Abstract
Polymer gel sensors on 96-well plates were successfully used to detect four different multi-explosives, including 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), nitrite, and perchlorate. The products of reactions between the explosives and the polymer gel sensors were digitally captured, and the images were analyzed by a developed Red–Green–Blue (RGB) analyzer program on a notebook computer. RGB color analysis provided the basic color data of the reaction products for the quantification of the explosives. The results provided good linear range, sensitivity, limit of detection, limit of quantitation, specificity, interference tolerance, and recovery. The method demonstrated great potential to detect explosives by colorimetric analysis of digital images of samples on 96-well plates. It is possible to apply the proposed method for quantitative on-site field screening of multi-explosives.
1. Introduction
Explosions can inflict serious, even life-threatening injuries on many people at the same time [1]. Explosions cause damage in several different ways: by the blast wave, the shock wave, heat, fragmentation, or the blast wind. Explosives are commonly classified as high and low explosives, according to the type and velocity of the reaction involved [2]. Homemade explosive devices or improvised explosive devices (IEDs) are simple to make using widely accessible materials. The increased controls placed on commercial and military explosives has made the use of IEDs more prevalent. IEDs can vary widely in size; most have at least four components: a power source, a main charge, an initiator, and a switch. In addition, a casing or container may be used, as well as a booster and added fragmentation or shrapnel, such as nails or screws [3]. The use of IEDs has increased dramatically in recent years in the southern border region of Thailand. In Yala, Pattani, Narathiwat provinces, and some districts of Songkhla province, violent conflict has frequently involved the use of IEDs.
Terrorist activities have generated an enormous demand for the rapid identification of explosive compounds at the site of the terrorist act. Builders of IEDs will modify a device to efficiently use the available materials, and a mix of high explosives and low explosives may be used. The available data may help to identify a terrorist group or bomber by linking the explosive evidence to known designs or materials used in previous explosions [4]. Field analysis of explosives needs a rapid, portable, real-time, low-cost, and reliable method of identification. The need for quantification of trace explosives has become a matter of urgency due to the increasing threat from terrorist attacks [5].
A variety of detection methods for the quantitative analysis of explosives has been reported. The instrumental techniques used include gas sensors [6,7,8,9,10], high-performance liquid chromatography [11,12], capillary electrophoresis [13,14], ion chromatography [15,16], and electrochemistry [17,18]. However, some of these methods are typically laboratory-based, time-consuming, require sample extraction and preparation, and require expert knowledge for effective results.
In recent years, rapid quantification by colorimetric analysis of digital images has received growing interest. This method has been used for the quantitative analysis of explosives [19,20,21] and other applications [22,23,24]. The method is based on the measurement of the intensity of red, green, and blue (RGB) basic color data in a digital image of a reaction product. The RGB intensity values obtained from a color image can be used to generate calibration equations for quantitative analysis. The colorimetric method is an interesting approach because it is inexpensive and does not require expert analysis. For example, the development of rapid quantitative determination of 2,4,6-trinitrotoluene (TNT) and ammonium nitrate explosives made use of a software application installed on a mobile phone [25,26]. The response was fast, processing was possible in real time, the procedure was simple, and the device was portable. Nevertheless, only one sample could be analyzed at a time. When a large number of samples had to be analyzed, the process took a long time, but the rapid quantitative analysis of multiple samples has recently been reported. A microplate spectrophotometer was used to analyze samples on 96-well microplates, which could simultaneously analyze many samples with high accuracy, but the equipment was both large and laboratory-based.
Polymer gel materials, which are composed of long macromolecular chains with a 3D cross-linked structure, have been increasingly investigated [27,28,29,30]. Sodium carboxymethyl cellulose (CMC) is a kind of high-polymer cellulose ether with properties of stability, water absorption, and thickening, bulking, and gelation abilities [31], whereas polyethylene glycol (PEG) can be used as a plasticizer that is soluble in water and many organic solvents. PEG accelerated the normal gelation process and was approved as a nontoxic product by the U.S. Food and Drug Administration [32,33]. The porous network of a polymer gel could be used to entrap reagents to produce a polymer gel sensor for the detection of explosives. Then, the colorimetric products of the reaction within the polymer gel sensor can be placed on 96-well plates and processed into digital images for analysis. An RGB analyzer program can measure the intensity of red, green, and blue (RGB) color values from the digital images, which can provide a simple quantification.
This work describes the development of a new, simple, and rapid field analysis method using 96-well plates containing sensors based on a binary polymer composite gel entrapping appropriate reagents. The work consisted of two parts. Firstly, we designed and fabricated a photographic box and created a software program for the analysis of RGB color intensity of the digital images and studied the analytical performance of the proposed method. Secondly, we applied the method for the high-throughput determination of multi-explosives. Digital image colorimetric analysis was applied for the determination of 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), nitrite, and perchlorate, which have been used in the three southernmost provinces of Thailand [4]. The portable method demonstrated rapid, accurate, and real-time simultaneous quantitative analysis of multi-explosives.
2. Materials and Methods
2.1. Chemicals and Materials
Sodium carboxymethyl cellulose (CMC, Mw ≈90,000 g mol−1), 2,4,6-trinitrotoluene (TNT, 96%), and 2,4-dinitrotoluene (DNT, 97%) were purchased from Sigma-Aldrich (Steinheim, Germany). Sodium nitrite (NaNO2, ≥97.0%) was analytical grade from Ajax Finechem (Auckland, New Zealand). Potassium hydroxide (KOH, ≥85.0%), potassium iodide (KI, ≥99.5%), methylene blue (≥82%), sulfuric acid (H2SO4, 95.0–97.0%), ethanol (≥99.9%), and acetone (≥99.8%) were of analytical grade, and all were purchased from Merck KGaA (Darmstadt, Germany). Potassium perchlorate (KClO4, ≥99%) was from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were analytical grade. Deionized water was used for the preparation of all solutions (18.2 MΩ∙cm) (BarnsteadTM Easy PureTM II water purification system, Thermo Fisher ScientificTM, Marietta, OH, USA). A UV/Vis microplate spectrophotometer (Thermo Fisher Scientific, Model 51119200, MultiskanTM GO, Vantaa, Uusimaa, Finland) was used for absorbance measurements. Clear polystyrene (PS) 96-well Microwell (Thermo Scientific Nunc®, Part No: NAL-167008, Suzhou, Jiangsu, China) was used for the preparation of the polymer gel sensor.
2.2. Preparation of Polymer Gel Sensor
Sodium carboxymethyl cellulose (CMC) was dissolved in distilled water (2.5% w/v), Then, 2 mL of polyethylene glycol (PEG-400) was added, and the mixture solution was magnetically stirred for an hour at room temperature. A transparent viscous gel was obtained. The polymer gel sensors for the determination of each explosive were prepared by mixing polymer gel and the appropriate reagent in a 1:1 ratio under stirring for 30 min. Then, the wells of 96-well plates were filled with 200 µL of the sensor mixture, and the plates were kept at room temperature overnight. During the gelling process, the reagents were entrapped inside the pores of the polymer gel.
2.3. Color Test of Explosives
First, 2,4,6-trinitrotoluene (TNT) and 2,4-dinitrotoluene (DNT) were prepared with 1:1 acetone–water [25,34]. TNT concentrations were in the range of 0.250 to 100 mg L−1 and DNT concentrations were in the range of 0.025 to 0.90 mg mL−1. One hundred µL of TNT and DNT standard solutions of each concentration were tested. The polymer gel sensor was prepared by mixing the polymer gel with 0.25 mol L−1 of potassium hydroxide in ethanol (1:1 ratio). This reagent changed the colorless sensor to a red-brown color in the presence of TNT and a blue color in the presence of DNT.
To test nitrite, a range of nitrite standard solutions (0.25–80 mg L−1) were prepared in deionized water, and 100 µL of each nitrite standard was tested with a sensor of polymer gel mixed with the two reagents (0.07 mol L−1 sulfuric acid and 0.07 mol L−1 potassium iodide) in a 1:1 ratio. The colorless gel sensor turned yellow in the presence of nitrite. To test perchlorate, standard perchlorate solutions were prepared in deionized water to appropriate concentrations in the range of 0.0050 to 1.0 mg mL−1. The polymer gel was mixed with methylene blue (MB) at 0.025% w/v in a ratio of 1:1, which turned violet in the presence of perchlorate.
