HyperSeed: An End-to-End Method to Process Hyperspectral Images of Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Imaging System
2.3. Image Acquisition
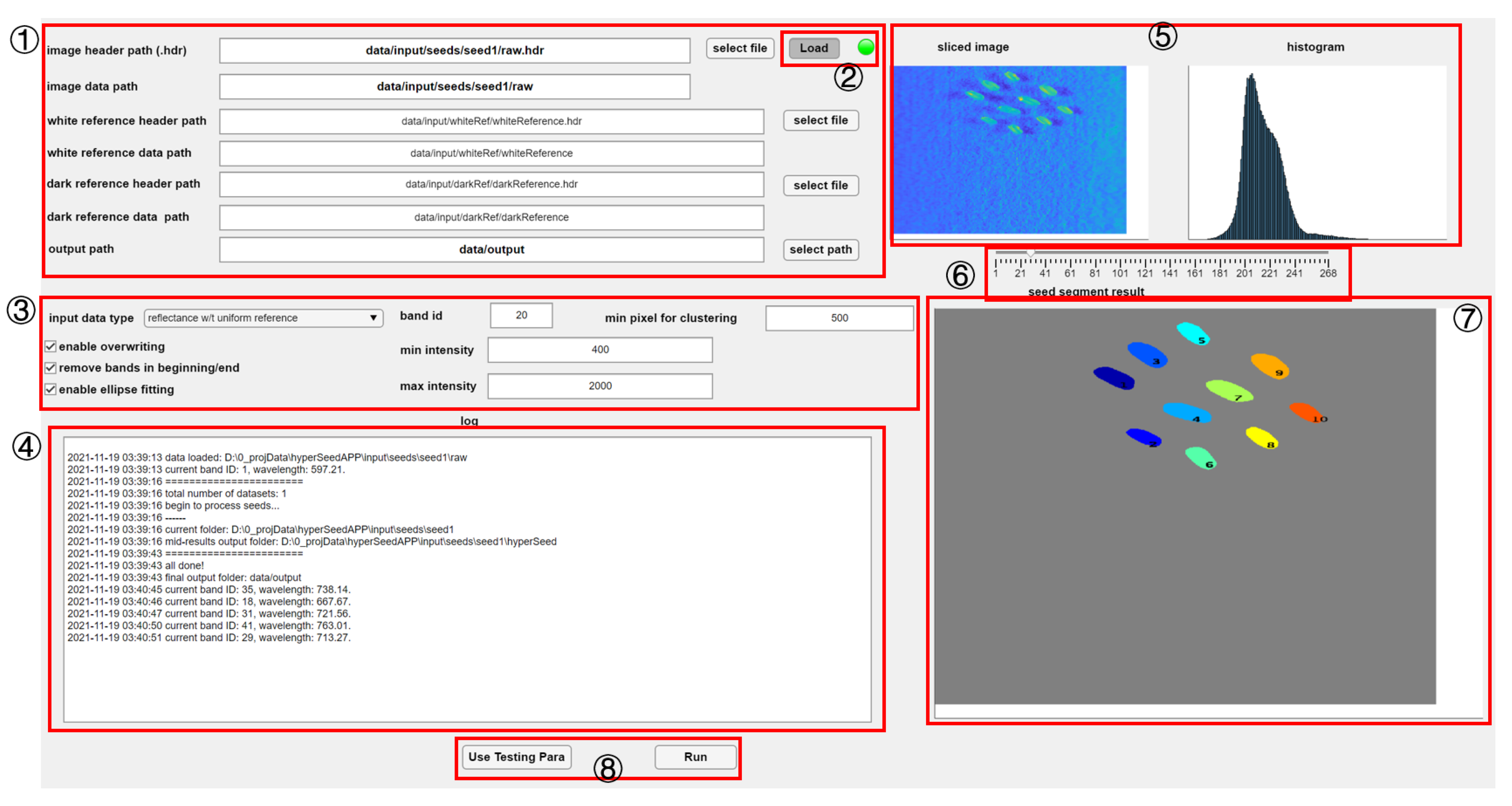
2.4. Software Implementation
2.4.1. Path Specification
2.4.2. Data Visualization
2.4.3. Parameters Setting
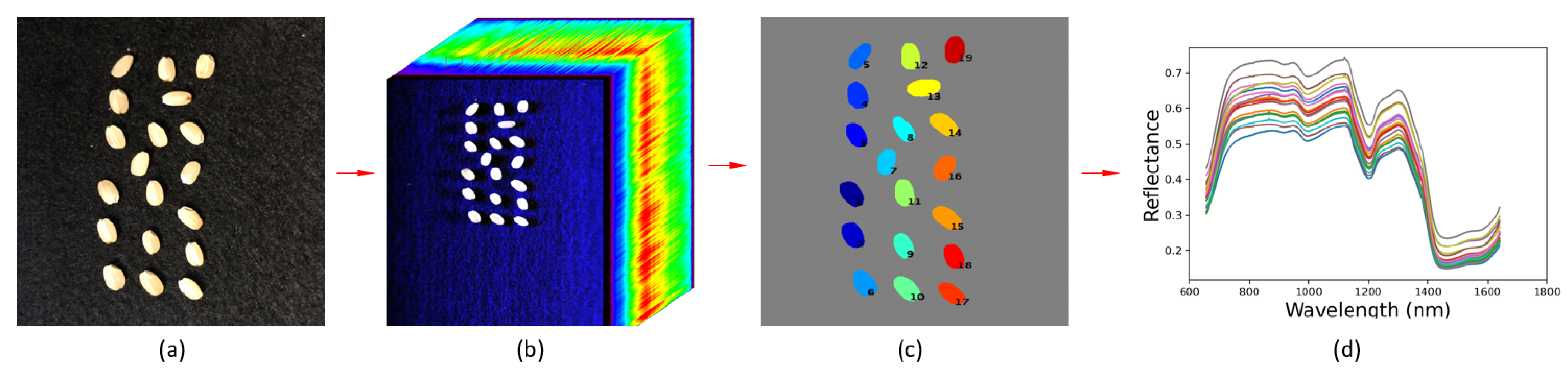
2.4.4. Hypercube Processing
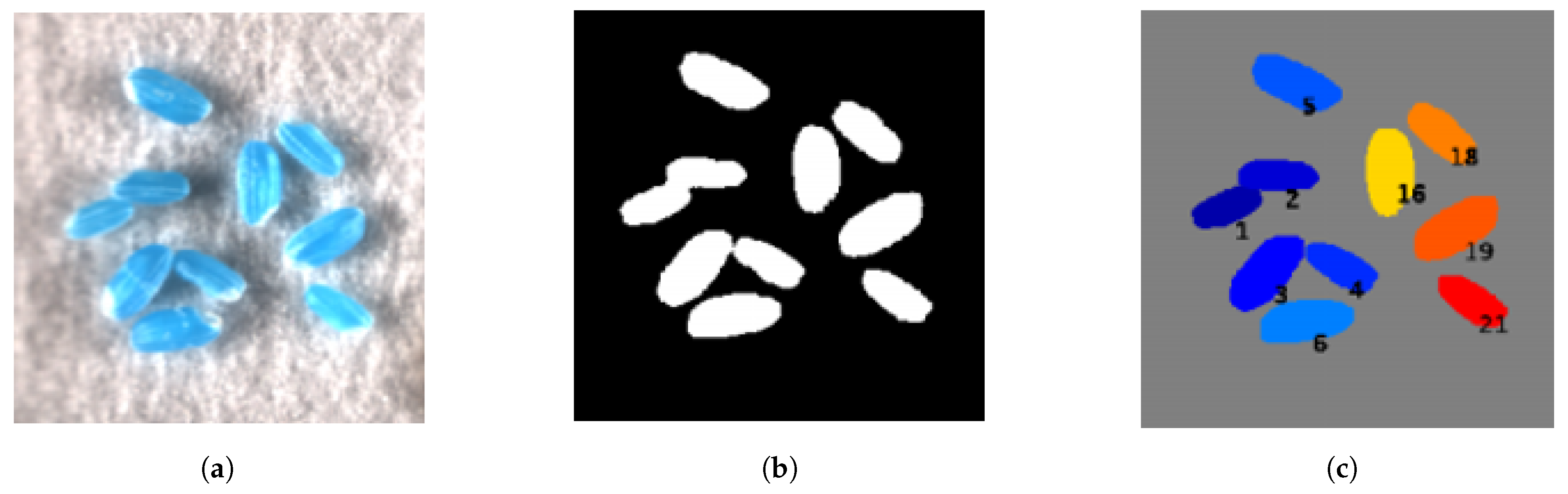
2.4.5. Initial Seed Segmentation
2.4.6. Refined Seed Segmentation
2.4.7. Spectral Data Extraction
2.4.8. Results Calibration
2.5. Seed Classification and Wavelength Analysis
2.5.1. Support Vector Machine (SVM)
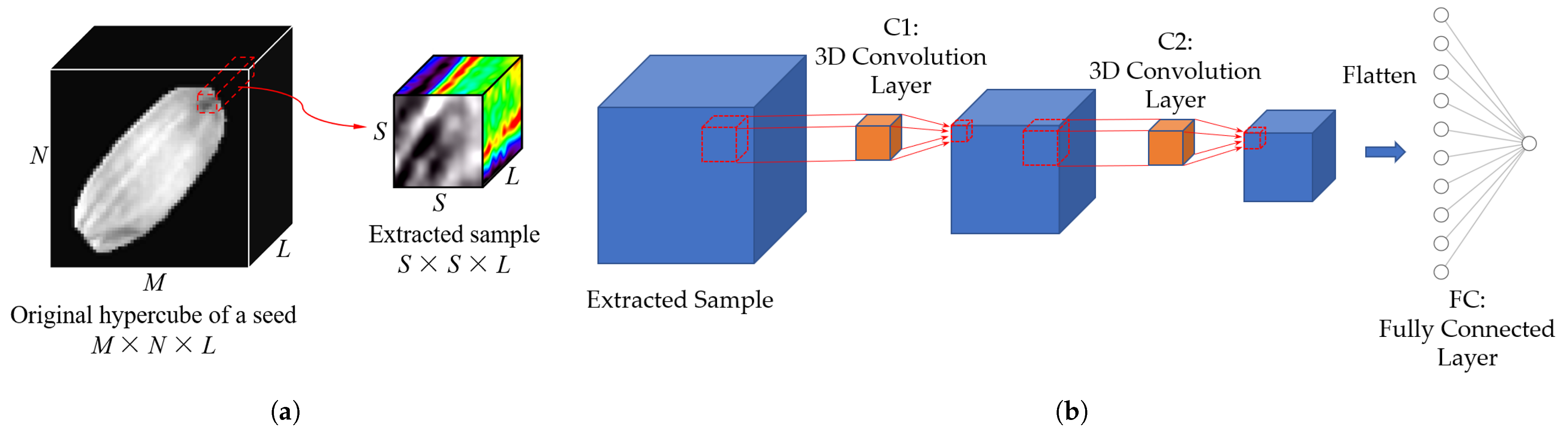
2.5.2. Neural Network Models—3D Convolutional Neural Network (3D CNN)
2.5.3. Dataset for Classification
2.5.4. Metrics for Classification
2.5.5. LightGBM for Feature Importance Analysis
3. Results
3.1. Performance Testing
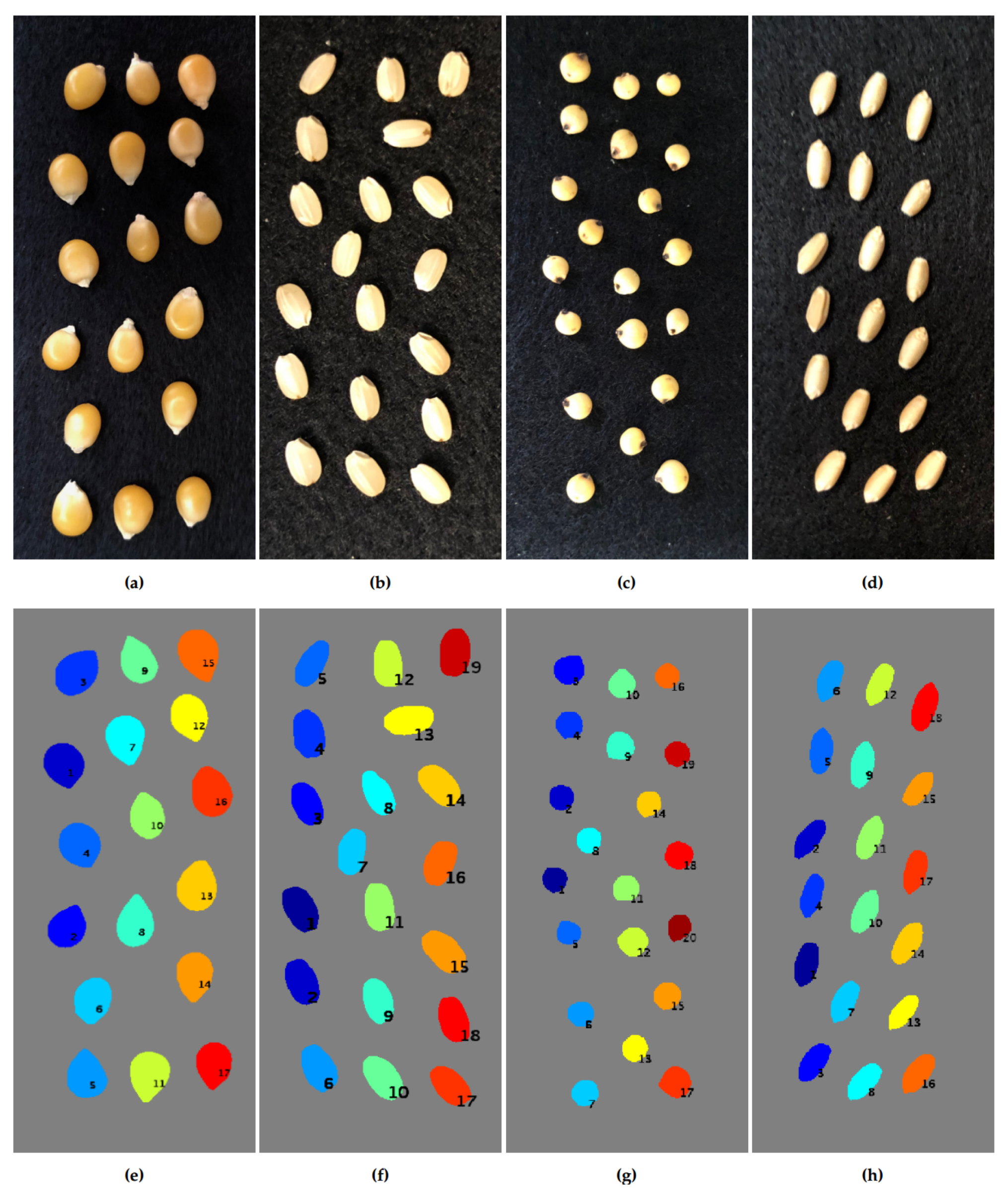
3.2. Segmentation Results Using Seeds from Various Plant Species
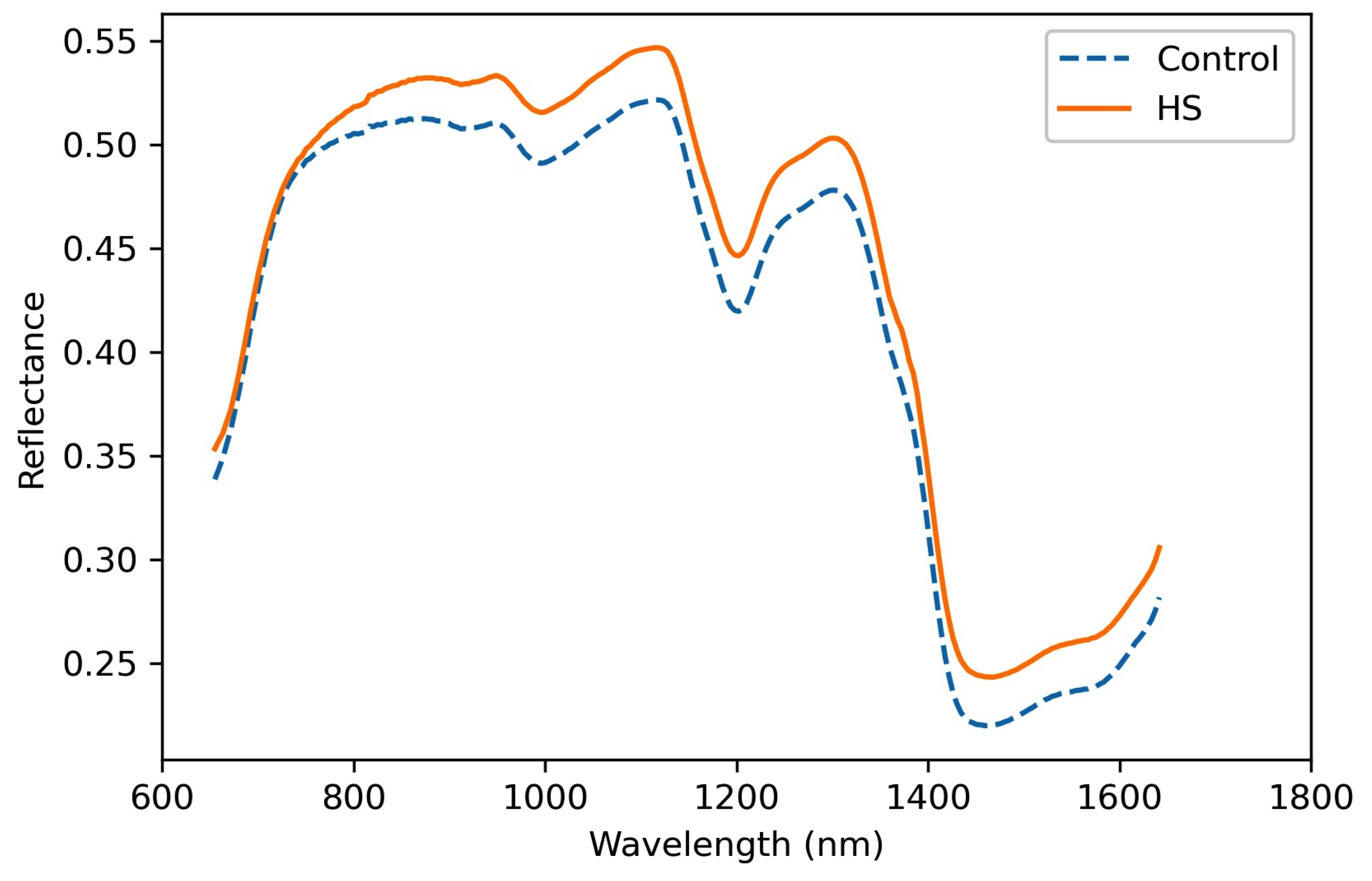
3.3. Spectral Analysis
3.4. Classification
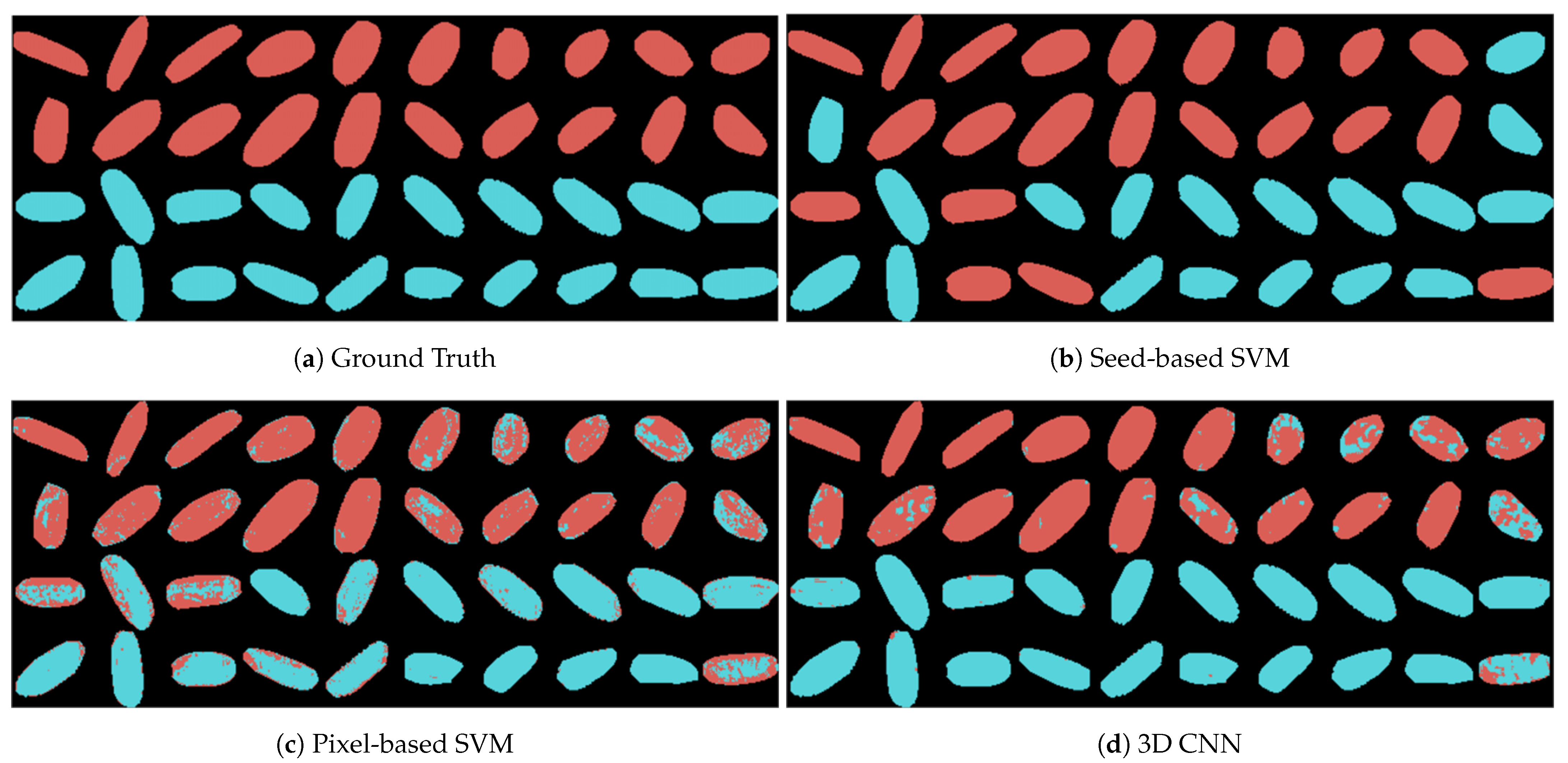
3.4.1. Seed-Based Support Vector Machine (Seed-Based SVM)
3.4.2. Pixel-Based Support Vector Machine (Pixel-Based SVM)
3.4.3. 3D Convolutional Neural Network (3D CNN)
