Can Electrochemical Sensors Be Used for Identification and Phylogenetic Studies in Lamiaceae?
Abstract
:1. Introduction
2. Materials and Methods
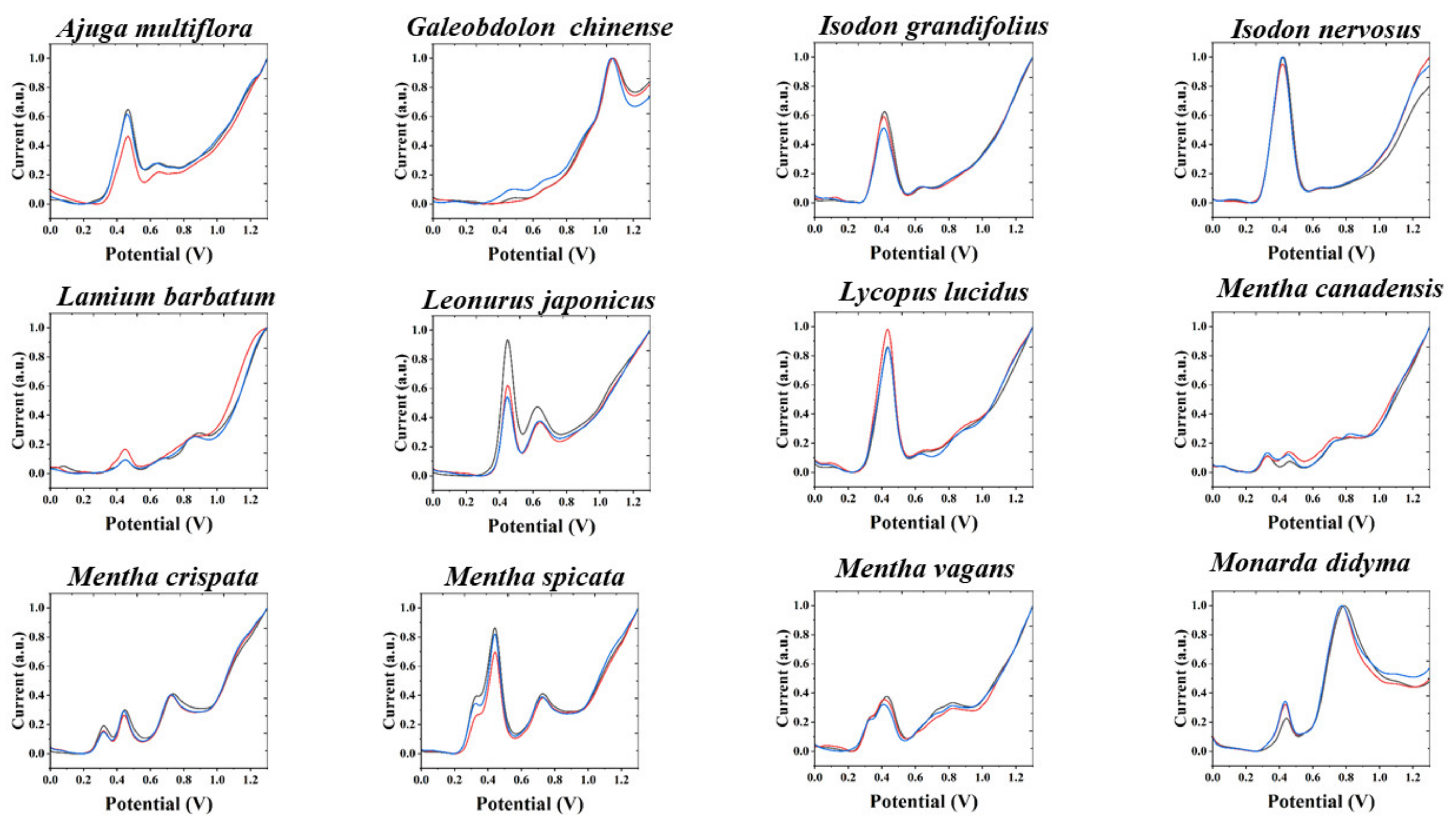
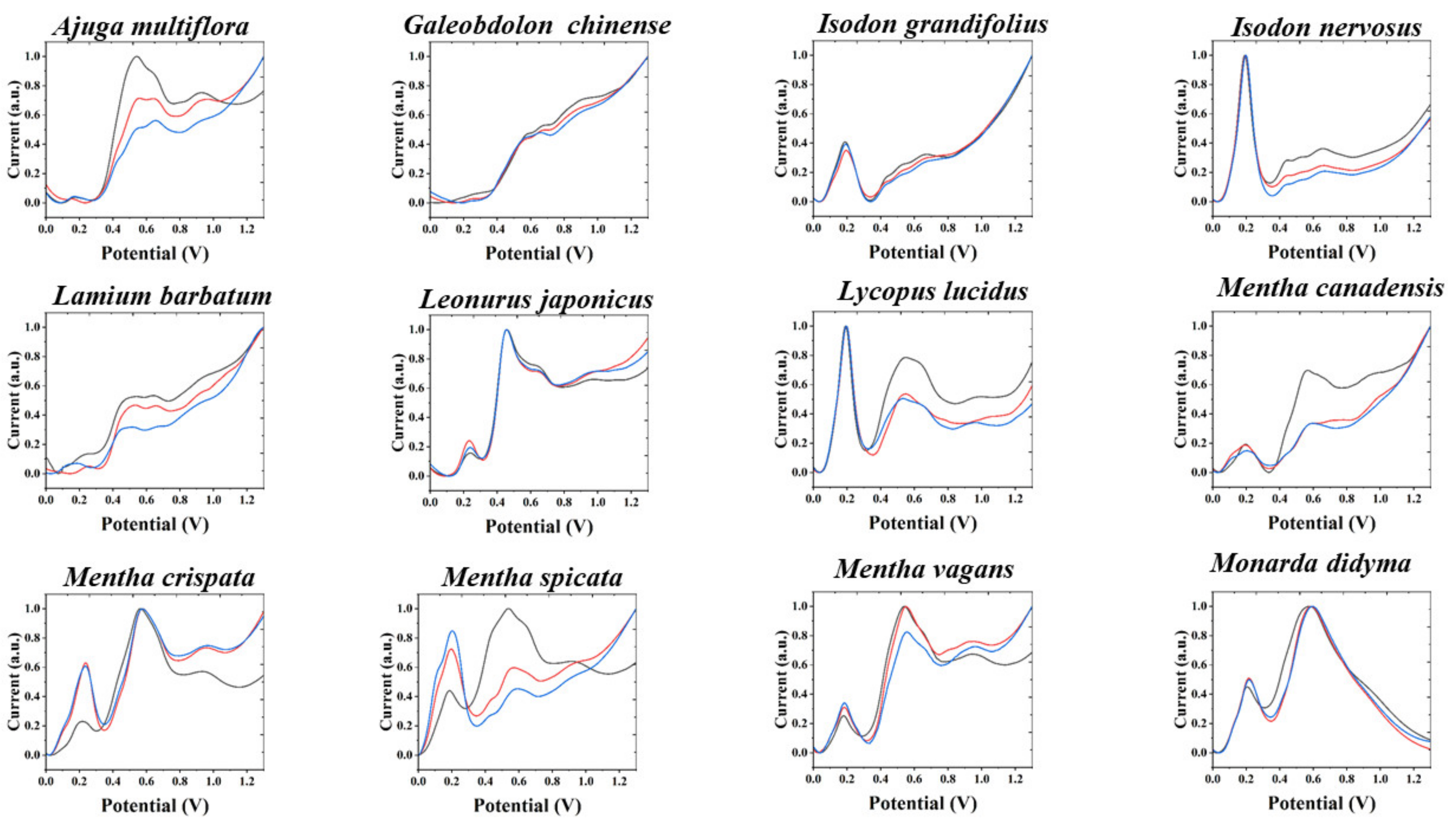
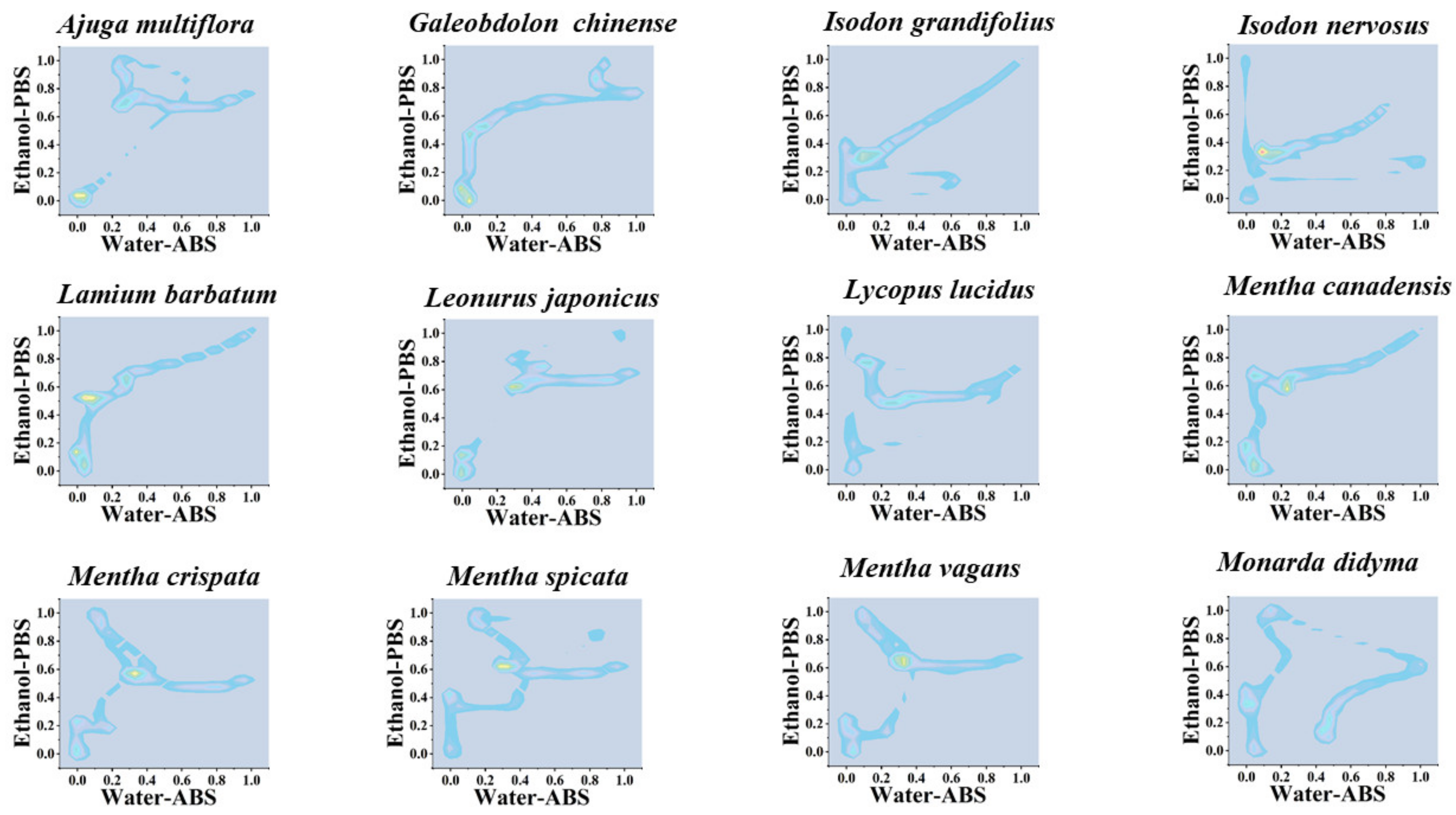
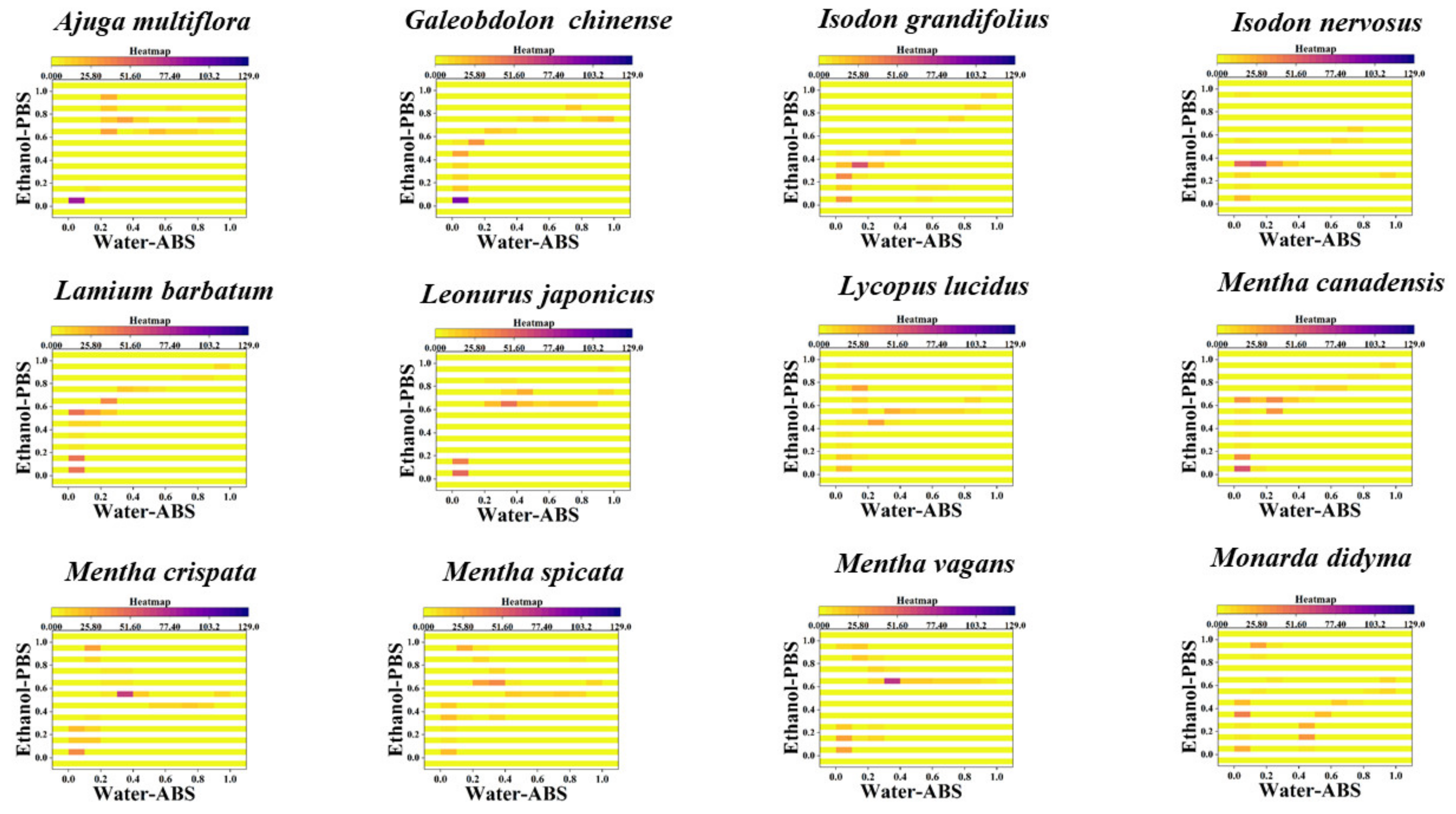
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doménech-Carbó, A.; Doménech-Carbó, M.T.; Ferragud-Adam, X.; Ortiz-Miranda, A.S.; Montoya, N.; Pasíes-Oviedo, T.; Peiró-Ronda, M.A.; Vives-Ferrándiz, J.; Marco, Y.C. Identification of Vegetal Species in Wooden Objects Using in Situ Microextrac-tion-Assisted Voltammetry of Microparticles. Anal. Methods 2017, 9, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Fan, B.; Wang, Q.; Wu, W.; Zhou, Q.; Li, D.; Xu, Z.; Fu, L.; Zhu, J.; Karimi-Maleh, H.; Lin, C.-T. Electrochemical Fingerprint Biosensor for Natural Indigo Dye Yielding Plants Analysis. Biosensors 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Zhuang, W.; Zhang, H.; Wang, A.; Su, W.; Yu, J.; Lin, C.-T. Enhanced Electrochemical Voltammetric Fingerprints for Plant Taxonomic Sensing. Biosens. Bioelectron. 2018, 120, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Ramírez-Barat, B.; Petiti, C.; Goidanich, S.; Doménech-Carbó, M.T.; Cano, E. Characterization of traditional artificial patinas on copper using the voltammetry of immobilized particles. J. Electroanal. Chem. 2020, 877, 114494. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Donnici, M.; Álvarez-Romero, C.; Daniele, S.; Doménech-Carbó, M.T. Multiple-scan voltammetry of immobilized particles of ancient copper/bronze coins. J. Solid State Electrochem. 2021, 25, 195–206. [Google Scholar] [CrossRef]
