Validity and Sensitivity of an Inertial Measurement Unit-Driven Biomechanical Model of Motor Variability for Gait
Abstract
:1. Introduction
2. Methods
2.1. Participants
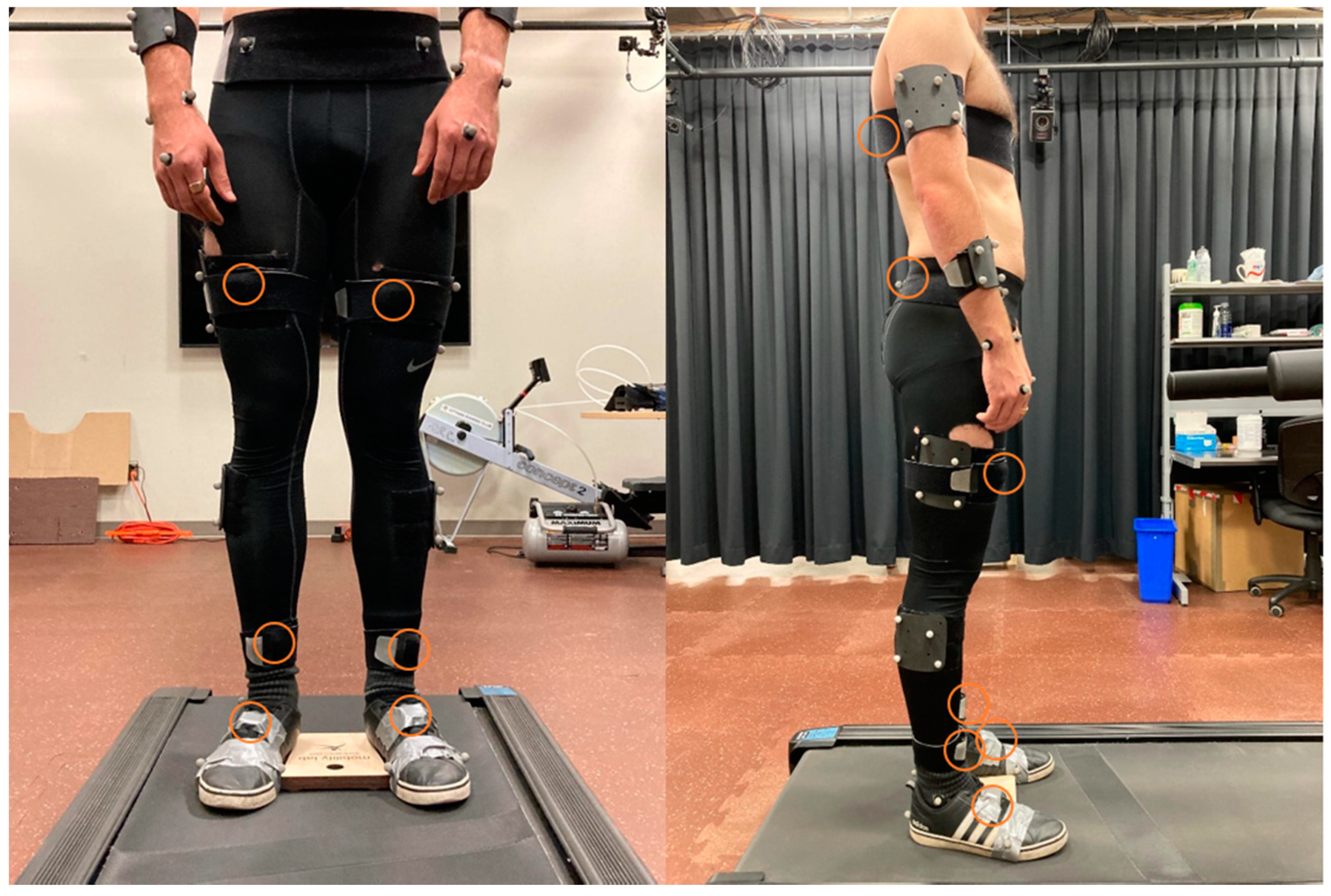
2.2. Instrumentation
2.3. Experimental Procedure
2.4. Data Analysis
2.4.1. Optoelectronic-Based Biomechanical Modelling
2.4.2. IMU-Based Biomechanical Modelling
2.5. Calculation of Kinematic Outcomes
2.5.1. Range of Motion (ROM)
2.5.2. Mean Standard Deviation (meanSD)
2.5.3. Maximum Finite-Time Lyapunov Exponent (λmax)
2.5.4. Detrended Fluctuation Analysis Scaling Exponent (DFAα)
2.5.5. Sample Entropy (SaEn)
2.6. Statistical Analyses
2.6.1. Analyses of IMU-Model Validity
2.6.2. Analyses of IMU-Model Sensitivity
3. Results
3.1. Participant Characteristics
3.2. IMU-Model Validity
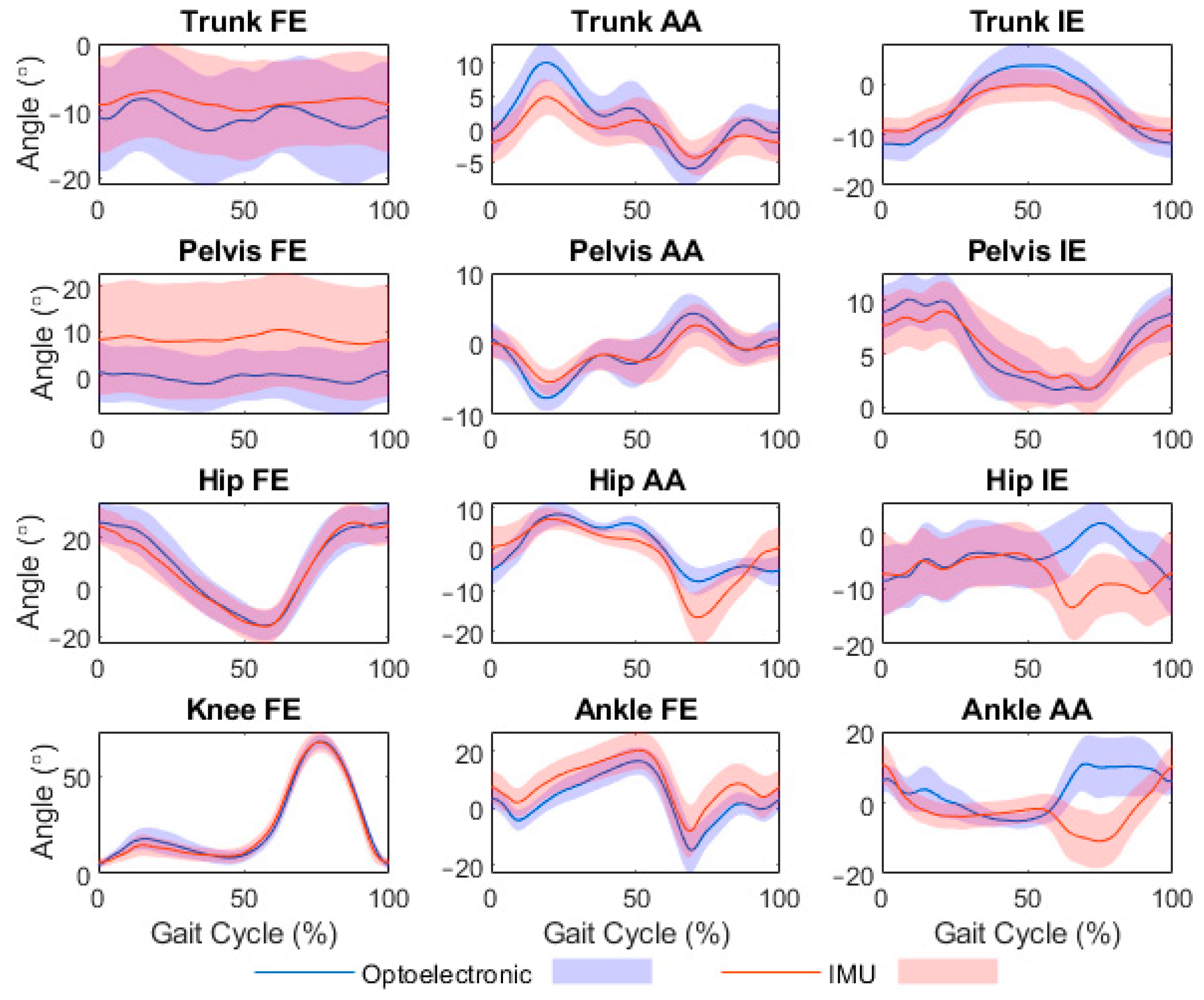
3.2.1. Validity of Joint Angle Time Series
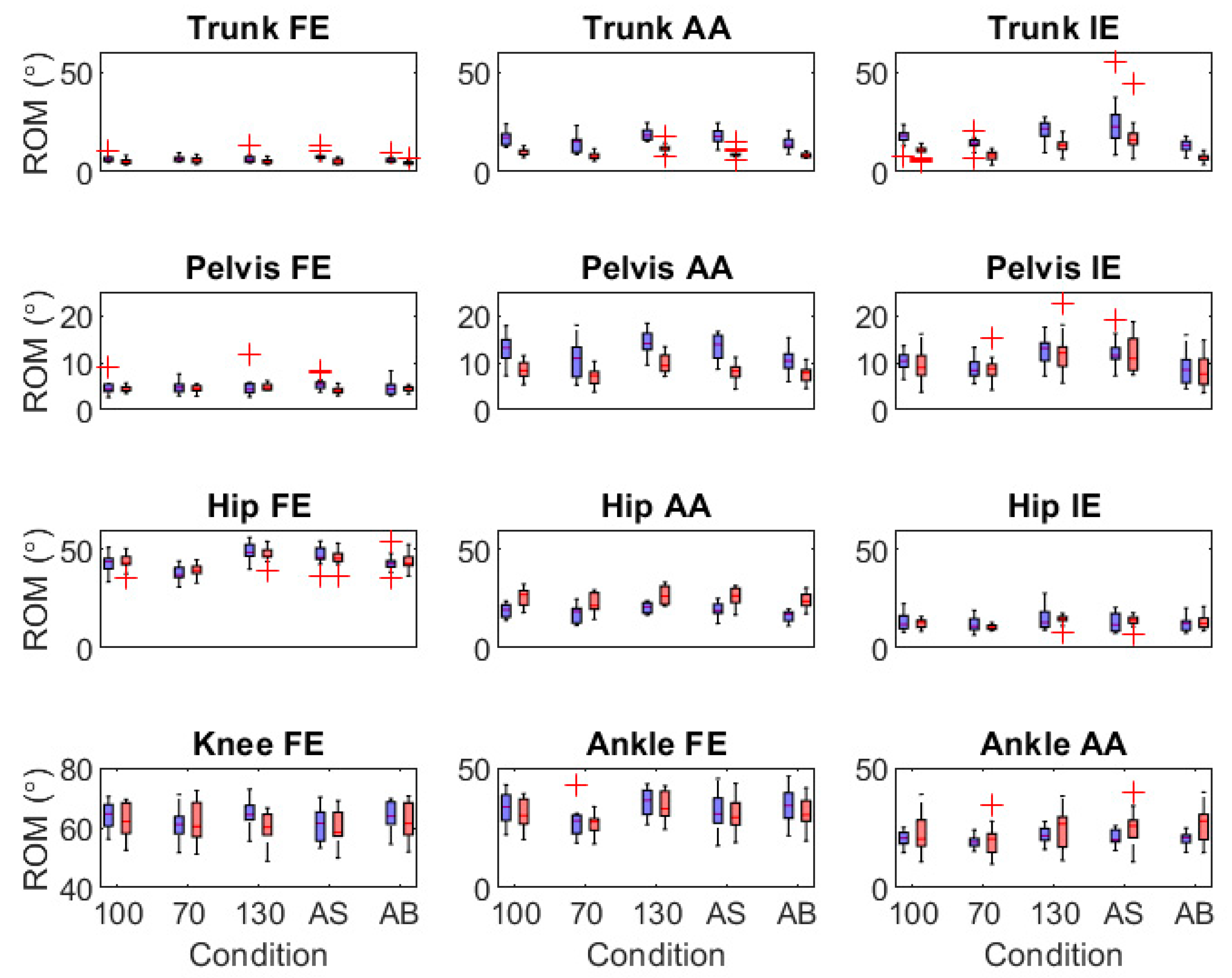
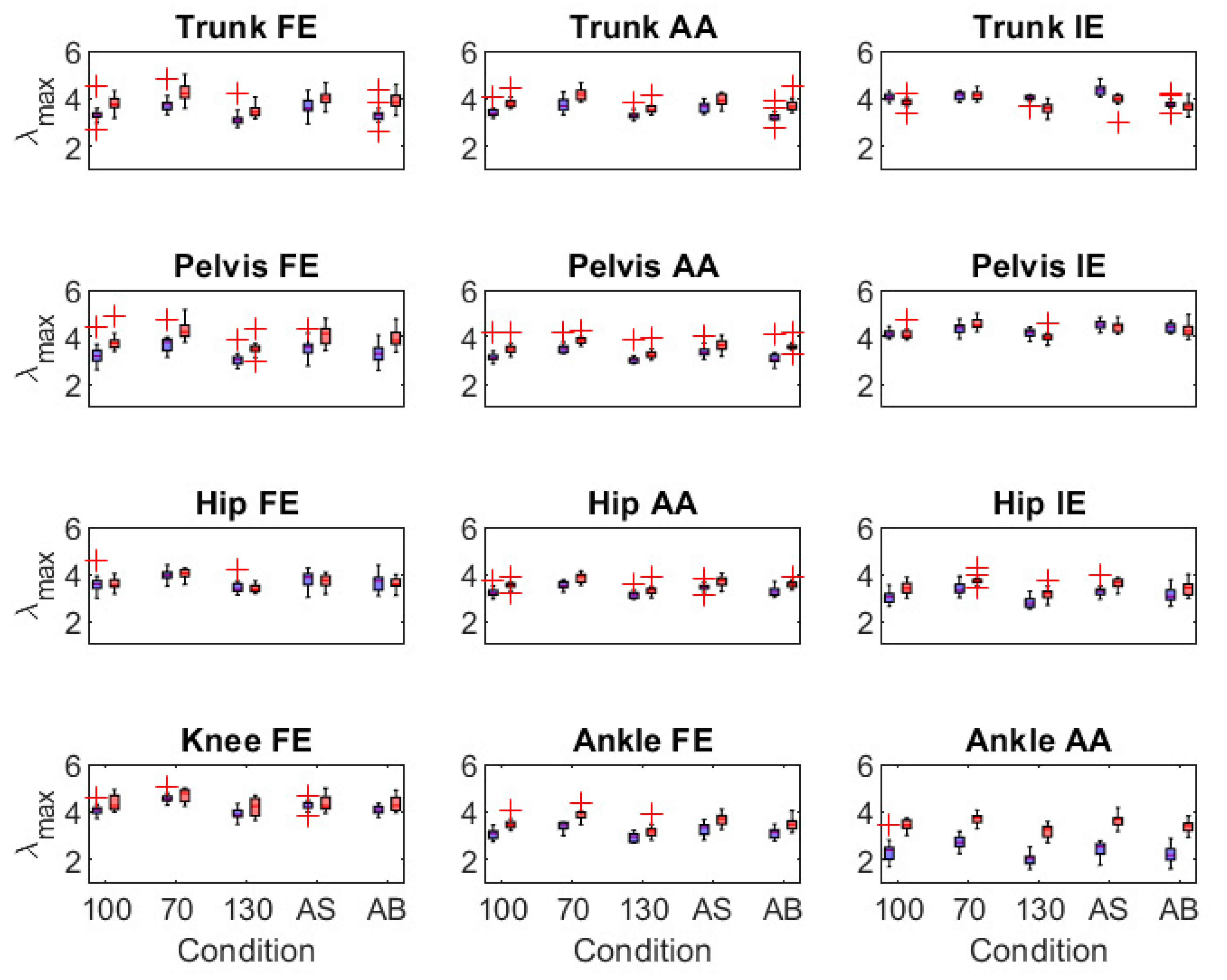
3.2.2. Validity of Joint Angle Range of Motion and Motor Variability Outcomes
3.3. IMU-Model Sensitivity
4. Discussion
4.1. Validity of IMU-Modelled Joint Angle Time Series
4.2. Validity of IMU-Modelled Joint Angle Outcomes
4.3. Sensitivity of IMU-Modelled Joint Angle Outcomes to Within-Participant Effects
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newell, K.M.; Slifkin, A.B. The Nature of Movement Variability. In Motor Behavior and Human Skill: A Multidisciplinary Perspective; Human Kinetics: Champaign, IL, USA, 1998; pp. 143–160. [Google Scholar]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzi, U.H.; Stergiou, N.; Kurz, M.; Hageman, P.; Heidel, J. Nonlinear dynamics indicates aging affects variability during gait. Clin. Biomech. 2003, 18, 435–443. [Google Scholar] [CrossRef]
- Kurz, M.J.; Stergiou, N. The aging humans neuromuscular system expresses less certainty for selecting joint kinematics during gait. Neurosci. Lett. 2003, 348, 155–158. [Google Scholar] [CrossRef]