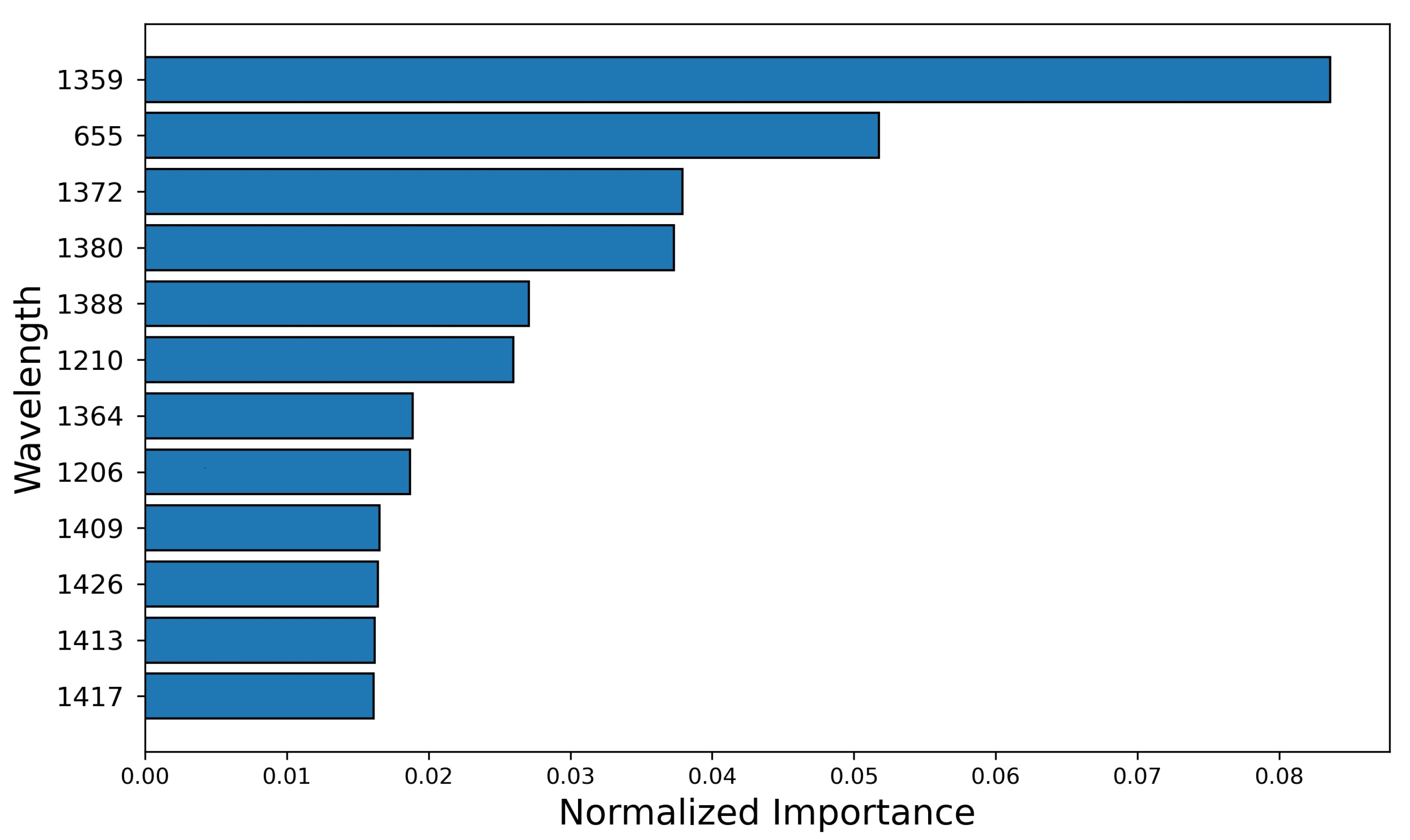
3.5. Wavelengths Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- TeKrony, D.M.; Egli, D.B. Relationship of seed vigor to crop yield: A review. Crop Sci. 1991, 31, 816–822. [Google Scholar] [CrossRef]
- Dell’Aquila, A. Digital imaging information technology applied to seed germination testing: A review. Agron. Sustain. Dev. 2009, 29, 213–221. [Google Scholar] [CrossRef]
- Sandhu, J.; Zhu, F.; Paul, P.; Gao, T.; Dhatt, B.K.; Ge, Y.; Staswick, P.; Yu, H.; Walia, H. PI-Plat: A high-resolution image-based 3D reconstruction method to estimate growth dynamics of rice inflorescence traits. Plant Methods 2019, 15, 162. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Zhu, F.; Paul, P.; Sandhu, J.; Doku, H.A.; Sun, J.; Pan, Y.; Staswick, P.; Walia, H.; Yu, H. Novel 3D Imaging Systems for High-Throughput Phenotyping of Plants. Remote Sens. 2021, 13, 2113. [Google Scholar] [CrossRef]
- Gao, T.; Sun, J.; Zhu, F.; Doku, H.A.; Pan, Y.; Walia, H.; Yu, H. Plant Event Detection from Time-Varying Point Clouds. In Proceedings of the 2019 IEEE International Conference on Big Data (Big Data), Los Angeles, CA, USA, 9–12 December 2019; pp. 3321–3329. [Google Scholar]