- Novak, I.; Mlakar, M.; Komorsky-Lovrić, Š. Voltammetry of Immobilized Particles of Cannabinoids. Electroanalysis 2013, 25, 2631–2636. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Y.; Zhang, J.; Karimi-Maleh, H.; Xu, Y.; Zhou, Q.; Fu, L.; Wu, W. Characterization of the Electrochemical Profiles of Lycoris Seeds for Species Identification and Infrageneric Relationships. Anal. Lett. 2020, 53, 2517–2528. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, B.; Zhang, M.; Du, X.; Wu, W.; Fu, L.; Zhou, W.; Zheng, Y. Electrochemical Profile Recording for Pueraria Variety Identification. Anal. Sci. 2020, 36, 1237–1241. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, Y.; Zhang, P.; Wang, Y.; Zheng, Y.; Fu, L.; Zhang, H.; Lin, C.-T.; Yu, A. Infrageneric phylogenetics investigation of Chimonanthus based on electroactive compound profiles. Bioelectrochemistry 2020, 133, 107455. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, D.; Li, X.; Wang, Z.; Zhou, Q.; Fu, L.; Yin, Y.; Creech, D. Biometric Identification of Taxodium spp. and Their Hybrid Progenies by Electrochemical Fingerprints. Biosensors 2021, 11, 403. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A Green and Sensitive Guanine-Based DNA Biosensor for Idarubicin Anticancer Monitoring in Biological Samples: A Simple and Fast Strategy for Control of Health Quality in Chemotherapy Procedure Confirmed by Docking Investigation. Chemosphere 2021, 132928. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules. 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briquet, J.-L.; Sawicki, F. L’analyse Localisée Du Politique. Politix. The localized analysis of politics 1989, 2, 6–16. [Google Scholar] [CrossRef]
