantarflexion power ↑ (p ≥ 0.05) |
| Bucklitsch et al. 2019 Germany Single arm trial | Baseline n = 8 (2 men; 6 women) Mean age (years) = 58.1 ± 6.3 Side of hemiplegia (2 right; 6 left) Type of stroke (6 ischemic; 1 hemorrhage) Time since stroke (years) = 15.3 ± 10.6 Immediate effect (n = 8) Note: This study included multiple sclerosis (n = 1) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Stimulation specification not reported | General anesthesia (n = 8) Implant location (the electrode cuff): Around the peroneal nerve. Implant location (the stimulator): Lateral femoral fascia 4- to 5-cm incision above the knee Curved incision at the upper leg Adverse events not reported | Plantar pressure Step width Effective foot length Double support phase | Compared to baseline When the stimulation was on Plantar pressure ↓ (p ≥ 0.05) Step width ↓ (p ≥ 0.05) Effective foot length ↑ (p < 0.05) Double support phase ↓ (p ≥ 0.05) |
| Buentjen et al. 2019 Germany Single arm trial | Baseline n = 45 (24 men; 21 women) Mean age (years) = 52.0 ± 12.0 Side of hemiplegia (26 right; 19 left) Type of stroke (15 ischemic; 26 hemorrhage) Time since stroke (years) = 5.9 ± 6.1 1-day (n = 33) 3-month (n = 33) 12-month (n = 33) Note: This study included multiple sclerosis (n = 4) | ActiGait (4-channel peroneal nerve stimulator; 12-contact electrode cuff; an external control unit; a heel switch) Simulation pulse: No voltage information reported, 30–250 µs, 15–45 Hz | General anesthesia (n = 45) Surgical procedure is same as Burridge et al. (2007) Adverse events not reported | Maximum and comfortable gait speed (10-m walkway) | Compared to 1-day (no baseline data were provided except walking speed): At 3-month Maximum gait speed ↑ (p < 0.05) Comfortable gait speed ↑ (p ≥ 0.05) At 12-month Maximum gait speed ↑ (p < 0.05) Comfortable gait speed ↑ (p ≥ 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.E.; Frederick, R.; Nunley, B.; Lavery, L.; Dhaher, Y.; Najafi, B.; Cogan, S. The Effect of Implanted Functional Electrical Stimulation on Gait Performance in Stroke Survivors: A Systematic Review. Sensors 2021, 21, 8323. https://doi.org/10.3390/s21248323
Kang GE, Frederick R, Nunley B, Lavery L, Dhaher Y, Najafi B, Cogan S. The Effect of Implanted Functional Electrical Stimulation on Gait Performance in Stroke Survivors: A Systematic Review. Sensors. 2021; 21(24):8323. https://doi.org/10.3390/s21248323
Chicago/Turabian StyleKang, Gu Eon, Rebecca Frederick, Brandon Nunley, Lawrence Lavery, Yasin Dhaher, Bijan Najafi, and Stuart Cogan. 2021. "The Effect of Implanted Functional Electrical Stimulation on Gait Performance in Stroke Survivors: A Systematic Review" Sensors 21, no. 24: 8323. https://doi.org/10.3390/s21248323
APA StyleKang, G. E., Frederick, R., Nunley, B., Lavery, L., Dhaher, Y., Najafi, B., & Cogan, S. (2021). The Effect of Implanted Functional Electrical Stimulation on Gait Performance in Stroke Survivors: A Systematic Review. Sensors, 21(24), 8323. https://doi.org/10.3390/s21248323