- Tanabata, T.; Shibaya, T.; Hori, K.; Ebana, K.; Yano, M. SmartGrain: High-throughput phenotyping software for measuring seed shape through image analysis. Plant Physiol. 2012, 160, 1871–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Paul, P.; Hussain, W.; Wallman, K.; Dhatt, B.K.; Sandhu, J.; Irvin, L.; Morota, G.; Yu, H.; Walia, H. SeedExtractor: An open-source GUI for seed image analysis. Front. Plant Sci. 2020, 11, 581546. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, B.; Marcos-Filho, J. Relationship between germination and bell pepper seed structure assessed by the X-ray test. Sci. Agric. 2011, 68, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Junior, F.; Yagushi, J.; Belini, U.; Cicero, S.; Tomazello-Filho, M. X-ray densitometry to assess internal seed morphology and quality. Seed Sci. Technol. 2012, 40, 102–107. [Google Scholar] [CrossRef]
- Dumont, J.; Hirvonen, T.; Heikkinen, V.; Mistretta, M.; Granlund, L.; Himanen, K.; Fauch, L.; Porali, I.; Hiltunen, J.; Keski-Saari, S.; et al. Thermal and hyperspectral imaging for Norway spruce (Picea abies) seeds screening. Comput. Electron. Agric. 2015, 116, 118–124. [Google Scholar] [CrossRef]
- Yang, X.; Hong, H.; You, Z.; Cheng, F. Spectral and image integrated analysis of hyperspectral data for waxy corn seed variety classification. Sensors 2015, 15, 15578–15594. [Google Scholar] [CrossRef] [Green Version]