- Zimowska, B.; Okoń, S.; Becchimanzi, A.; Krol, E.D.; Nicoletti, R. Phylogenetic Characterization of Botryosphaeria Strains Associated with Asphondylia Galls on Species of Lamiaceae. Divers 2020, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Bunsawat, J.; Elliott, N.E.; Hertweck, K.L.; Sproles, E.; Alice, L.A. Phylogenetics of Mentha (Lamiaceae): Evidence from Chloroplast DNA Sequences. Syst. Bot. 2004, 29, 959–964. [Google Scholar] [CrossRef]
- Drew, B.T.; Sytsma, K.J. Phylogenetics, biogeography, and staminal evolution in the tribe Mentheae (Lamiaceae). Am. J. Bot. 2012, 99, 933–953. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-P.; Drew, B.T.; Li, B.; Soltis, D.E.; Soltis, P.S.; Xiang, C.-L. Resolving the Phylogenetic Position of Ombrocharis (La-miaceae), with Reference to the Molecular Phylogeny of Tribe Elsholtzieae. Taxon 2016, 65, 123–136. [Google Scholar] [CrossRef] [Green Version]
- Drew, B.T.; Sytsma, K.J. The South American radiation of Lepechinia (Lamiaceae): Phylogenetics, divergence times and evolution of dioecy. Bot. J. Linn. Soc. 2013, 171, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Grauso, L.; de Falco, B.; Motti, R.; Lanzotti, V. Corn poppy, Papaver rhoeas L.: A critical review of its botany, phytochemistry and pharmacology. Phytochem. Rev. 2021, 20, 227–248. [Google Scholar] [CrossRef]
- Wakawa, A.I.; Audu, B.; Sulaiman, Y. Phytochemistry and Proximate Composition of Root, Stem Bark, Leaf and Fruit of Desert Date, Balanites Aegyptiaca. J. Phytopharm. 2018, 7, 464–470. [Google Scholar] [CrossRef]
- Geng, R.; Ma, L.; Liu, L.; Xie, Y. Influence of Bovine Serum Albumin-Flavonoid Interaction on the Antioxidant Activity of Dietary Flavonoids: New Evidence from Electrochemical Quantification. Molecules 2018, 24, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiorcea-Paquim, A.; Enache, T.A.; Gil, E.D.S.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-M.; Zhi-Bin, W.; Ping, X.; Qiu-Hong, W.; He, B.; Kuang, H.