All test samples were left in the 96-well plates for 5 min prior to observation of the resultant color. Each color test was repeated three times. The RGB intensity of the colored products was detected while the samples were on the 96-well plates using the RGB analyzer program (PSU RGB Analyzer Plus 3.0) on a notebook.
2.4. Photographic System
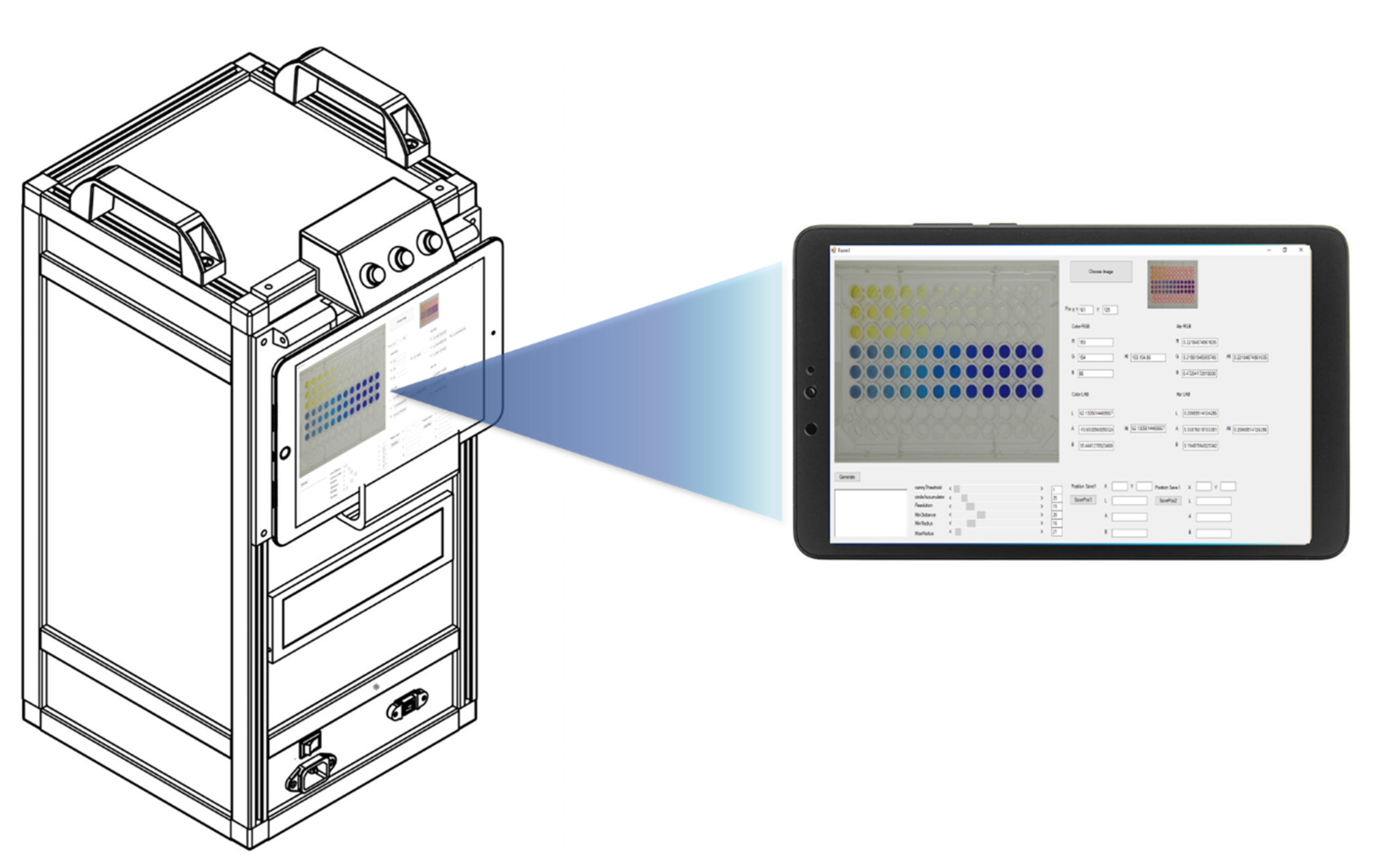
A photographic box measuring 24.0 cm × 29.0 cm × 53.0 cm was designed and fabricated from opaque black acrylic sheet with a white internal background (Figure 1). The photographic box was equipped with a camera, power supply, battery, two microcontrollers, and two motors. Twelve white high-intensity light-emitting diodes (LEDs) were used as the light sources inside the box. The images were captured 5 min after the addition of the standard/sample solution. The images were captured with an industrial video camera (ELP USB 8MP high-resolution SONY IMX179) within the photographic box. Each image was 64.0 KB (640 × 480 pixels) in size and was saved as a JPEG (24-bits) on a 2-in-1 notebook in tablet mode (Acer One 10, HD (1280 × 800), IPS).
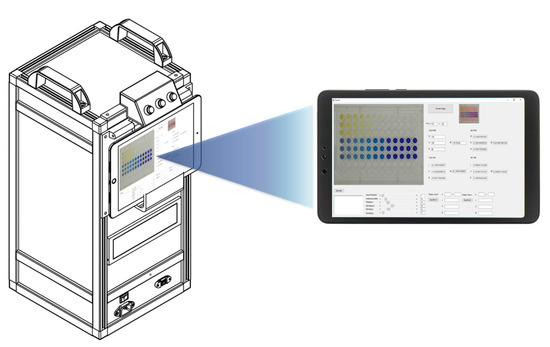
Figure 1.
RGB analysis system for the determination of multi-explosives.
Once the images were captured, they were automatically transferred to the computer with the program command. The RGB values of the colorimetric product were analyzed with the in-house designed RGB analysis program. Each colorimetric test was repeated three times from three wells of the microplate. The average intensities of the red, green, blue, and total RGB (T; T = R + G + B) from the three replications were used as single data points in graphs.