- Bock, C.; Poole, G.; Parker, P.; Gottwald, T. Plant disease severity estimated visually, by digital photography and image analysis, and by hyperspectral imaging. Crit. Rev. Plant Sci. 2010, 29, 59–107. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, W.; Zhao, B.; Wang, R.; Li, M.; Yang, L.; Wang, J.; Sun, Q. A reliable methodology for determining seed viability by using hyperspectral data from two sides of wheat seeds. Sensors 2018, 18, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.; Chen, J.; Zhao, Y.; Zhu, S.; He, Y.; Zhang, C. Variety identification of single rice seed using hyperspectral imaging combined with convolutional neural network. Appl. Sci. 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Huang, W.; Yang, G.; Wang, Q.; Li, J.; Chen, L. Determination of starch content in single kernel using near-infrared hyperspectral images from two sides of corn seeds. Infrared Phys. Technol. 2020, 110, 103462. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [Green Version]
- Dhatt, B.K.; Paul, P.; Sandhu, J.; Hussain, W.; Irvin, L.; Zhu, F.; Adviento-Borbe, M.A.; Lorence, A.; Staswick, P.; Yu, H.; et al. Allelic variation in rice Fertilization Independent Endosperm 1 contributes to grain width under high night temperature stress. New Phytol. 2021, 229, 335. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Dhatt, B.K.; Sandhu, J.; Hussain, W.; Irvin, L.; Morota, G.; Staswick, P.; Walia, H. Divergent phenotypic response of rice accessions to transient heat stress during early seed development. Plant Direct 2020, 4, e00196. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhang, M.; Zhou, Y.; Wang, Y.; Shen, J.; Chen, H.; Zhang, L.; Lü, B.; Liang, G.; Liang, J. The Rice G protein γ subunit DEP1/qPE9–1 positively regulates grain-filling process by increasing Auxin and Cytokinin content in Rice grains. Rice 2019, 12, 91. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.; Jiang, H.; Wang, Z.; Wu, F.; Xiong, Y.; Yao, J. A heat stress responsive NAC transcription factor heterodimer plays key roles in rice grain filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef]
- Misra, G.; Badoni, S.; Parween, S.; Singh, R.K.; Leung, H.; Ladejobi, O.; Mott, R.; Sreenivasulu, N. Genome-wide association coupled gene to gene interaction studies unveil novel epistatic targets among major effect loci impacting rice grain chalkiness. Plant Biotechnol. J. 2021, 19, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Shen, Q. Spectral–spatial classification of hyperspectral imagery with 3D convolutional neural network. Remote Sens. 2017, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Burges, C.J. A tutorial on support vector machines for pattern recognition. Data Min. Knowl. Discov. 