-X. Phytochemistry and Pharmacology of Genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Fu, L.; Su, W.; Chen, F.; Zhao, S.; Zhang, H.; Karimi-Maleh, H.; Yu, A.; Yu, J.; Lin, C.-T. Early sex determination of Ginkgo biloba based on the differences in the electrocatalytic performance of extracted peroxidase. Bioelectrochemistry 2021, 140, 107829. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, R.; Li, Z.; Zhang, M.; Wang, Q.; Xu, Y.; Fu, L.; Du, J.; Zheng, Y.; Zhu, J. Electroanalytical Study of Infrage-neric Relationship of Lagerstroemia Using Glassy Carbon Electrode Recorded Voltammograms. Rev. Mex. Ing. Quím. 2020, 19, 281–291. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Zhang, M.; Zheng, Y.; Wu, M.; Lan, Z.; Pu, J.; Zhang, H.; Chen, F.; Su, W. Electrochemical Sex Determina-tion of Dioecious Plants Using Polydopamine-Functionalized Graphene Sheets. Front. Chem. 2020, 8, 92. [Google Scholar] [CrossRef]
- Yang, R.; Fan, B.; Wang, S.; Li, L.; Li, Y.; Li, S.; Zheng, Y.; Fu, L.; Lin, C.-T. Electrochemical Voltammogram Recording for Identifying Varieties of Ornamental Plants. Micromachines 2020, 11, 967. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhu, J.; Zhang, H.; Zhang, L.; Su, W. Embedding Leaf Tissue in Graphene Ink to Improve Signals in Electrochemistry-Based Chemotaxonomy. Electrochem. Commun. 2018, 92, 39–42. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Wang, A.; Zhang, P.; Ding, S.; Wu, W.; Zhou, Q.; Chen, F.; Zhao, S. Identification of medicinal herbs in Asteraceae and Polygonaceae using an electrochemical fingerprint recorded using screen-printed electrode. J. Herb. Med. 2021, 30, 100512. [Google Scholar] [CrossRef]