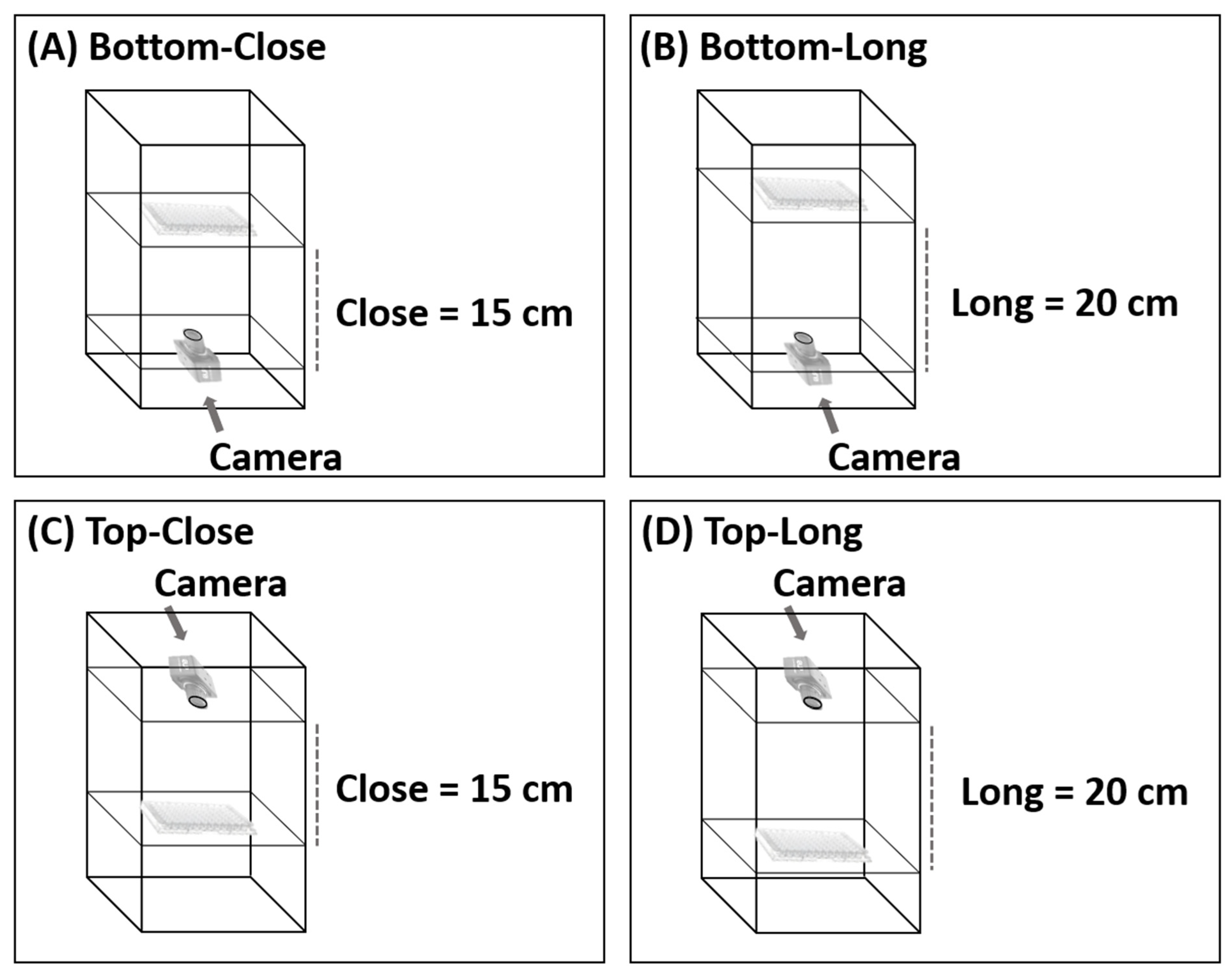
To find the best camera position in the photographic system, images were captured from above and below the 96-well plates and from a distance of 15 cm and 20 cm (Figure 2). If the distance was less than 15 cm, the camera could not focus. Various conditions were optimized. The condition considered optimal was that which produced the lowest relative standard deviation (%RSD) of different concentrations (20, 40, 60, 80, and 100 mg L−1) of four food colorings (red, green, yellow, and black). To study the precision of the proposed method, entire 96-well plates of each food coloring were analyzed at a concentration of 80 mg L−1 (n = 96), and the results were compared with the results obtained from analysis with the spectrophotometer.
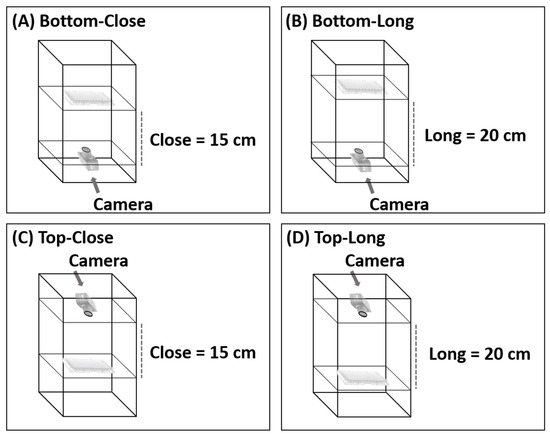
Figure 2.
Various configurations of the photographic system; (A) Bottom-Close, (B) Bottom-Long, (C) Top-Close and (D) Top-Long.
2.5. The RGB Analyzer Program
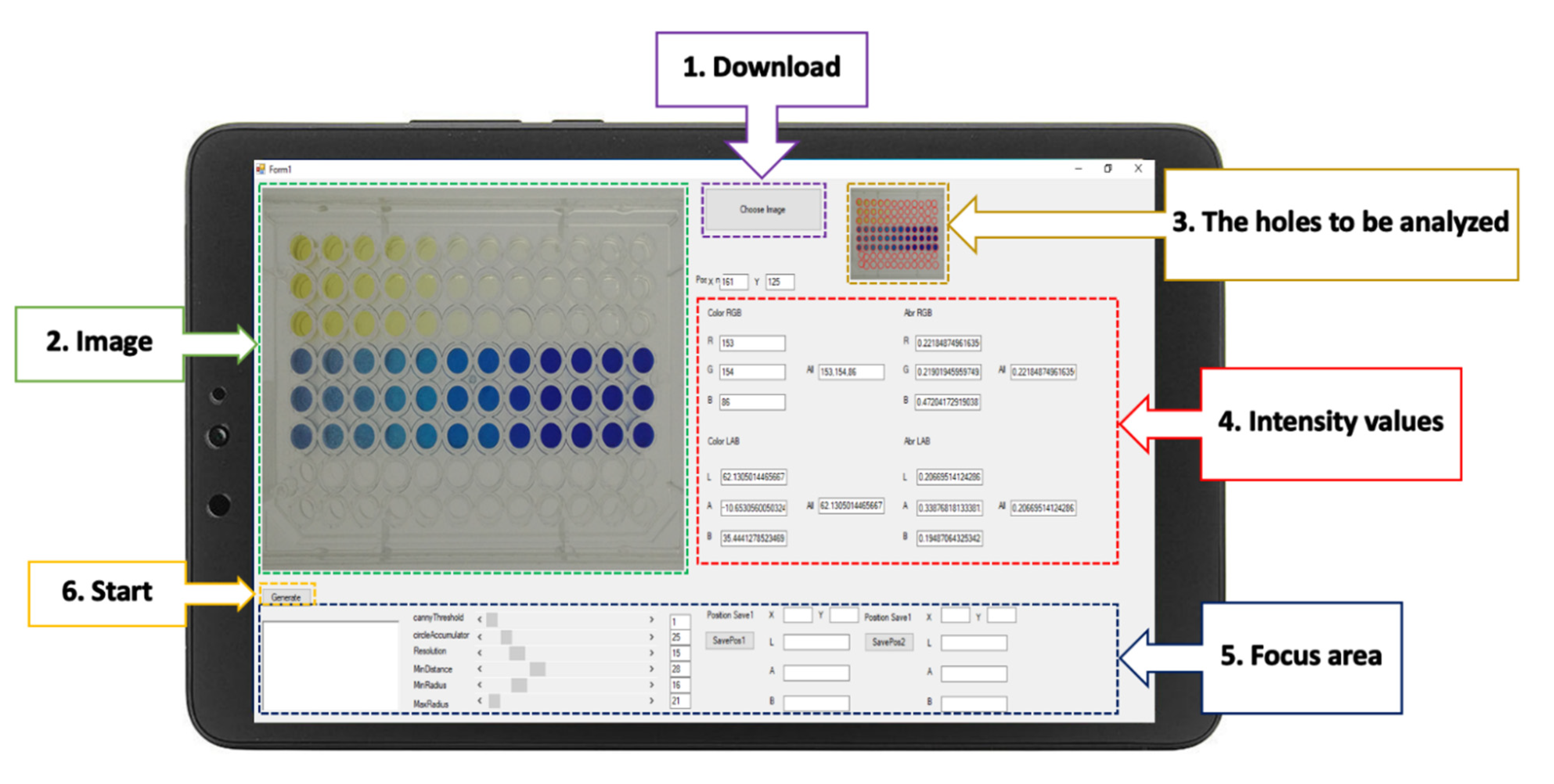
The RGB analyzer program was designed for multi-explosives determination. This program consists of six functions, and the interface presents six elements (Figure 3): the download command to input the images to the program; the image presented after download; a screen that shows the wells to be analyzed; the intensity values displayed in real time; a focus adjustment for better analysis of a specific area; and the start command of the RGB analyzer program. After the start command has been initiated, the analytical data will automatically appear in the Excel program. Data can be transferred to connected colleagues on a network system or by Bluetooth.
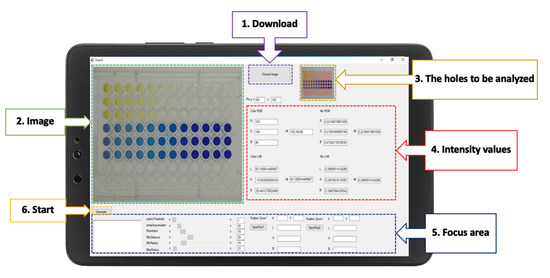
Figure 3.
