Advanced Optical Sensing of Phenolic Compounds for Environmental Applications
Abstract
:1. Introduction
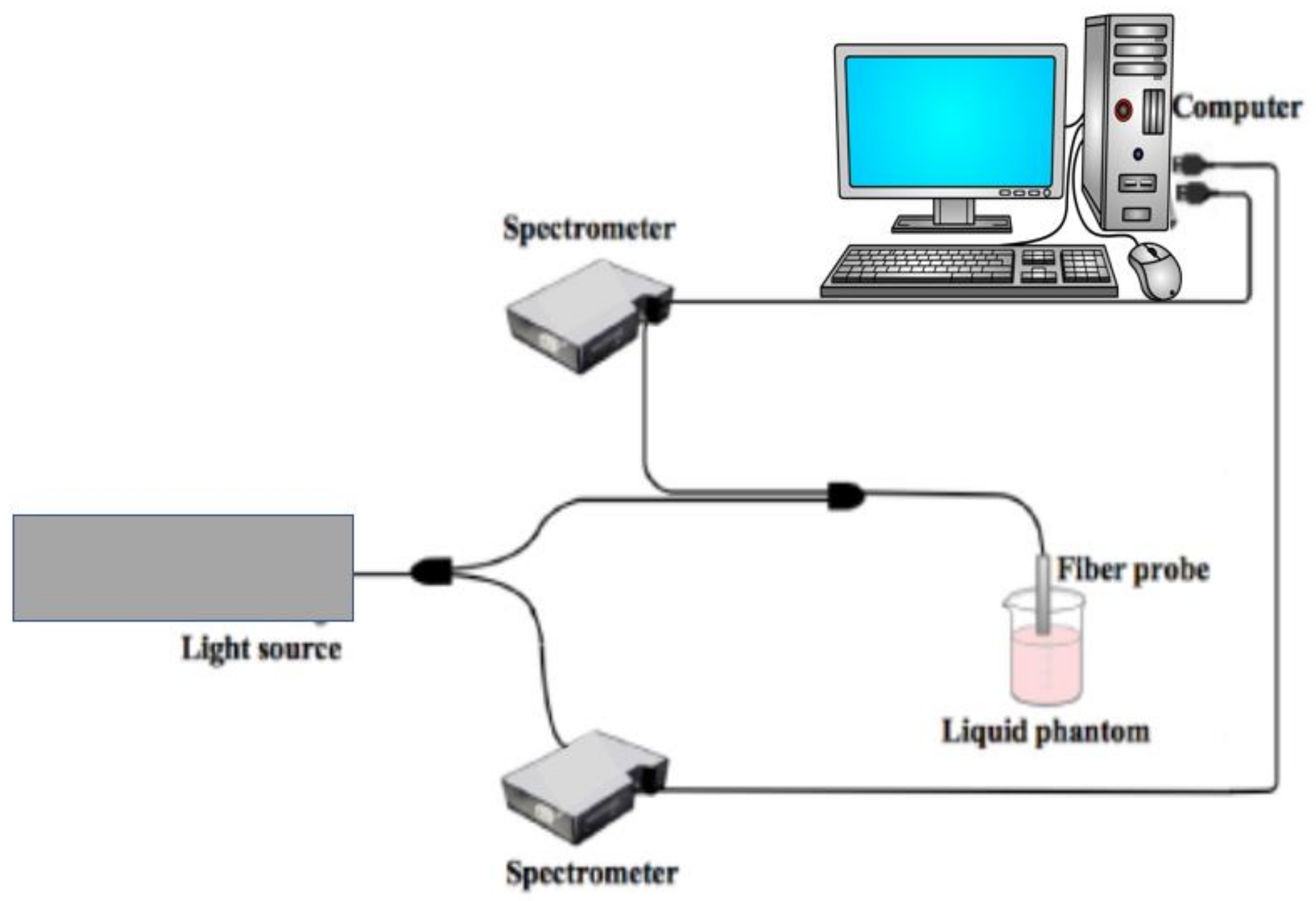
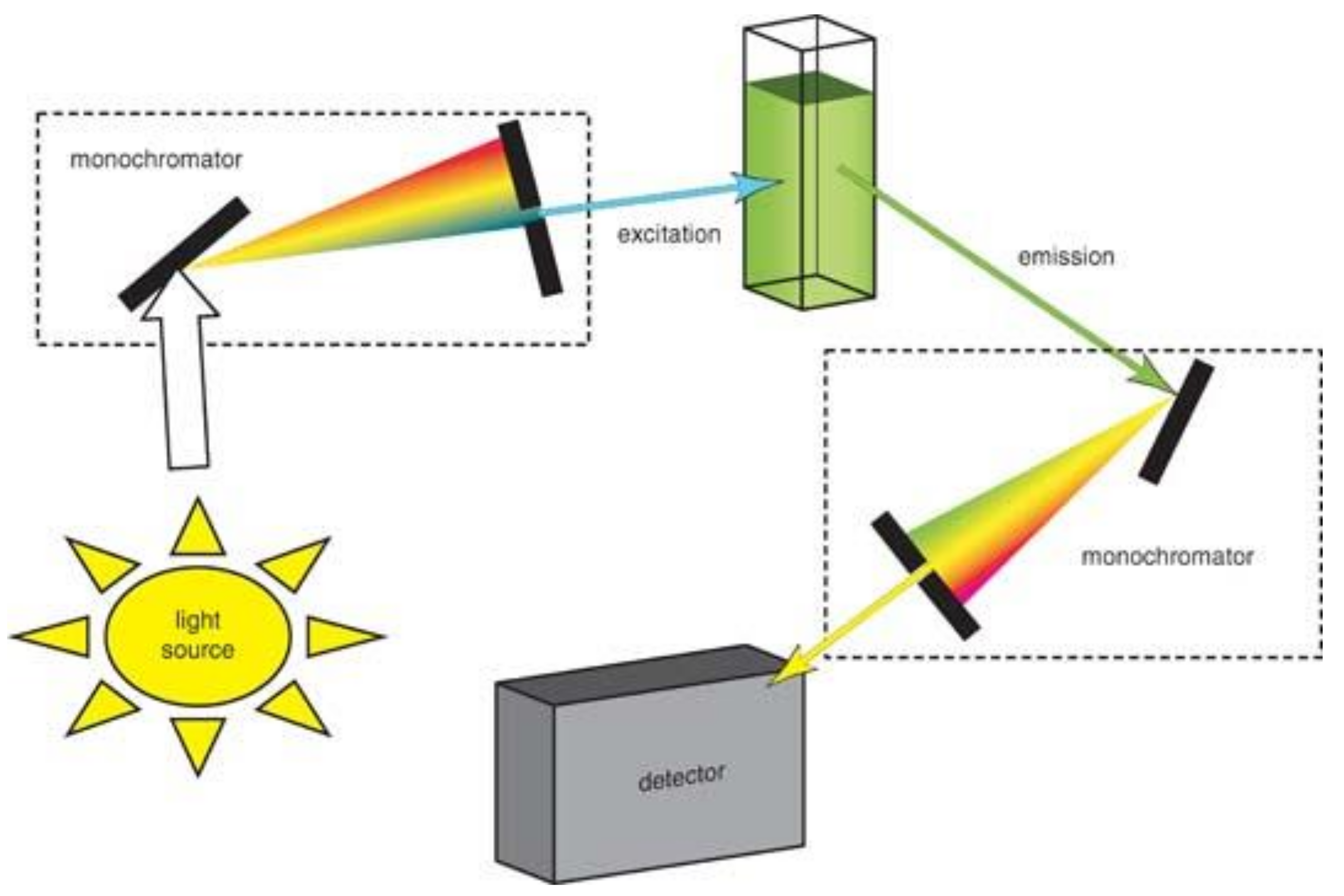