- Macias, F.A.; Galindo, J.L.; Galindo, J.C. Evolution and Current Status of Ecological Phytochemistry. Phytochemistry 2007, 68, 2917–2936. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Giuliani, C.; Foddai, S.; Cianfaglione, K.; Maggi, F.; Fico, G.; Guiso, M.; Nicoletti, M.; Bianco, A.; et al. Occurrence of flavonoids in different Lamiaceae taxa for a preliminary study on their evolution based on phytochemistry. Biochem. Syst. Ecol. 2021, 96, 104247. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Spampinato, G.; Fuentes, J.P.; Gomes, C.P.; Quinto-Canas, R.; Cano, E. Taxonomy, Ecology and Distribution of Juniperus oxycedrus L. Group in the Mediterranean Basin Using Bioclimatic, Phytochemical and Morphometric Approaches, with Special Reference to the Iberian Peninsula. Forests 2021, 12, 703. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.L.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine Herbicide Monitoring as a Hazardous Substance by a DNA Nanostructure Biosensor. J. Hazard. Mater. 2022, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; Levitt, J.; McGinley, J.; Chandler, P.; Guenther, P.; Huybrechts, I.; Playdon, M. Measuring Dietary Botanical Diversity as a Proxy for Phytochemical Exposure. Nutrients 2021, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Ayaz, A.; Saqib, S.; Zaman, W.; Butt, M.A.; Ullah, A. Silene Conoidea L.: A Review on Its Systematic, Ethnobota-ny and Phytochemical Profile. Plant Sci. Today 2019, 6, 373–382. [Google Scholar] [CrossRef]
- Roma-Marzio, F.; Najar, B.; Alessandri, J.; Pistelli, L.; Peruzzi, L. Taxonomy of prickly juniper (Juniperus oxycedrus group): A phytochemical–morphometric combined approach at the contact zone of two cryptospecies. Phytochemistry 2017, 141, 48–60. [Google Scholar] [CrossRef]
- Ayaz, A.; Zaman, W.; Saqib, S.; Ullah, F.; Mahmood, T. Phylogeny and diversity of lamiaceae based on rps14 gene in Pakistan. Genetika 2020, 52, 435–452. [Google Scholar] [CrossRef]