The graphical interface of the RGB analyzer program for the determination of multi-explosives.
2.6. Analytical Performance and Method Validation
The linear range, limit of detection (LOD), precision, accuracy, interference effect, and specificity of the method were investigated. The LOD was calculated by using LOD = 3(Sa/b), where Sa is the standard deviation of the intercept and b is the slope of the calibration curve. Precision was indicated by the relative standard deviation percentage (%RSD) for each RGB value. The accuracy of the method in terms of percent recovery was evaluated by analyzing the known concentration of each explosive standard solution against the established calibration curve; specificity was tested toward each explosive singly and toward mixtures of two, three, and four explosives.
2.7. Interference Effects
Next, we assessed the specificity of the proposed method for the measurement of the studied explosives. The interference effects of the developed method for multi-explosives determination were investigated by testing some species (urea, Zn2+, Ba2+, Cu2+, Pb2+, Ni2+, Mg2+, Fe2+, Sb3+, SO42−, Cl−, and NO3−) that were previously reported to be present in real samples [18,21,35]. The tolerance limits for the potential interfering substances were defined as the maximum concentration that produced a signal error less than ±5% compared with the signal from standard explosives determined by mixing different concentrations of these possible interference species with 10 mg L−1 TNT, 0.5 mg mL−1 DNT, 2 mg L−1 nitrite, and 0.7 mg mL−1 perchlorate under the optimal conditions.
2.8. Application for Multi-Explosives Sensing
To simulate the forensic conditions encountered in real-case scenarios, six different material samples were used as substrates during the collection of explosive residues. These materials were a table, a tile floor, a mobile phone, a windowsill, a PVC pipe, and a wall clock. The samples were collected from substrate surfaces by swabbing with a cotton swab (15 cm size L, United Medicine Instruments Co., Ltd., Bangkok, Thailand). Then, the cotton swab was inserted into a microtube containing an appropriate solvent solution for each explosive. For 2,4,6-trinitrotoluene (TNT) and 2,4-dinitrotoluene (DNT), the solvent solution was 1:1 acetone–water [4,5]. Deionized water was used as the solvent solution for nitrite and perchlorate. Then, the solution was dropped onto the polymer gel sensors in the plates. Final sample concentrations were 10, 15, 20, 25, and 30 mg L−1 for TNT, 0.25, 0.30, 0.35, 0.40, and 0.45 mg mL−1 for DNT, 1.5, 2.0, 2.5, 3.0, and 3.5 mg L−1 for nitrite, and 0.55, 0.60, 0.65, 0.70, and 0.75 mg mL−1 for perchlorate. The recoveries were evaluated according to the AOAC guideline.
3. Results and Discussion
3.1. Optimization of the Photographic System
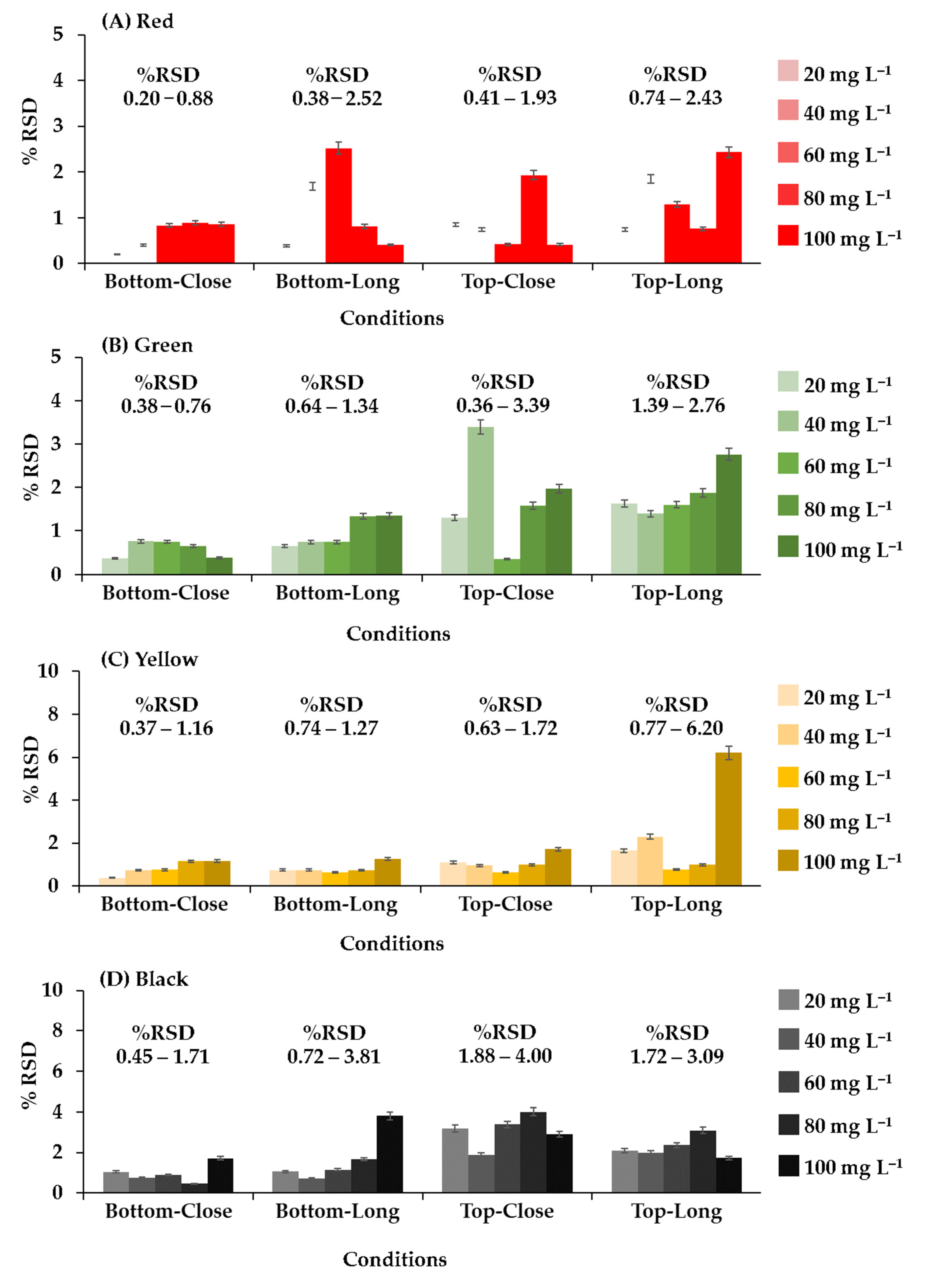
The position and distance of the camera relative to the 96-well plate was adjusted, as shown in Figure 4. The system analyzed red, green, yellow, and black food coloring at different concentrations (20, 40, 60, 80, and 100 mg L−1) with the camera in each position. In the initial study, food coloring was chosen for the optimum condition of the camera position in the photographic system and the precision (n = 96) of the proposed method because it is inexpensive, nontoxic, harmless, and stable. This constant property eliminates color shifts over time. Different results were obtained from every configuration, but the best results were obtained when the camera was positioned below the plate at a distance of 15 cm (Figure 2A). The relative standard deviations (RSDs) (n = 3) were the lowest, and the RSD ranges were the narrowest at all concentrations and colors of food coloring (0.20–0.88%RSD of red (Figure 4A), 0.38–0.76%RSD of green (Figure 4B), 0.37–1.16%RSD of yellow (Figure 4C), and 0.45–1.71%RSD of black (Figure 4D)). These results showed that the capturing position and the distance between the plate and camera affected the results of analysis. Therefore, in further experiments, images were captured from underneath the 96-well plates at a distance of 15 cm. The relevant AOAC guideline for food coloring specifies an RSD of <5.3% at a concentration of 80 mg L−1 [36]. The RSDs of the results of analysis of the entire 96-well plate using the microplate spectrophotometer ranged from 1.67 to 2.20% (Table 1) and therefore were acceptable. The RSDs (n = 96) of the results obtained for the same analysis using the proposed method ranged from 3.24 to 3.61%. The results of the proposed method were acceptable and demonstrated excellent performance and high throughput (96 samples at a time) of the proposed method.