2. Optical Detection Techniques
3. Biosensors
3.1. Enzymatic Biosensors
3.1.1. Laccase-Based Biosensors
3.1.2. Tyrosinase-Based Biosensors
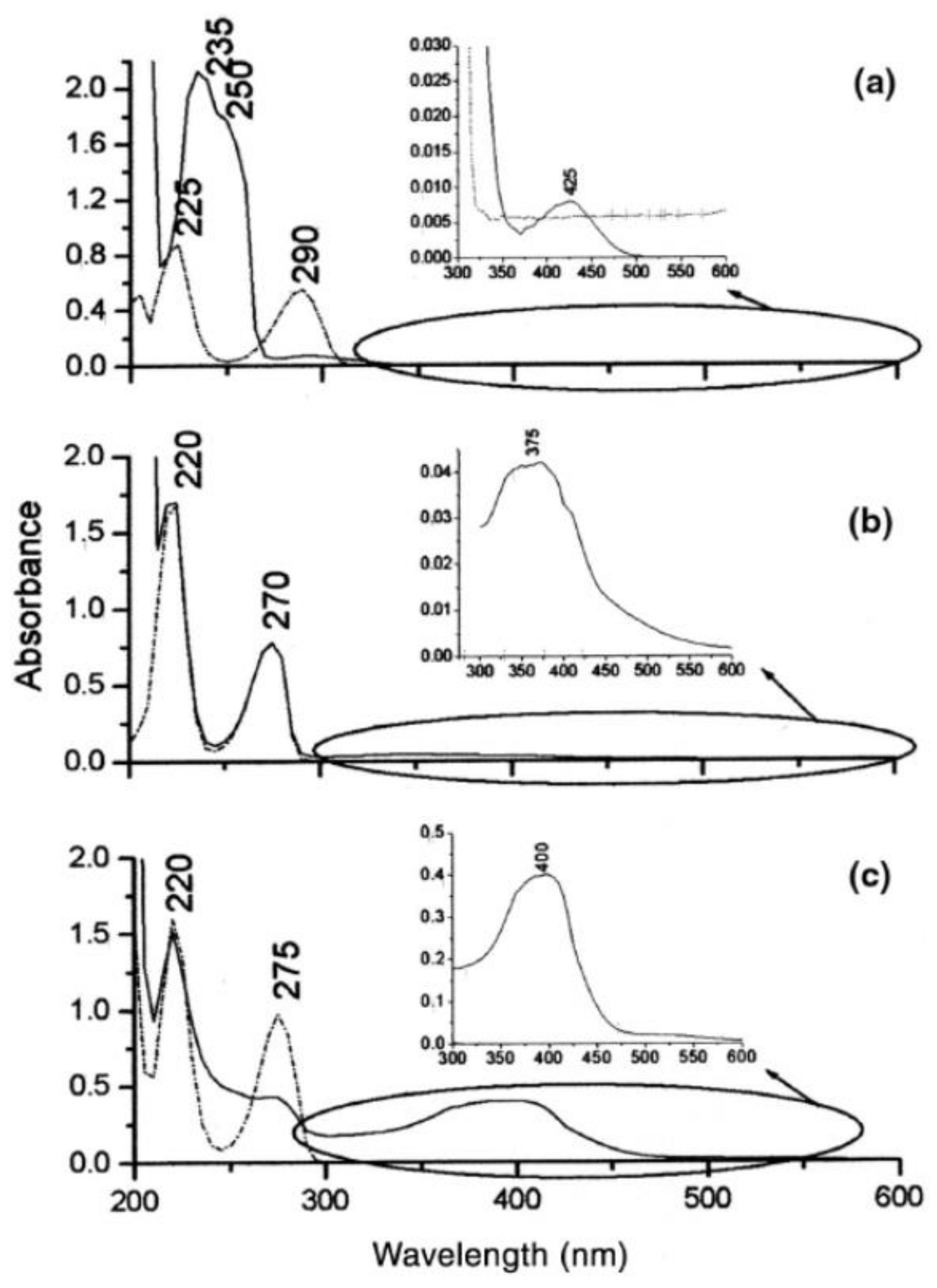