- Bailey, C.A.; Porta, M.; Pilloni, G.; Arippa, F.; Côté, J.N.; Pau, M. Does variability in motor output at individual joints predict stride time variability in gait? Influences of age, sex, and plane of motion. J. Biomech. 2019, 99, 109574. [Google Scholar] [CrossRef]
- Ihlen, E.A.F.; van Schooten, K.S.; Bruijn, S.M.; van Dieën, J.H.; Vereijken, B.; Helbostad, J.L.; Pijnappels, M. Improved Prediction of Falls in Community-Dwelling Older Adults Through Phase-Dependent Entropy of Daily-Life Walking. Front. Aging Neurosci. 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Picerno, P. 25 years of lower limb joint kinematics by using inertial and magnetic sensors: A review of methodological approaches. Gait Posture 2017, 51, 239–246. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Novak, A.; Brouwer, B.; Li, Q. Concurrent validation of Xsens MVN measurement of lower limb joint angular kinematics. Physiol. Meas. 2013, 34, N63–N69. [Google Scholar] [CrossRef]
- Palermo, E.; Rossi, S.; Marini, F.; Patane, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Measurement 2014, 52, 145–155. [Google Scholar] [CrossRef]
- Zihajehzadeh, S.; Park, E.J. A Novel Biomechanical Model-Aided IMU/UWB Fusion for Magnetometer-Free Lower Body Motion Capture. IEEE Trans. Syst. Man Cybern. Syst. 2016, 47, 927–938. [Google Scholar] [CrossRef]
- Teufl, W.; Miezal, M.; Taetz, B.; Fröhlich, M.; Bleser, G. Validity of inertial sensor based 3D joint kinematics of static and dynamic sport and physiotherapy specific movements. PLoS ONE 2019, 14, e0213064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapp, E.; Shin, S.; Thomsen, W.; Ferber, R.; Halilaj, E. Estimation of kinematics from inertial measurement units using a combined deep learning and optimization framework. J. Biomech. 2021, 116, 110229. [Google Scholar] [CrossRef] [PubMed]