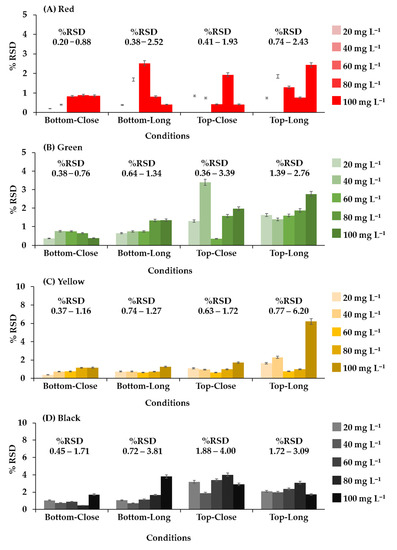
Figure 4.
The relative standard deviation of each system configuration from analysis of food coloring; (A) red, (B) green, (C) yellow, and (D) black.

Table 1.
The relative standard deviations of the proposed method and microplate spectrophotometry for four food colorings at a concentration of 80 mg L−1.
3.2. Colorimetric Test of Explosives
3.2.1. Colorimetric Test of TNT and DNT
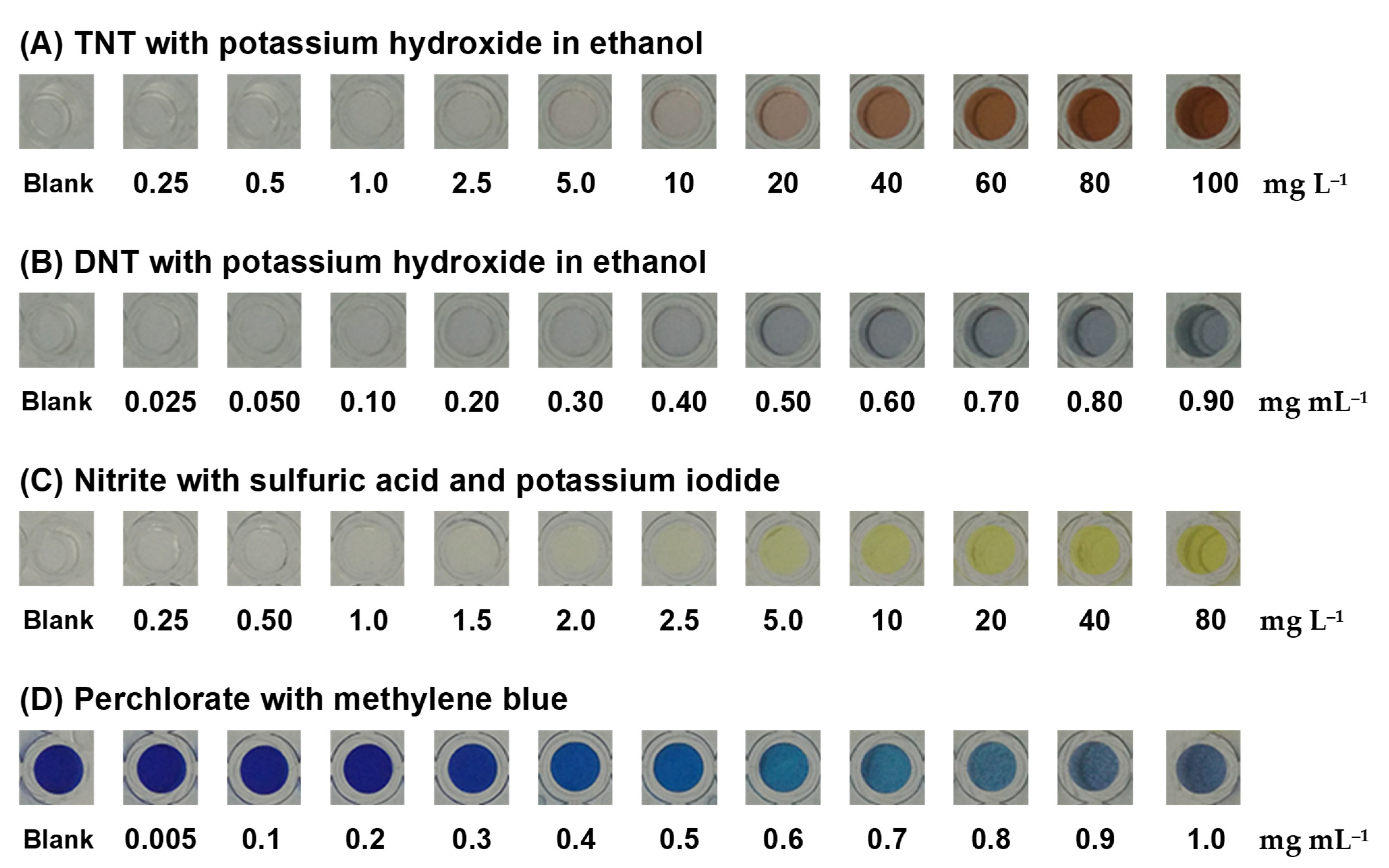
A colorimetric test of TNT and DNT with resulting production of a Janowsky complex by the nitro-aromatic rings of TNT and DNT with alkaline acetone was proceeded with potassium hydroxide (KOH) [34,37,38,39]. Clear TNT and DNT standard solutions were added into wells containing KOH in the polymer gel. TNT produced a red-brown product (Figure 5A) and DNT produced a blue product (Figure 5B). The product color intensity increased with increasing TNT or DNT concentrations. By the naked eye, the colored product of 10 mg L−1 TNT and 0.5 mg mL−1 DNT was visible, indicating that the polymer gel sensor was sensitive enough. These color products were reacted for 5 min prior to capturing the image.
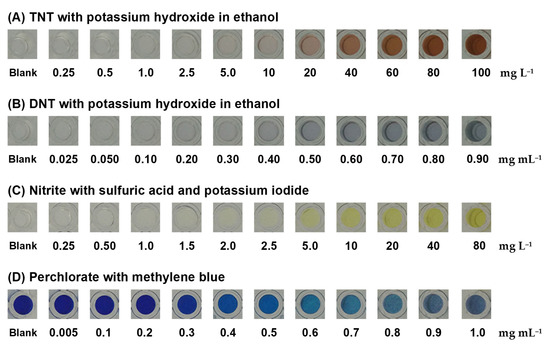
Figure 5.
Colorimetric products of (A) TNT and (B) DNT were produced with potassium hydroxide in ethanol. Colorimetric products of (C) nitrite were produced with sulfuric acid and potassium iodide and colorimetric products of (D) perchlorate were produced with methylene blue.
3.2.2. Colorimetric Test of Nitrite
Nitrite reacted with sulfuric acid and potassium iodide in the polymer gel sensor to produce a yellow product of iodine (Figure 5C), and it could produce nitric oxide, potassium sulfate, sodium sulfate, and water, in which the nitrite in the acidic solution selectively reacted with iodide to form iodine [40,41,42,43]. The homogeneous yellow color of the product in the polymer gel sensor intensified with increasing nitrite concentrations, which indicated a homogenous reagent entrapment within the polymer gel matrix.
3.2.3. Colorimetric Test of Perchlorate
Perchlorate was detected through the use of MB in the polymer gel sensor with the precipitation of a violet methylene blue perchlorate complex [44,45,46,47,48]. The increment of the violet precipitate produced by the reaction increased with the concentration of perchlorate, allowing a semiquantitative analysis to be developed. When the concentration of perchlorate was increased, the blue color of MB became lighter, as shown in Figure 5D.