- Liu, S.; Feng, S.; Huang, Y.; An, W.; Yang, Z.; Xie, C.; Zheng, X. Characterization of the Complete Chloroplast Genome of Buddleja Lindleyana. J. AOAC Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Zhao, Y.; Qin, M. The complete chloroplast genome of a Chinese medicinal plant, Peristrophe japonica (Thunb.) Bremek. (Lamiales: Acanthaceae) from Nanjing, China. Mitochondrial DNA Part B Resour. 2021, 6, 1888–1889. [Google Scholar] [CrossRef]
- Pulotova, T. Phenolic Compounds of Some Species of Mint. Uzb. Biol. Zh 1973, 17, 17–19. [Google Scholar]
- Tucker, A.O.; Hendriks, H.; Bos, R.; Fairbrothers, D.E. The Origin of Mentha—Gracilis (Lamiaceae). II. Essential Oils. Econ. Bot. 1991, 45, 200–215. [Google Scholar] [CrossRef]
- Murray, M.; Lincoln, D.; Marble, P. Oil Composition of Mentha Aquatica x Mentha Spicata F1 Hybrids in Relation to the Origin of x Mentha Piperita. Can. J. Genet. Cytology 1972, 14, 13–29. [Google Scholar] [CrossRef]
- Khanuja, S.; Shasany, A.; Srivastava, A.; Kumar, S. Assessment of Genetic Relationships in Mentha Species. Euphytica 2000, 111, 121–125. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Wilson, T.C.; Zhou, Y.-D.; Wang, Z.-H.; Liu, E.-D.; Peng, H.; Xiang, C.-L. Isodon Hsiwenii (Lamiaceae: Nepe-toideae), a New Species from Yunnan, China. Syst. Bot. 2019, 44, 913–922. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Huang, C.-Z.; Zhao, Y.; Xiang, C.-L. Molecular and Morphological Evidence for a New Species of Isodon (La-miaceae) from Southern China. Plant Diversity 2021, 43, 54–62. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Maki, M.; Drew, B.T.; Paton, A.J.; Li, H.-W.; Zhao, J.-L.; Conran, J.G.; Li, J. Phylogeny and Historical Biogeogra-phy of Isodon (Lamiaceae): Rapid Radiation in South-West China and Miocene Overland Dispersal into Africa. Mol. Phyloge-net. Evol. 2014, 77, 183–194. [Google Scholar] [CrossRef]