- Slade, P.; Habib, A.; Hicks, J.L.; Delp, S.L. An open-source and wearable system for measuring 3D human motion in real-time. IEEE Trans. Biomed. Eng. 2021, 1. [Google Scholar] [CrossRef] [PubMed]
- Al Borno, M.; O’Day, J.; Ibarra, V.; Dunne, J.; Seth, A.; Habib, A.; Ong, C.; Hicks, J.; Uhlrich, S.; Delp, S. OpenSense: An open-source tool box for Inertial-Measurement-Unit-based measurement of lower extremity kinematics over long durations. bioRxiv 2021, 1–26. [Google Scholar] [CrossRef]
- Ferrari, A.; Cutti, A.G.; Garofalo, P.; Raggi, M.; Heijboer, M.; Cappello, A.; Davalli, A. First in vivo assessment of “Outwalk”: A novel protocol for clinical gait analysis based on inertial and magnetic sensors. Med Biol. Eng. Comput. 2009, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, M.; Nicholas, K.; Button, K.; Sparkes, V.; Sheeran, L.; Davies, J.L. Inertial Measurement Units for Clinical Movement Analysis: Reliability and Concurrent Validity. Sensors 2018, 18, 719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Villa, S.G.; Munoz Diaz, E.; Ahmed, D.B.; Jimenez Martin, A.; Dominguez, J.J.G. IMU-based Characterization of the Leg for the Implementation of Biomechanical Models. In Proceedings of the 2019 International Conference on Indoor Positioning and Indoor Navigation (IPIN), Pisa, Italy, 30 September–3 October 2019; Volume 17, pp. 1–8. [Google Scholar]
- Weygers, I.; Kok, M.; Konings, M.; Hallez, H.; De Vroey, H.; Claeys, K. Inertial Sensor-Based Lower Limb Joint Kinematics: A Methodological Systematic Review. Sensors 2020, 20, 673. [Google Scholar] [CrossRef] [Green Version]
- Roetenberg, D.; Luinge, H.; Slycke, P. Xsens MVN: Full 6DOF Human Motion Tracking Using Miniature Inertial Sensors. Xsens Motion Technologies BV, Tech. Rep 1. 2009, pp. 1–7. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.569.9604&rep=rep1&type=pdf (accessed on 21 June 2021).