3.3. Digital Image Colorimetry for Explosives Quantification
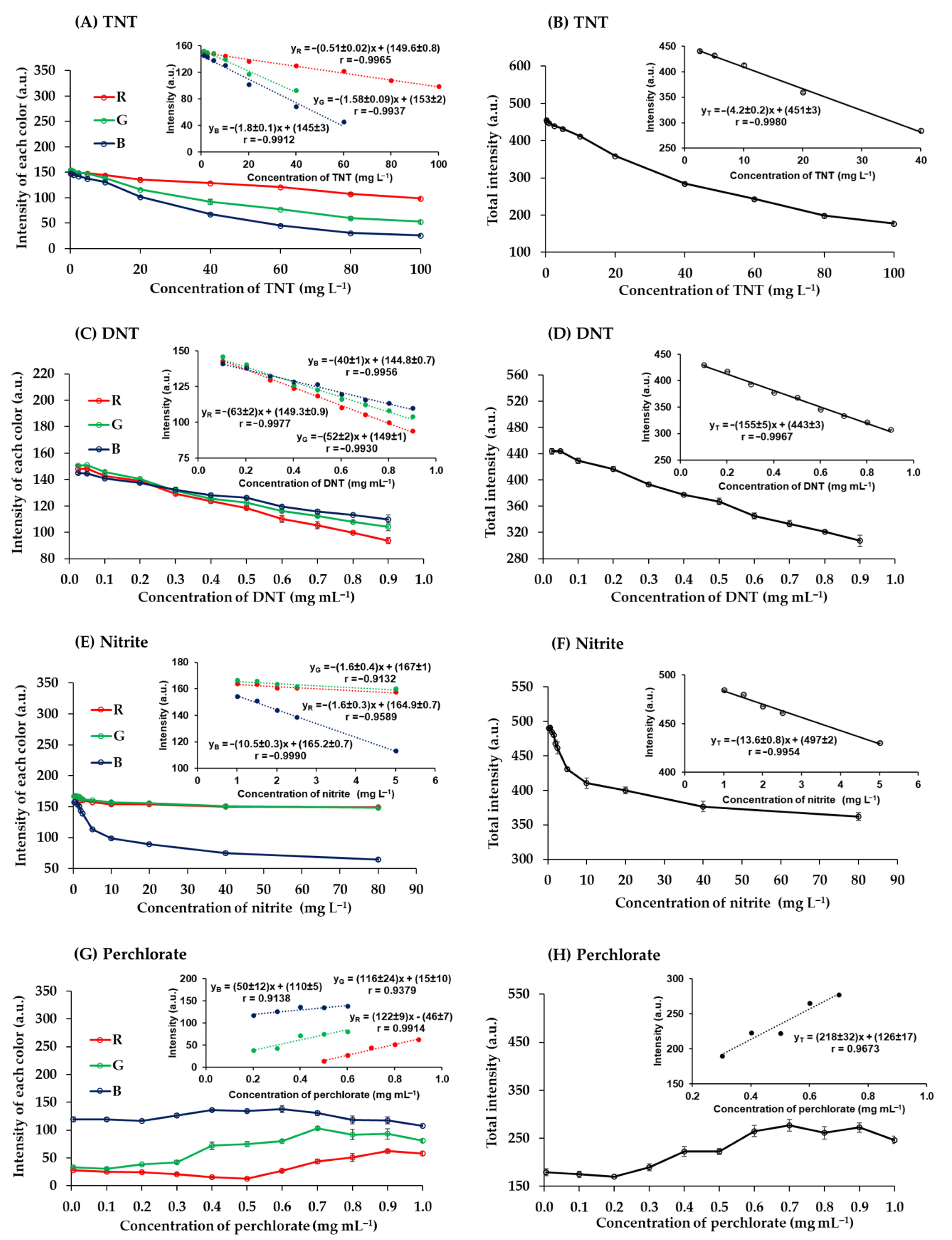
The quantitative analysis of the studied explosives was achieved using digital image colorimetry. Digital images of color products were analyzed using the RGB analyzer program on a notebook in real time. The program determined RGB intensity values ranging from 0 to 255 for each color. A white image would produce R, G, and B values of 255, 255, and 255, and a black image would produce R, G, and B values of 0, 0, and 0. These images were captured with an industrial video camera within the photographic box. Calibration curves were plotted of color intensities against explosive concentrations (Figure 6).
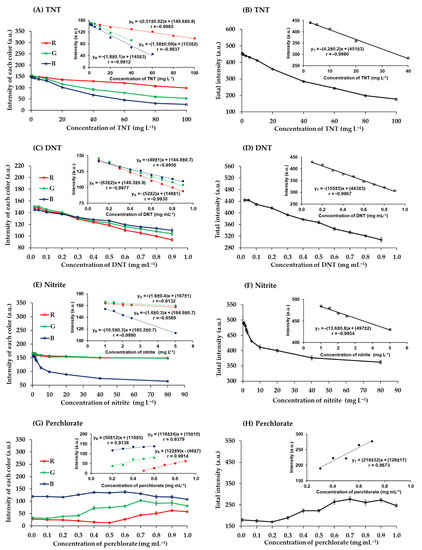
Figure 6.
Relationship between the intensity of each color and the concentration of (A) TNT, (C) DNT, (E) nitrite, and (G) perchlorate and the relationship between the total intensity and the concentration of (B) TNT, (D) DNT, (F) nitrite, and (H) perchlorate.
The red-brown products of TNT reacted with potassium hydroxide were captured and analyzed. The RGB intensity values were correlated with the concentrations of TNT (Figure 6A). The red intensity was higher than green and blue intensities, which is to be expected given the red-brown color of the product, and it remained higher in the concentration range analyzed. The green and blue intensities decreased with increasing TNT concentrations from 0.25 to 100 mg L−1, as previously reported. The relationships derived from the combination of the total RGB intensity values (T = R + G + B) (Figure 6B). It was found that the total intensity was a more sensitive measure than the individual RGB values.
The quantification of DNT with potassium hydroxide gave blue products. Blue intensity was lower than red and green intensities at DNT concentrations below 0.3 mg mL−1. However, blue intensity was the highest at higher concentrations (Figure 6C) because the products were dark blue [34]. The sensitivity of the total values was higher than the sensitivity of each color intensity (Figure 6D).
Nitrite was quantified by reaction with sulfuric acid and potassium iodide to produce a yellow product. The red and green intensities of this product were similar, while the blue intensity was the lowest at all concentrations of nitrite (0.25 to 80 mg L−1) (Figure 6E) because the product was yellow, which is a color combination of red and green [49]. The individual RGB values had a lower sensitivity than the total intensity (Figure 6F).
The quantitative analysis of perchlorate with MB produced a violet precipitate product associated with the MB perchlorate complex. The blue intensity was relatively stable but decreased slightly as the concentration of chlorate increased >0.6 mg mL−1 (Figure 6G) due to the increasing presence of the MB perchlorate complex. Conversely, when the concentration of perchlorate increased, the red and green intensity values increased. At perchlorate concentrations from 0.5 to 0.9 mg mL−1, red intensity increased more than total RGB intensity, indicating greater sensitivity in this concentration range (Figure 6G,H).
3.4. Analytical Performance and Method Validation
The calibration equations, including the linear range and linearity for the quantification of TNT, DNT, nitrite, and perchlorate, are summarized in Table 2. Although the total RGB intensity provided higher sensitivity than the individual RGB values of all explosives (−4.2 ± 0.2 a.u. L mg−1 of TNT, −155 ± 5 a.u. mL mg−1 of DNT, −13.6 ± 0.8 a.u. L mg−1 of nitrite and 218 ± 32 a.u. mL mg−1 of perchlorate), the correlation coefficient (r) must be better than 0.995 [50]. Consequently, perchlorate determination was based on the red intensity, and the determination of TNT, DNT, and nitrite was based on the total intensity. The analytical performance of the proposed method was presented in Table 3. The LODs of TNT, DNT, nitrite, and perchlorate were 2.28 mg L−1, 0.0523 mg mL−1, 0.512 mg L−1, and 0.163 mg mL−1, respectively. More importantly, even after 30 days of preparation, the polymer gel sensor continued to produce consistent colors for the tests with TNT, DNT, nitrite, and perchlorate. As a result, it was found that the proposed polymer gel sensor has a usable time of at least one month.

Table 2.
The calibration equations for the quantification of the four explosives studied.

Table 3.