1998, 2, 121–167. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. LightGBM: A highly efficient gradient boosting decision tree. Adv. Neural Inf. Process. Syst. 2017, 30, 3146–3154. [Google Scholar]
- Zhang, L.; Rao, Z.; Ji, H. Hyperspectral imaging technology combined with multivariate data analysis to identify heat-damaged rice seeds. Spectrosc. Lett. 2020, 53, 207–221. [Google Scholar] [CrossRef]
- Haralock, R.M.; Shapiro, L.G. Computer and Robot Vision; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1991; Volume 1, pp. 28–48. [Google Scholar]
- Soille, P. Morphological Image Analysis: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–174. [Google Scholar]
- Panagiotakis, C.; Argyros, A. Region-based Fitting of Overlapping Ellipses and its application to cells segmentation. Image Vis. Comput. 2020, 93, 103810. [Google Scholar] [CrossRef]
- Wakholi, C.; Kandpal, L.M.; Lee, H.; Bae, H.; Park, E.; Kim, M.S.; Mo, C.; Lee, W.H.; Cho, B.K. Rapid assessment of corn seed viability using short wave infrared line-scan hyperspectral imaging and chemometrics. Sens. Actuators B Chem. 2018, 255, 498–507. [Google Scholar] [CrossRef]
- Polder, G.; van der Heijden, G.W.; Keizer, L.P.; Young, I.T. Calibration and characterisation of imaging spectrographs. J. Near Infrared Spectrosc. 2003, 11, 193–210. [Google Scholar] [CrossRef]
- Polder, G.; van der Heijden, G.W.; Young, I.T. Spectral image analysis for measuring ripeness of tomatoes. Trans. ASAE 2002, 45, 1155. [Google Scholar] [CrossRef]
- Zhu, F.; Pan, Y.; Gao, T.; Walia, H.; Yu, H. Interactive Visualization of Hyperspectral Images based on Neural Networks. IEEE Comput. Graph. Appl. 2021, 41, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Zisserman, A. Very deep convolutional networks for large-scale image recognition. arXiv 2014, arXiv:1409.1556. [Google Scholar]
- Zhu, S.; Zhou, L.; Gao, P.; Bao, Y.; He, Y.; Feng, L. Near-infrared hyperspectral imaging combined with deep learning to identify cotton seed varieties. Molecules 2019, 24, 3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvao, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemom. Intell. Lab. Syst. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]

| Reflectance Type | Total Number | Training Set | Validation Set | Test Set | |||
|---|---|---|---|---|---|---|---|
| Control | HS | Control | HS | Control | HS | ||
| Seed-based | 200 | 80 | 80 | N/A | N/A | 20 | 20 |