- Madgwick, S.O.H.; Harrison, A.J.L.; Vaidyanathan, R. Estimation of IMU and MARG orientation using a gradient descent algorithm. In Proceedings of the 2011 IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–7. [Google Scholar]
- Šlajpah, S.; Kamnik, R.; Munih, M. Kinematics based sensory fusion for wearable motion assessment in human walking. Comput. Methods Programs Biomed. 2014, 116, 131–144. [Google Scholar] [CrossRef]
- O’Donovan, K.J.; Kamnik, R.; O’Keeffe, D.T.; Lyons, G.M. An inertial and magnetic sensor based technique for joint angle measurement. J. Biomech. 2007, 40, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Ibata, Y.; Kitamura, S.; Motoi, K.; Sagawa, K. Measurement of three-dimensional posture and trajectory of lower body during standing long jumping utilizing body-mounted sensors. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4891–4894. [Google Scholar]
- De Vries, W.; Veeger, D.; Baten, C.; van der Helm, F. Magnetic distortion in motion labs, implications for validating inertial magnetic sensors. Gait Posture 2009, 29, 535–541. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z.; Wu, J.-K.; Wong, L. Hierarchical Information Fusion for Global Displacement Estimation in Microsensor Motion Capture. IEEE Trans. Biomed. Eng. 2013, 60, 2052–2063. [Google Scholar] [CrossRef]
- Kok, M.; Hol, J.D.; Schön, T. An optimization-based approach to human body motion capture using inertial sensors. IFAC Proc. Vol. 2014, 47, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Tagliapietra, L.; Modenese, L.; Ceseracciu, E.; Mazzà, C.; Reggiani, M. Validation of a model-based inverse kinematics approach based on wearable inertial sensors. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 834–844. [Google Scholar] [CrossRef] [PubMed]
- McGinley, J.L.; Baker, R.; Wolfe, R.; Morris, M.E. The reliability of three-dimensional kinematic gait measurements: A systematic review. Gait Posture 2009, 29, 360–369. [Google Scholar] [CrossRef]
- Delp, S.L.; Anderson, F.C.; Arnold, A.S.; Loan, P.; Habib, A.; John, C.T.; Guendelman, E.; Thelen, D.G. OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Trans. Biomed. Eng. 2007, 54, 1940–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seth, A.; Hicks, J.L.; Uchida, T.K.; Habib, A.; Dembia, C.L.; Dunne, J.J.; Ong, C.; Demers, M.S.; Rajagopal, A.; Millard, M.; et al. OpenSim: Simulating musculoskeletal dynamics and neuromuscular control to study human and animal movement. PLoS Comput. Biol. 2018, 14, e1006223. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Dembia, C.; DeMers, M.; Delp, D.D.; Hicks, J.L.; Delp, S.L. Full-Body Musculoskeletal Model for Muscle-Driven Simulation of Human Gait. IEEE Trans. Biomed. Eng. 2016, 63, 2068–2079. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Marin, L.C. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J. Biomech. 2006, 39, 444–452. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J. Separating the effects of age and walking speed on gait variability. Gait Posture 2008, 27, 572–577. [Google Scholar] [CrossRef]
- Kang, H.G.; Dingwell, J. Effects of walking speed, strength and range of motion on gait stability in healthy older adults. J. Biomech. 2008, 41, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Nantel, J. The effects of arm swing amplitude and lower-limb asymmetry on gait stability. PLoS ONE 2019, 14, e0218644. [Google Scholar] [CrossRef] [Green Version]
- Siragy, T.; Mezher, C.; Hill, A.; Nantel, J. Active arm swing and asymmetric walking leads to increased variability in trunk kinematics in young adults. J. Biomech. 2019, 99, 109529. [Google Scholar] [CrossRef]
- Bailey, C.A.; Hill, A.; Graham, R.B.; Nantel, J. Effects of arm swing amplitude and lower limb asymmetry on motor variability patterns during treadmill gait. J. Biomech. 2021, 130, 110855. [Google Scholar] [CrossRef]
- Riva, F.; Bisi, M.C.; Stagni, R. Gait variability and stability measures: Minimum number of strides and within-session reliability. Comput. Biol. Med. 2014, 50, 9–13. [Google Scholar] [CrossRef]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar] [PubMed]