Analytical performances of the proposed method from the colorimetric test of the explosives studied.
3.5. Specificity
The specificity of the proposed method toward the four explosives was studied using the explosives singly and in mixtures of two, three, and four explosives (Table 4). The results indicated good specificity. Products of reactions between TNT/DNT and KOH were purple, which is a combination of red/brown and blue. Sulfuric acid/potassium iodide and MB only reacted with nitrite and perchlorate and so could only be used for the qualitative analysis of an explosive mixture to indicate the likely presence of some kind of explosive substance containing nitrite or perchlorate. It should be noted that only nitrite and a mixture of nitrite + perchlorate can be detected. In contrast, the mixed sample containing TNT + nitrite, DNT + nitrite, TNT + DNT + nitrite, TNT + nitrite + perchlorate, DAT + nitrite + perchlorate, and TNT + DNT + nitrite + perchlorate was undetectable. This may be due to decreased nitrite solubility because TNT and DNT use acetone (1:1 acetone–water) as a solvent. The revised manuscript includes these details in Section 3.5.

Table 4.
Specificity of single explosives and multiple combinations of explosives to reagents.
3.6. Interference
The influence of various foreign species on the determination of the studied multi-explosives using the proposed method was evaluated under the same experimental conditions. The potential interferences comprised urea, Zn2+, Ba2+, Cu2+, Pb2+, Ni2+, Mg2+, Fe2+, Sb3+, SO42−, Cl−, and NO3−. The tolerance limit was assigned as the concentration of the interfering species which caused a relative error of intensity change below ±5% (Table 5). These experimental results suggested that the proposed method provided good selectivity when applied for the simultaneous measurement of TNT, DNT, nitrite, and perchlorate.

Table 5.
Tolerance limits of potential interferences on the determination of 10 mg L−1 TNT, 0.5 mg mL−1 DNT, 2 mg L−1 nitrite, and 0.7 mg mL−1 perchlorate.
3.7. Analysis of Case Work Sample
The correlations between the optimal RGB values and the concentration of TNT, DNT, nitrite, and perchlorate was also investigated using six different substrate samples (tables, tile floors, mobile phones, windowsills, PVC pipes, and wall clocks). Percentage recoveries ranged from 83 ± 3 to 106 ± 2% for TNT, from 92 ± 2 to 104 ± 3% for DNT, from 89 ± 2 to 108 ± 2% for nitrite, and from 79.3 ± 0.6 to 118 ± 3% for perchlorate (Table 6). The recoveries of TNT, DNT, and nitrite were acceptable according to AOAC guidelines [36], but perchlorate was slightly above the limit because the violet precipitate product affected the quantitative analysis. The overall results indicated that the proposed method could be applied to qualitative and quantitative screening of multi-explosives.

Table 6.
Recoveries from six different substrate samples.
4. Conclusions
The determination of multi-explosives detected with specific polymer gel sensors on 96-well plates was based on the colorimetric analysis of digital images. RGB values were produced from images of reaction products and were successfully applied to quantify the explosive compounds in the reactions. A simple photographic box and a software program were designed to create a portable system that can transmit data wirelessly. The proposed method was rapid (96 samples at a time), accurate, and portable. The real-time analysis enables the use of the method in forensic cases and shows great potential to rapidly progress criminal investigations.
Author Contributions
Conceptualization, Y.T., A.T., A.P., S.T., W.J., S.J., K.T. and W.L.; methodology, Y.T., A.T., A.P. and W.L.; validation, Y.T., A.T., A.P., S.T., W.J., S.J., K.T. and W.L.; formal analysis, Y.T., A.T., A.P., S.T., W.J., S.J., K.T. and W.L.; writing—original draft preparation, Y.T. and W.L.; writing—review and editing, Y.T., A.T., A.P., S.T., W.J., S.J., K.T. and W.L.; project administration, W.L.; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded by the National Research Council of Thailand (grant number SCI6305166b).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This paper was supported by the forensic science innovation and service center, the police forensic science center 9, the Center of Excellence for Trace Analysis and Biosensors (TAB-CoE), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), the Office of the Higher Education Commission, Division of Health and Applied Sciences, Graduate School and Faculty of Science, Prince of Songkla University, Hat Yai, Thailand. The authors would like to thank Thomas Duncan Coyne for his assistance with the English.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DePalma, R.G.; Aharonson-Daniel, L. Blast Injuries. N. Engl. J. Med. 2005, 352, 1335–1342. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, S.; Jain, P. Importance of Forensic Investigation in Explosion: A Case study. J. Forensic Res. 2016, 7, 5. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Reducing the Threat of Improvised Explosive Device Attacks by Restricting Access to Explosive Precursor Chemicals; National Academies Press: Washington, DC, USA, 2018; p. 214. [Google Scholar]
- Boonpanya, J.; Chaikum, N.; Kontrong, P.; Wilairat, P.; Tiensuwan, M. Forensic Investigation of Explosion Cases in the Restive South. R. Thai Army Med. J. 2007, 60, 91–100. [Google Scholar]
- Steinfeld, J.I.; Wormhoudt, J. Explosives detection: A Challenge for Physical Chemistry. Annu. Rev. Phys. Chem. 1998, 49, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Perr, J.M.; Furton, K.G.; Almirall, J.R. Gas chromatography positive chemical ionization and tandem mass spectrometry for the analysis of organic high explosives. Talanta 2005, 67, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Pinnaduwage, L.A.; Thundat, T.; Hawk, J.; Hedden, D.; Britt, P.; Houser, E.; Stepnowski, S.; McGill, R.; Bubb, D. Detection of 2,4-dinitrotoluene using microcantilever sensors. Sens. Actuators B Chem. 2004, 99, 223–229. [Google Scholar] [CrossRef]
- Hao, M.; Zeng, W.; Li, Y.-Q.; Wang, Z.-C. Three-dimensional graphene and its composite for gas sensors. Rare Met. 2021, 40, 1494–1514. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Zhang, J.-W.; Li, X.-G.; Rumyantseva, M.N.; Gaskov, A.M. ZnSe/NiO heterostructure-based chemiresistive-type sensors for low-concentration NO2 detection. Rare Met. 2020, 40, 1632–1641. [Google Scholar] [CrossRef]
- Zhao, Q.-N.; Zhang, Y.-J.; Duan, Z.-H.; Wang, S.; Liu, C.; Jiang, Y.-D.; Tai, H.-L. A review on Ti3C2Tx-based nanomaterials: Synthesis and applications in gas and humidity sensors. Rare Met. 2021, 40, 1459–1476. [Google Scholar] [CrossRef]
- Gaurav; Kaur, V.; Kumar, A.; Malik, A.K.; Rai, P.K. SPME-HPLC: A new approach to the analysis of explosives. J. Hazard. Mater. 2007, 147, 691–697. [Google Scholar] [CrossRef]
- Gaurav; Malik, A.K.; Rai, P.K. Development of a new SPME–HPLC–UV method for the analysis of nitro explosives on reverse phase amide column and application to analysis of aqueous samples. J. Hazard. Mater. 2009, 172, 1652–1658. [Google Scholar] [CrossRef]
- Sarazin, C.; Delaunay, N.; Varenne, A.; Vial, J.; Costanza, C.; Eudes, V.; Minet, J.-J.; Gareil, P. Identification and determination of inorganic anions in real extracts from pre- and post-blast residues by capillary electrophoresis. J. Chromatogr. A 2010, 1217, 6971–6978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Sun, Y.; Zeng, L.; Mei, H.; Xu, J.; Guo, B.; Hao, H. Detection of Three Common Organic Explosives Using Capillary Electrophoresis. J. Mater. Sci. Chem. Eng. 2016, 4, 17–25. [Google Scholar] [CrossRef][Green Version]