| Pixel-based | 274,641 | 104,517 | 104,719 | 5501 | 5512 | 27,527 | 26,865 |
| Model | Metrics on Test Samples | Seed Group Prediction Accuracy | |||
|---|---|---|---|---|---|
| Accuracy | Precision | Recall | F-score | ||
| Seed-based SVM | 80.00% | 75.00% | 83.33% | 78.94% | 80.00% |
| Pixel-based SVM | 85.67% | 86.36% | 84.30% | 85.32% | 92.50% |
| 3D CNN | 94.21% | 90.83% | 98.18% | 94.37% | 97.50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Chandran, A.K.N.; Paul, P.; Walia, H.; Yu, H. HyperSeed: An End-to-End Method to Process Hyperspectral Images of Seeds. Sensors 2021, 21, 8184. https://doi.org/10.3390/s21248184
Gao T, Chandran AKN, Paul P, Walia H, Yu H. HyperSeed: An End-to-End Method to Process Hyperspectral Images of Seeds. Sensors. 2021; 21(24):8184. https://doi.org/10.3390/s21248184
Chicago/Turabian StyleGao, Tian, Anil Kumar Nalini Chandran, Puneet Paul, Harkamal Walia, and Hongfeng Yu. 2021. "HyperSeed: An End-to-End Method to Process Hyperspectral Images of Seeds" Sensors 21, no. 24: 8184. https://doi.org/10.3390/s21248184
APA StyleGao, T., Chandran, A. K. N., Paul, P., Walia, H., & Yu, H. (2021). HyperSeed: An End-to-End Method to Process Hyperspectral Images of Seeds. Sensors, 21(24), 8184. https://doi.org/10.3390/s21248184