- Woltring, H.J. A Fortran package for generalized, cross-validatory spline smoothing and differentiation. Adv. Eng. Softw. (1978) 1986, 8, 104–113. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Liu, A.-M.; Xiao, F.; Wang, Y.-Z.; Hu, F.; Chen, J.-L.; Dai, K.-R.; Gu, D.-Y. Effect of active arm swing to local dynamic stability during walking. Hum. Mov. Sci. 2016, 45, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-K.; Buldyrev, S.; Goldberger, A.L.; Havlin, S.; Sciortino, F.; Simons, M.; Stanley, H.E. Long-range correlations in nucleotide sequences. Nature 1992, 356, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M.; Peng, C.-K.; Ladin, Z.; Wei, J.Y.; Goldberger, A.L. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J. Appl. Physiol. 1995, 78, 349–358. [Google Scholar] [CrossRef]
- Dingwell, J.B.; Cusumano, J.P. Identifying Stride-To-Stride Control Strategies in Human Treadmill Walking. PLoS ONE 2015, 10, e0124879. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Peng, C.-K.; Goldberger, A.L.; Hausdorff, J.M. Multiscale entropy analysis of human gait dynamics. Phys. A Stat. Mech. Its Appl. 2003, 330, 53–60. [Google Scholar] [CrossRef]
- McCamley, J.D.; Denton, W.; Arnold, A.; Raffalt, P.C.; Yentes, J.M. On the Calculation of Sample Entropy Using Continuous and Discrete Human Gait Data. Entropy 2018, 20, 764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ko, S.-U.; Tolea, M.I.; Hausdorff, J.M.; Ferrucci, L. Sex-specific differences in gait patterns of healthy older adults: Results from the Baltimore Longitudinal Study of Aging. J. Biomech. 2011, 44, 1974–1979. [Google Scholar] [CrossRef] [Green Version]
- Beange, K.H.E.; Chan, A.D.C.; Graham, R.B. Evaluation of wearable IMU performance for orientation estimation and motion tracking. In Proceedings of the IEEE International Workshop on Medical Measurement and Applications, Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Beange, K.H.E.; Chan, A.D.C.; Graham, R.B. Wearable sensor performance for motion tracking of the lumbar spine. In Proceedings of the 42nd Canadian Medical and Biological Engineering Conference, Ottawa, ON, Canada, 21–24 May 2019; pp. 1–4. [Google Scholar]
- El-Zayat, B.F.; Efe, T.; Heidrich, A.; Anetsmann, R.; Timmesfeld, N.; Fuchs-Winkelmann, S.; Schofer, M.D. Objective assessment, repeatability, and agreement of shoulder ROM with a 3D gyroscope. BMC Musculoskelet. Disord. 2013, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Beange, K.H.; Chan, A.D.; Beaudette, S.M.; Graham, R.B. Concurrent validity of a wearable IMU for objective assessments of functional movement quality and control of the lumbar spine. J. Biomech. 2019, 97, 109356. [Google Scholar] [CrossRef] [PubMed]
- Pietraszewski, B.; Winiarski, S.; Jaroszczuk, S. Three-dimensional human gait pattern—reference data for normal men. Acta Bioeng. Biomech. 2012, 14, 9–16. [Google Scholar] [CrossRef]
- Gates, D.H.; Dingwell, J.B. Comparison of different state space definitions for local dynamic stability analyses. J. Biomech. 2009, 42, 1345–1349. [Google Scholar] [CrossRef] [Green Version]
- Pacher, L.; Chatellier, C.; Vauzelle, R.; Fradet, L. Sensor-to-Segment Calibration Methodologies for Lower-Body Kinematic Analysis with Inertial Sensors: A Systematic Review. Sensors 2020, 20, 3322. [Google Scholar] [CrossRef] [PubMed]
- Dingwell, J.; Cusumano, J.P.; Cavanagh, P.R.; Sternad, D. Local Dynamic Stability Versus Kinematic Variability of Continuous Overground and Treadmill Walking. J. Biomech. Eng. 2000, 123, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollman, J.H.; Watkins, M.K.; Imhoff, A.C.; Braun, C.E.; Akervik, K.A.; Ness, D.K. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture 2016, 43, 204–209. [Google Scholar] [CrossRef]
- König, N.; Singh, N.; von Beckerath, J.; Janke, L.; Taylor, W. Is gait variability reliable? An assessment of spatio-temporal parameters of gait variability during continuous overground walking. Gait Posture 2014, 39, 615–617. [Google Scholar] [CrossRef] [PubMed]

| Angle | Gait Condition | ||||||
|---|---|---|---|---|---|---|---|
| Preferred Speed, Preferred Swing | 70% Preferred Speed, Preferred Swing | 130% Preferred Speed, Preferred Swing | Preferred Speed, Active Swing | Preferred Speed, Arms Bound | Pooled | ||
| RMSD (°) | Trunk FE | 3.8 [2.1, 5.5] | 4.1 [2.1, 5.5] | 4.2 [2.2, 6.2] | 4.2 [2.8, 5.6] | 3.4 [2.0, 4.8] | 4.0 [2.3, 5.6] |