- Johns, C.; Shellie, R.; Potter, O.G.; O’Reilly, J.W.; Hutchinson, J.P.; Guijt, R.; Breadmore, M.; Hilder, E.; Dicinoski, G.W.; Haddad, P.R. Identification of homemade inorganic explosives by ion chromatographic analysis of post-blast residues. J. Chromatogr. A 2008, 1182, 205–214. [Google Scholar] [CrossRef]
- Tyrrell, É.; Dicinoski, G.W.; Hilder, E.F.; Shellie, R.A.; Breadmore, M.C.; Pohl, C.A.; Haddad, P.R. Coupled reversed-phase and ion chromatographic system for the simultaneous identification of inorganic and organic explosives. J. Chromatogr. A 2011, 1218, 3007–3012. [Google Scholar] [CrossRef]
- Marinović, V.; Marinović, S.; Jovanović, M.; Jovanović, J.; Štrbac, S. The electrochemical reduction of trinitrotoluene on a platinum wire modified by chemisorbed acetonitrile. J. Electroanal. Chem. 2010, 648, 1–7. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Nitrite amperometric sensor for gunshot residue screening. Electrochim. Acta 2019, 331, 135309. [Google Scholar] [CrossRef]
- Arshad, A.; Wang, H.; Bai, X.; Jiang, R.; Xu, S.; Wang, L. Colorimetric paper sensor for sensitive detection of explosive nitroaromatics based on Au@Ag nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 16–22. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Visual detection of peroxide-based explosives using novel mimetic Ag nanoparticle/ZnMOF nanocomposite. J. Hazard. Mater. 2018, 360, 233–242. [Google Scholar] [CrossRef]
- Thipwimonmas, Y.; Jaidam, J.; Samoson, K.; Khunseeraksa, V.; Phonchai, A.; Thiangchanya, A.; Chang, K.; Abdullah, A.; Limbut, W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes. Chemosensors 2021, 9, 161. [Google Scholar] [CrossRef]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; NicDaeid, N. A sol–gel colorimetric sensor for methamphetamine detection. Sens. Actuators B Chem. 2015, 215, 553–560. [Google Scholar] [CrossRef]
- Kaewnu, K.; Samoson, K.; Thiangchanya, A.; Phonchai, A.; Limbut, W. A novel colorimetric indicator for ethanol detection in preserved baby mangoes. Food Chem. 2022, 369, 130769. [Google Scholar] [CrossRef]
- Arts, R.; Hartog, I.D.; Zijlema, S.; Thijssen, V.; Van Der Beelen, S.H.E.; Merkx, M.M. Detection of Antibodies in Blood Plasma Using Bioluminescent Sensor Proteins and a Smartphone. Anal. Chem. 2016, 88, 4525–4532. [Google Scholar] [CrossRef] [PubMed]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; NicDaeid, N. Using the iPhone as a device for a rapid quantitative analysis of trinitrotoluene in soil. Talanta 2013, 115, 143–149. [Google Scholar] [CrossRef]
- Choodum, A.; Boonsamran, P.; NicDaeid, N.; Wongniramaikul, W. On-site semi-quantitative analysis for ammonium nitrate detection using digital image colourimetry. Sci. Justice 2015, 55, 437–445. [Google Scholar] [CrossRef]
- Jirasek, A. Experimental investigations of polymer gel dosimeters. J. Phys. Conf. Ser. 2007, 56, 23. [Google Scholar] [CrossRef]
- Li, X.; Nakagawa, S.; Tsuji, Y.; Watanabe, N.; Shibayama, M. Polymer gel with a flexible and highly ordered three-dimensional network synthesized via bond percolation. Sci. Adv. 2019, 5, eaax8647. [Google Scholar] [CrossRef]
- Philippova, O.E.; Khokhlov, A.R. 1.13-Polymer Gels, in Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 339–366. [Google Scholar]
- Hassan, P.A.; Verma, G.; Ganguly, R. 1-Soft Materials—Properties and Applications, in Functional Materials; Banerjee, S., Tyagi, A.K., Eds.; Elsevier: London, UK, 2012; pp. 1–59. [Google Scholar]
- Zheng, J.; Zeng, R.; Zhang, F.; Kan, J. Effects of sodium carboxymethyl cellulose on rheological properties and gelation behaviors of sodium alginate induced by calcium ions. LWT 2019, 103, 131–138. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef]
- Choodum, A.; Malathong, K.; NicDaeid, N.; Limsakul, W.; Wongniramaikul, W. A cost effective hydrogel test kit for pre and post blast trinitrotoluene. Forensic Sci. Int. 2016, 266, 202–208. [Google Scholar] [CrossRef]
- Hassanzadeh, J.; Khataee, A.; Bagheri, N.; Lotfi, R. Sensitive chemiluminescence determination method for 2,4,6-trinitrotoluene based on the catalytic activity of amine-capped gold nanoparticles. New J. Chem. 2017, 41, 134–141. [Google Scholar] [CrossRef]
- AOAC. Appendix F: Guidelines for Standard Method Performance Requirements; AOAC: Rockville, MD, USA, 2016. [Google Scholar]
- Jenkins, T.F.; Walsh, M.E. Development of field screening methods for TNT, 2,4-DNT and RDX in soil. Talanta 1992, 39, 419–428. [Google Scholar] [CrossRef]
- Üzer, A.; Erçağ, E.; Apak, R. Selective colorimetric determination of TNT partitioned between an alkaline solution and a strongly basic Dowex 1-X8 anion exchanger. Forensic Sci. Int. 2008, 174, 239–243. [Google Scholar] [CrossRef]
- Erçağ, E.; Üzer, A.; Eren, Ş.; Sağlam, Ş.; Filik, H.; Apak, R. Rapid detection of nitroaromatic and nitramine explosives on chromatographic paper and their reflectometric sensing on PVC tablets. Talanta 2011, 85, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, R.; Sornambikai, S.; Karthikeyan, N.S.; Sathiyanarayanan, K.; Kumar, A.S. A Simple Colorimetric Screening of Nitrite Using Iodide in an Acidic pH Solution (Invited article). Austin J. Anal. Pharm. Chem. 2014, 1, 2. [Google Scholar]
- Miura, Y.; Hamada, H. Ion chromatography of nitrite at the ppb level with photometric measurement of iodine formed by post-column reaction of nitrite with iodide. J. Chromatogr. A 1999, 850, 153–160. [Google Scholar] [CrossRef]
- Grgur, B.N.; Gvozdenović, M.; Stevanovic, J.; Jugovic, Z.K.; Trišović, T. Electrochemical oxidation of iodide in aqueous solution. Chem. Eng. J. 2006, 124, 47–54. [Google Scholar] [CrossRef]
- Rastegarzadeh, S.; Kalantaripour, M. Development of an optical redox chemical sensor for nitrite determination. Química Nova 2012, 35, 766–770. [Google Scholar] [CrossRef][Green Version]
- Peters, K.L.; Corbin, I.; Kaufman, L.M.; Zreibe, K.; Blanes, L.; McCord, B.R. Simultaneous colorimetric detection of improvised explosive compounds using microfluidic paper-based analytical devices (μPADs). Anal. Methods 2015, 7, 63–70. [Google Scholar] [CrossRef]
- Urbansky, E.T. Perchlorate Chemistry: Implications for Analysis and Remediation. Bioremediat. J. 1998, 2, 81–95. [Google Scholar] [CrossRef]
- Nabar, G.M.; Ramachandran, C.R. Quantitative Determination of Perchlorate Ion in Solution. Anal. Chem. 1959, 31, 263–265. [Google Scholar] [CrossRef]
- Urbansky, E.T. Quantitation of Perchlorate Ion: Practices and Advances Applied to the Analysis of Common Matrices. Crit. Rev. Anal. Chem. 2000, 30, 311–343. [Google Scholar] [CrossRef]
- Chemical Confirmation Tests. In Field Confirmation Testing for Suspicious Substances; CRC Press: Boca Raton, FL, USA, 2009; pp. 75–351.
- Li, G.; Yuan, R.; Yang, Z.; Huang, X. A yellow license plate location method based on RGB model of color image and texture of plate. In Proceedings of the Second Workshop on Digital Media and its Application in Museum & Heritages (DMAMH 2007), Chongqing, China, 10–12 December 2007. [Google Scholar]
- Bruce, P.; Minkkinen, P.; Riekkola, M.-L. Practical method validation: Validation sufficient for an analysis method. Microchim. Acta 1998, 128, 93–106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).