- Zhong, J.-S.; Li, J.; Li, L.; Conran, J.G.; Li, H.-W. Phylogeny of Isodon (Schrad. Ex Benth.) Spach (Lamiaceae) and Related Genera Inferred from Nuclear Ribosomal ITS, TrnL–TrnF Region, and Rps16 Intron Sequences and Morphology. Syst. Bot. 2010, 35, 207–219. [Google Scholar] [CrossRef]
- Zhao, F.; Drew, B.T.; Chen, Y.-P.; Hu, G.-X.; Li, B.; Xiang, C.-L. The Chloroplast Genome of Salvia: Genomic Characteriza-tion and Phylogenetic Analysis. Int. J. Plant Sci. 2020, 181, 812–830. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Zhou, B.; Yue, Z. The Complete Chloroplast Genomes of Lycopus Lucidus and Agastache Rugosa, Two Herbal Species in Tribe Mentheae of Lamiaceae Family. Mitochondrial DNA Part B 2021, 6, 89–90. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Li, D.; Fu, L.; Zheng, Y.; Gu, Y.; Chen, F.; Zhao, S. Can Electrochemical Sensors Be Used for Identification and Phylogenetic Studies in Lamiaceae? Sensors 2021, 21, 8216. https://doi.org/10.3390/s21248216
Wang D, Li D, Fu L, Zheng Y, Gu Y, Chen F, Zhao S. Can Electrochemical Sensors Be Used for Identification and Phylogenetic Studies in Lamiaceae? Sensors. 2021; 21(24):8216. https://doi.org/10.3390/s21248216
Chicago/Turabian StyleWang, Da, Dongling Li, Li Fu, Yuhong Zheng, Yonghua Gu, Fei Chen, and Shichao Zhao. 2021. "Can Electrochemical Sensors Be Used for Identification and Phylogenetic Studies in Lamiaceae?" Sensors 21, no. 24: 8216. https://doi.org/10.3390/s21248216
APA StyleWang, D., Li, D., Fu, L., Zheng, Y., Gu, Y., Chen, F., & Zhao, S. (2021). Can Electrochemical Sensors Be Used for Identification and Phylogenetic Studies in Lamiaceae? Sensors, 21(24), 8216. https://doi.org/10.3390/s21248216