| Trunk AA | 3.6 [2.8, 4.3] | 3.6 [2.7, 4.5] | 3.9 [3.1, 4.7] | 4.3 [3.3, 5.2] | 3.4 [2.8, 4.0] | 3.7 [2.9, 4.5] | |
| Trunk IE | 4.0 [3.1, 5.0] | 2.9 [2.5, 3.3] | 3.8 [3.2, 4.4] | 6.7 [1.9, 11.4] | 2.9 [2.5, 3.4] | 4.1 [2.6, 5.5] | |
| Pelvis FE | 10.6 [7.7, 13.5] | 9.6 [6.7, 12.5] | 8.1 [5.2, 10.9] | 9.2 [6.1, 12.2] | 8.8 [5.4, 12.2] | 9.2 [6.2, 12.3] | |
| Pelvis AA | 2.1 [1.7, 2.5] | 2.3 [1.7, 2.8] | 2.2 [1.9, 2.5] | 2.4 [1.9, 2.5] | 2.0 [1.6, 2.4] | 2.2 [1.8–2.6] | |
| Pelvis IE | 1.9 [1.5, 2.2] | 1.7 [1.3, 2.2] | 2.1 [1.7, 2.5] | 2.0 [1.6, 2.4] | 1.8 [1.5, 2.2] | 1.9 [1.5, 2.3] | |
| Hip FE | 4.4 [3.5, 5.2] | 3.7 [3.0, 4.4] | 5.8 [4.9, 6.8] | 4.2 [3.4, 4.9] | 4.5 [3.0, 6.0] | 4.5 [3.6, 5.5] | |
| Hip AA | 5.5 [4.6, 6.4] | 4.8 [4.0, 5.6] | 5.8 [4.9, 6.7] | 5.4 [4.4, 6.4] | 5.5 [4.6, 6.5] | 5.4 [4.5, 6.3] | |
| Hip IE | 6.1 [5.4, 6.8] | 5.8 [4.9, 6.6] | 6.5 [5.7, 7.2] | 5.8 [5.0, 6.7] | 6.7 [5.5, 7.9] | 6.2 [5.3, 7.0] | |
| Knee FE | 4.6 [3.7, 5.5] | 3.7 [3.1, 4.3] | 4.8 [3.6, 5.9] | 4.0 [3.6, 4.4] | 4.7 [3.7, 5.6] | 4.3 [3.5, 5.1] | |
| Ankle FE | 6.7 [5.6, 7.9] | 6.2 [4.9, 7.5] | 7.5 [6.2, 8.8] | 6.9 [5.4, 8.4] | 7.4 [6.0, 8.8] | 6.9 [5.6, 8.3] | |
| Ankle AA | 12.0 [10.3, 13.6] | 10.2 [8.8, 11.5] | 12.0 [10.7, 13.2] | 11.5 [10.1, 12.9] | 12.7 [10.9, 14.5] | 11.7 [10.2, 13.2] | |
| Pooled (all) | 5.4 [5.0, 5.9] | 4.9 [4.5, 5.2] | 5.6 [5.2, 5.9] | 5.5 [5.0, 6.0] | 5.3 [4.9, 5.8] | 5.3 [4.9, 5.8] | |
| Pooled (without Ankle AA) | 4.8 [4.4, 5.3] | 4.4 [4.1, 4.7] | 5.0 [4.6, 5.4] | 5.0 [4.4, 5.6] | 4.6 [4.2, 5.1] | 4.8 [4.3, 5.2] | |
| CVrom (%) | Trunk FE | 25.5 [16.6, 34.3] | 27.9 [17.8, 38.1] | 29.4 [16.9, 42.0] | 27.7 [19.6, 35.8] | 28.2 [17.5, 38.9] | 27.7 [17.7, 37.8] |
| Trunk AA | 15.0 [13.0, 17.1] | 16.8 [13.7, 20.0] | 15.3 [13.0, 17.7] | 17.7 [14.5, 20.8] | 17.9 [15.1, 19.2] | 16.6 [13.9, 19.2] | |
| Trunk IE | 15.6 [14.2, 37.8] | 14.2 [12.0, 16.5] | 13.7 [11.3, 16.1] | 22.1 [3.8, 40.4] | 15.6 [13.7, 17.5] | 16.2 [10.5, 21.9] | |
| Pelvis FE | 120.3 [80.3, 160.3] | 94.2 [64.7, 123.7] | 95.3 [55.5, 135.0] | 92.9 [59.5, 126.2] | 107.3 [58.5, 156.0] | 102.0 [63.7, 140.2] | |
| Pelvis AA | 12.3 [10.5, 14.1] | 15.2 [11.0, 19.4] | 12.0 [10.5, 13.5] | 13.4 [11.8, 15.1] | 14.4 [11.2, 17.7] | 13.5 [11.0, 16.0] | |
| Pelvis IE | 13.4 [10.4, 16.3] | 12.9 [9.2, 16.5] | 12.8 [10.7, 14.9] | 11.7 [9.9, 13.6] | 13.2 [10.8, 15.7] | 12.8 [10.2, 15.4] | |
| Hip FE | 9.1 [7.5, 10.6] | 8.5 [6.6, 10.5] | 11.0 [9.3, 12.6] | 8.0 [6.6, 9.4] | 9.2 [6.7, 11.8] | 9.2 [7.3, 11.0] | |
| Hip AA | 23.3 [18.5, 28.2] | 21.9 [17.2, 26.5] | 22.9 [18.8, 27.0] | 22.1 [17.3, 27.0] | 28.2 [21.5, 35.0] | 23.7 [18.7, 28.7] | |
| Hip IE | 32.2 [27.5, 37.0] | 30.2 [25.0, 36.1] | 32.9 [29.6, 36.1] | 29.8 [25.4, 34.1] | 35.0 [29.2, 40.9] | 32.0 [27.3, 36.7] | |
| Knee FE | 6.7 [5.4, 8.0] | 5.5 [4.6, 6.4] | 7.0 [5.5, 8.5] | 6.0 [5.4, 6.7] | 6.8 [5.3, 8.3] | 6.4 [5.2, 7.6] | |
| Ankle FE | 16.2 [13.9, 18.5] | 17.2 [14.3, 20.2] | 17.7 [15.2, 20.2] | 16.6 [13.3, 19.9] | 17.6 [14.5, 20.6] | 17.1 [14.3, 19.9] | |
| Ankle AA | 46.1 [39.6, 52.5] | 41.4 [35.4, 47.4] | 46.1 [40.8, 51.3] | 43.5 [37.3, 49.6] | 49.8 [43.0, 56.5] | 45.3 [39.2, 51.5] | |
| Pooled (all) | 28.0 [23.9, 32.1] | 25.5 [22.5, 28.5] | 26.3 [22.7, 30.0] | 26.0 [22.2, 29.7] | 28.6 [24.2, 33.0] | 26.9 [23.1, 30.7] | |
| Pooled (without Ankle AA) | 26.3 [22.2, 30.5] | 24.1 [21.0, 27.1] | 24.5 [20.7, 28.4] | 24.4 [20.5, 28.3] | 26.7 [21.9, 31.5] | 25.2 [21.2, 29.2] | |
| Angle | ICC2,1 | RMSD | CVmean | Bias | LOA95% | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| ROM | Trunk FE | 0.13 [−0.11, 0.35] | 2.5 [2.1, 2.9] | 26.1 [22.3, 29.8] | −1.3 [−1.8, −0.8] * | −5.4 [−5.9, −4.9] | 2.8 [2.3, 3.3] |
| Trunk AA | 0.48 [0.28, 0.64] | 7.8 [7.0, 8.6] | 41.3 [38.3, 44.4] | −7.0 [−7.8, −6.2] * | −13.6 [−14.3, −12.8] | −0.5 [−1.3, 0.3] | |
| Trunk IE | 0.85 [0.77, 0.90] | 7.7 [6.8, 8.6] | 38.3 [34.8, 41.7] | −6.7 [−7.6, −5.8] * | −14.1 [−15.0, −13.2] | 0.7 [−0.2, 1.6] | |
| Pelvis FE | 0.03 [−0.26, 0.21] | 1.8 [1.5, 2.1] | 26.2 [20.8, 31.5] | −0.4 [−0.8, 0.0] * | −3.8 [−4.2, −3.4] | 3.0 [2.6, 3.4] | |
| Pelvis AA | 0.62 [0.46, 0.75] | 4.8 [4.2, 5.3] | 32.6 [28.5, 35.6] | −4.1 [−4.7, −3.6] * | −8.8 [−9.4, −8.2] | 0.5 [0.0, 1.1] | |
| Pelvis IE | 0.84 [0.75, 0.90] | 2.0 [1.7, 2.4] | 14.8 [12.1, 17.6] | −0.5 [−0.9, 0.0] * | −4.4 [−4.9, −3.9] | 3.5 [3.0, 4.0] | |
| Hip FE | 0.84 [0.75, 0.90] | 2.8 [2.4, 3.3] | 5.0 [3.8, 6.1] | 0.1 [−0.6, 0.8] | −5.5 [−6.2, −4.8] | 5.7 [5.0, 6.4] | |
| Hip AA | 0.24 [−0.01, 0.45] | 8.5 [7.5, 9.6] | 44.8 [36.9, 52.7] | 6.8 [5.5, 8.0] * | −3.5 [−4.9, −2.3] | 17.1 [15.8, 18.3] | |
| Hip IE | −0.11 [−0.35, −0.13] | 4.8 [4.2, 5.4] | 36.9 [30.4, 43.4] | 0.4 [−0.7, 1.6] | −9.1 [−10.2, −7.9] | 9.9 [8.7, 11.0] | |
| Knee FE | 0.26 [0.02, 0.47] | 6.9 [5.9, 7.9] | 8.8 [7.2, 10.3] | −1.4 [−3.0, 0.2] | −14.7 [−16.4, 13.1] | 11.9 [10.3, 13.5] | |
| Ankle FE | 0.76 [0.64, 0.85] | 4.7 [4.1, 5.4] | 12.4 [10.3, 14.5] | −1.2 [−2.3, −0.1] * | −10.3 [−11.4, −9.2] | 7.8 [6.7, 8.9] | |
| Ankle AA | 0.10 [−0.14, 0.34] | 8.4 [7.2, 9.6] | 33.0 [26.6, 39.3] | 3.1 [1.2, 5.0] * | −12.3 [−14.2, −10.4] | 18.5 [16.6, 20.3] | |
| meanSD | Trunk FE | 0.63 [0.46, 0.75] | 0.29 [0.25, 0.34] | 17.8 [14.1, 21.6] | 0.07 [0.00, 0.14] * | −0.49 [−0.56, −0.42] | 0.63 [0.56, 0.70] |
| Trunk AA | 0.79 [0.68, 0.86] | 0.18 [0.15, 0.22] | 10.9 [8.6, 13.1] | 0.02 [−0.02, 0.07] | −0.34 [−0.38, −0.29] | 0.38 [0.34, 0.43] | |
| Trunk IE | 0.80 [0.70, 0.87] | 0.60 [0.54, 0.66] | 36.3 [33.6, 39.1] | −0.52 [−0.59, −0.45] * | −1.10 [−1.17, −1.03] | 0.06 [−0.01, 0.13] | |
| Pelvis FE | 0.77 [0.65, 0.85] | 0.16 [0.13, 0.18] | 12.7 [10.1, 15.2] | 0.04 [0.00, 0.07] * | −0.26 [−0.30, −0.22] | 0.33 [0.30, 0.37] | |
| Pelvis AA | 0.60 [0.43, 0.73] | 0.14 [0.12, 0.17] | 12.7 [10.2, 15.2] | −0.08 [−0.11, −0.05] * | −0.32 [−0.35, −0.29] | 0.16 [0.13, 0.19] | |
| Pelvis IE | 0.64 [0.48, 0.76] | 0.28 [0.23, 0.32] | 19.7 [15.1, 24.2] | 0.20 [0.15, 0.24] * | −0.19 [−0.242, −0.15] | 0.59 [0.54, 0.63] | |
| Hip FE | 0.80 [0.69, 0.87] | 0.23 [0.20, 0.26] | 17.5 [14.4, 20.6] | 0.16 [0.12, 0.20] * | −0.16 [−0.20, −0.12] | 0.48 [0.44, 0.52] | |
| Hip AA | 0.70 [0.56, 0.81] | 0.14 [0.12, 0.16] | 12.6 [10.1, 15.0] | 0.06 [0.03, 0.09] * | −0.18 [−0.21, −0.15] | 0.30 [0.27, 0.33] | |
| Hip IE | 0.69 [0.54, 0.80] | 0.20 [0.17, 0.23] | 12.5 [9.9, 15.1] | 0.10 [0.06, 0.14] * | −0.24 [−0.28, −0.20] | 0.44 [0.40, 0.48] | |
| Knee FE | 0.48 [0.27, 0.65] | 0.40 [0.34, 0.46] | 20.7 [15.6, 25.9] | 0.19 [0.10, 0.27] * | −0.51 [−0.60, −0.43] | 0.89 [0.80, 0.97] | |
| Ankle FE | 0.68 [0.53, 0.79] | 0.61 [0.55, 0.66] | 50.0 [43.5, 56.5] | 0.56 [0.50, 0.62] * | 0.10 [−0.04, 0.16] | 1.02 [0.97, 1.08] | |
| Ankle AA | 0.24 [0.00, 0.46] | 0.38 [0.32, 0.43] | 25.6 [20.7, 30.5] | 0.20 [0.13, 0.28] * | −0.42 [−0.49, −0.34] | 0.83 [0.75, 0.90] | |
| λmax | Trunk FE | 0.72 [0.58, 0.82] | 0.54 [0.48, 0.60] | 14.3 [12.3, 16.2] | 0.44 [0.37, 0.52] * | −0.18 [−0.25, −0.10] | 1.06 [0.99, 1.14] |
| Trunk AA | 0.85 [0.77, 0.91] | 0.40 [0.36, 0.44] | 10.5 [9.3, 11.7] | 0.36 [0.32, 0.40] * | 0.04 [−0.00, 0.08] | 0.69 [0.65, 0.72] | |
| Trunk IE | 0.59 [0.40, 0.73] | 0.34 [0.29, 0.39] | 6.5 [5.3, 7.7] | −0.23 [−0.29, −0.17] * | −0.73 [−0.79, −0.67] | 0.27 [0.21, 0.33] | |
| Pelvis FE | 0.89 [0.82, 0.93] | 0.61 [0.56, 0.66] | 17.8 [16.1, 19.5] | 0.58 [0.52, 0.63] * | 0.16 [0.10, 0.21] | 1.00 [0.95, 1.05] | |
| Pelvis AA | 0.82 [0.71, 0.88] | 0.37 [0.32, 0.41] | 10.1 [8.6, 11.6] | 0.32 [0.27, 0.36] * | −0.05 [−0.10, −0.01] | 0.69 [0.64, 0.73] | |
| Pelvis IE | 0.67 [0.51, 0.78] | 0.22 [0.19, 0.25] | 3.9 [3.1, 4.7] | −0.04 [−0.09, 0.01] | −0.47 [−0.52, −0.42] | 0.39 [0.34, 0.44] | |
| Hip FE | 0.87 [0.80, 0.92] | 0.20 [0.17, 0.22] | 4.2 [3.5, 4.9] | −0.04 [−0.09, 0.01] | −0.42 [−0.47, −0.37] | 0.34 [0.29, 0.39] | |
| Hip AA | 0.78 [0.66, 0.86] | 0.31 [0.28, 0.34] | 8.4 [7.5, 9.4] | 0.27 [0.23, 0.30] * | −0.02 [−0.05, 0.02] | 0.56 [0.52, 0.59] | |
| Hip IE | 0.75 [0.62, 0.84] | 0.44 [0.39, 0.49] | 12.7 [10.9, 14.5] | 0.38 [0.33, 0.43] * | −0.06 [−0.11, 0.00] | 0.82 [0.77, 0.87] | |
| Knee FE | 0.74 [0.61, 0.84] | 0.40 [0.34, 0.46] | 7.4 [5.9, 8.9] | 0.20 [0.12, 0.28] * | −0.48 [−0.56, −0.40] | 0.88 [0.79, 0.96] | |
| Ankle FE | 0.74 [0.60, 0.83] | 0.45 [0.41, 0.50] | 13.0 [11.4, 14.5] | 0.40 [0.35, 0.45] * | −0.02 [−0.07, 0.03] | 0.82 [0.77, 0.87] | |
| Ankle AA | 0.37 [0.14, 0.56] | 1.17 [1.10, 1.25] | 51.1 [45.9, 56.4] | 1.13 [1.05, 1.21] * | 0.49 [0.41, 0.56] | 1.77 [1.69, 1.85] | |
| DFAα | Trunk FE | 0.40 [0.18, 0.58] | 0.21 [0.18, 0.24] | 22.7 [17.2, 28.2] | −0.03 [−0.08, 0.02] | −0.44 [−0.49, −0.39] | 0.38 [0.33, 0.43] |
| Trunk AA | 0.27 [0.04, 0.48] | 0.19 [0.16, 0.22] | 18.7 [14.8, 22.6] | −0.05 [−0.09, 0.00] | −0.42 [−0.46, −0.37] | 0.32 [0.28, 0.37] | |
| Trunk IE | 0.65 [0.49, 0.76] | 0.17 [0.15, 0.20] | 16.6 [13.0, 20.2] | −0.00 [−0.04, 0.04] | −0.34 [−0.38, −0.30] | 0.34 [0.30, 0.38] | |
| Pelvis FE | 0.36 [0.14, 0.55] | 0.20 [0.17, 0.23] | 25.5 [20.4, 30.7] | 0.01 [−0.04, 0.05] | −0.39 [−0.44, −0.25] | 0.40 [0.35, 0.45] | |
| Pelvis AA | 0.48 [0.28, 0.64] | 0.21 [0.18, 0.24] | 21.2 [17.7, 24.7] | −0.07 [−0.12, −0.03] * | −0.47 [−0.52, −0.42] | 0.32 [0.27, 0.37] | |
| Pelvis IE | 0.46 [0.25, 0.63] | 0.16 [0.14, 0.19] | 17.6 [13.3, 21.9] | 0.03 [−0.01, 0.06] | −0.29 [−0.33, −0.25] | 0.34 [0.30, 0.38] | |
| Hip FE | 0.50 [0.30, 0.66] | 0.20 [0.17, 0.23] | 21.0 [16.8, 25.3] | −0.02 [−0.06, 0.03] | −0.41 [−0.46, −0.36] | 0.38 [0.33, 0.43] | |
| Hip AA | 0.37 [0.14, 0.56] | 0.23 [0.20, 0.27] | 21.3 [17.4, 25.1] | −0.07 [−0.12, −0.01] * | −0.50 [−0.56, −0.45] | 0.37 [0.32, 0.43] | |
| Hip IE | 0.13 [−0.12, 0.36] | 0.24 [0.21, 0.27] | 26.7 [21.8, 31.6] | −0.04 [−0.10, 0.02] | −0.51 [−0.56, −0.45] | 0.43 [0.37, 0.48] | |
| Knee FE | 0.43 [0.21, 0.61] | 0.20 [0.17, 0.23] | 20.0 [16.1, 23.9] | 0.02 [−0.03, 0.07] | −0.37 [−0.42, −0.32] | 0.41 [0.36, 0.46] | |
| Ankle FE | 0.62 [0.44, 0.75] | 0.16 [0.13, 0.18] | 17.2 [13.4, 21.0] | 0.02 [−0.02, 0.06] | −0.29 [−0.33, −0.25] | 0.33 [0.29, 0.37] | |
| Ankle AA | −0.05 [−0.29, 0.20] | 0.31 [0.35, 0.26] | 35.9 [28.2, 43.7] | 0.12 [0.05, 0.19] * | −0.44 [−0.51, −0.38] | 0.68 [0.61, 0.75] | |
| SaEn | Trunk FE | 0.56 [0.38, 0.70] | 0.18 [0.15, 0.20] | 17.0 [13.8, 20.2] | 0.02 [−0.02, 0.06] | −0.33 [−0.37, −0.29] | 0.36 [0.32, 0.41] |
| Trunk AA | 0.61 [0.44, 0.74] | 0.19 [0.16, 0.21] | 29.8 [23.3, 36.3] | 0.15 [0.13, 0.18] * | −0.05 [−0.08, −0.03] | 0.36 [0.34, 0.39] | |
| Trunk IE | 0.48 [0.28, 0.64] | 0.10 [0.08, 0.12] | 16.1 [12.3, 19.9] | 0.04 [0.01, 0.06] * | −0.15 [−0.17, −0.13] | 0.22 [0.20, 0.24] | |
| Pelvis FE | 0.53 [0.34, 0.68] | 0.22 [0.19, 0.26] | 16.7 [14.1, 19.4] | −0.12 [−0.16, −0.07] * | −0.49 [−0.54, −0.45] | 0.26 [0.22, 0.31] | |
| Pelvis AA | 0.68 [0.54, 0.79] | 0.12 [0.10, 0.13] | 15.1 [12.1, 18.1] | 0.06 [0.03, 0.08] * | −0.15 [−0.17, −0.12] | 0.26 [0.23, 0.28] | |
| Pelvis IE | 0.64 [0.48, 0.76] | 0.24 [0.21, 0.27] | 63.1 [47.9, 78.4] | 0.19 [0.15, 0.22] * | −0.11 [−0.14, −0.07] | 0.48 [0.45, 0.52] | |
| Hip FE | 0.44 [0.23, 0.62] | 0.05 [0.04, 0.06] | 17.9 [14.3, 21.4] | 0.04 [0.03, 0.04] * | −0.04 [−0.05, −0.03] | 0.11 [0.10, 0.12] | |
| Hip AA | 0.17 [−0.08, 0.39] | 0.15 [0.13, 0.17] | 23.5 [20.0, 27.0] | −0.09 [−0.12, −0.06] * | −0.33 [−0.36, −0.30] | 0.15 [0.12, 0.18] | |
| Hip IE | −0.12 [−0.35, 0.12] | 0.25 [0.22, 0.29] | 21.3 [17.2, 25.4] | −0.04 [−0.10, −0.02] * | −0.53 [−0.59, −0.47] | 0.46 [0.40, 0.52] | |
| Knee FE | 0.74 [0.61, 0.83] | 0.07 [0.05, 0.08] | 16.5 [12.8, 20.2] | −0.02 [−0.04, −0.01] * | −0.14 [−0.16, −0.13] | 0.10 [0.08, 0.11] | |
| Ankle FE | 0.46 [0.24, 0.63] | 0.13 [0.11, 0.14] | 24.6 [20.2, 29.0] | 0.10 [0.08, 0.12] * | −0.06 [−0.08, −0.04] | 0.26 [0.24, 0.28] | |
| Ankle AA | 0.10 [−0.14, 0.34] | 0.19 [0.16, 0.22] | 26.8 [22.1, 31.6] | −0.03 [−0.08, −0.01] * | −0.40 [−0.44, −0.35] | 0.34 [0.29, 0.38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, C.A.; Uchida, T.K.; Nantel, J.; Graham, R.B. Validity and Sensitivity of an Inertial Measurement Unit-Driven Biomechanical Model of Motor Variability for Gait. Sensors 2021, 21, 7690. https://doi.org/10.3390/s21227690
Bailey CA, Uchida TK, Nantel J, Graham RB. Validity and Sensitivity of an Inertial Measurement Unit-Driven Biomechanical Model of Motor Variability for Gait. Sensors. 2021; 21(22):7690. https://doi.org/10.3390/s21227690
Chicago/Turabian StyleBailey, Christopher A., Thomas K. Uchida, Julie Nantel, and Ryan B. Graham. 2021. "Validity and Sensitivity of an Inertial Measurement Unit-Driven Biomechanical Model of Motor Variability for Gait" Sensors 21, no. 22: 7690. https://doi.org/10.3390/s21227690
APA StyleBailey, C. A., Uchida, T. K., Nantel, J., & Graham, R. B. (2021). Validity and Sensitivity of an Inertial Measurement Unit-Driven Biomechanical Model of Motor Variability for Gait. Sensors, 21(22), 7690. https://doi.org/10.3390/s21227690