3.2. Immunosensors
3.3. Receptor-Based Biosensors
3.4. Nucleic Acids-Based Biosensors
3.5. Whole-Cells Biosensors
3.6. Molecularly Imprinted Polymer-Based Sensors
4. Sensors
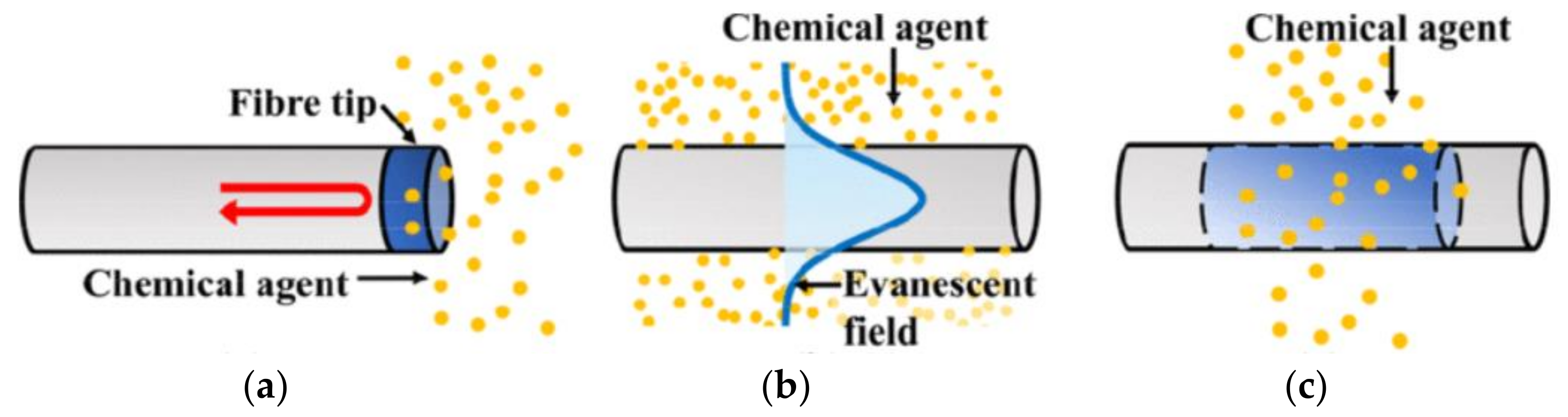
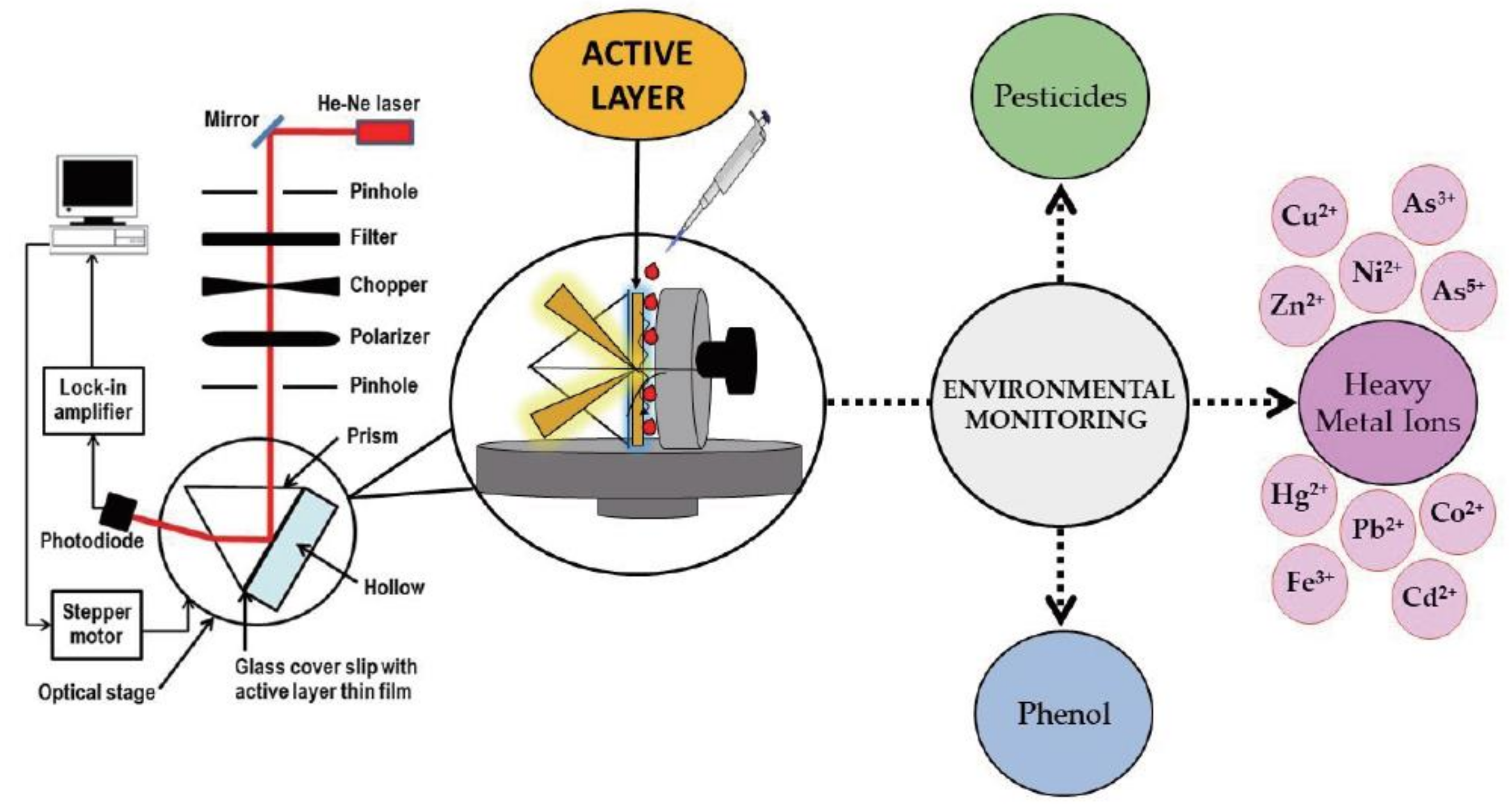
4.1. Optical Chemical Sensors
4.2. Nanostructure-Based Sensors
4.3. Photonic Crystal Fiber Sensors
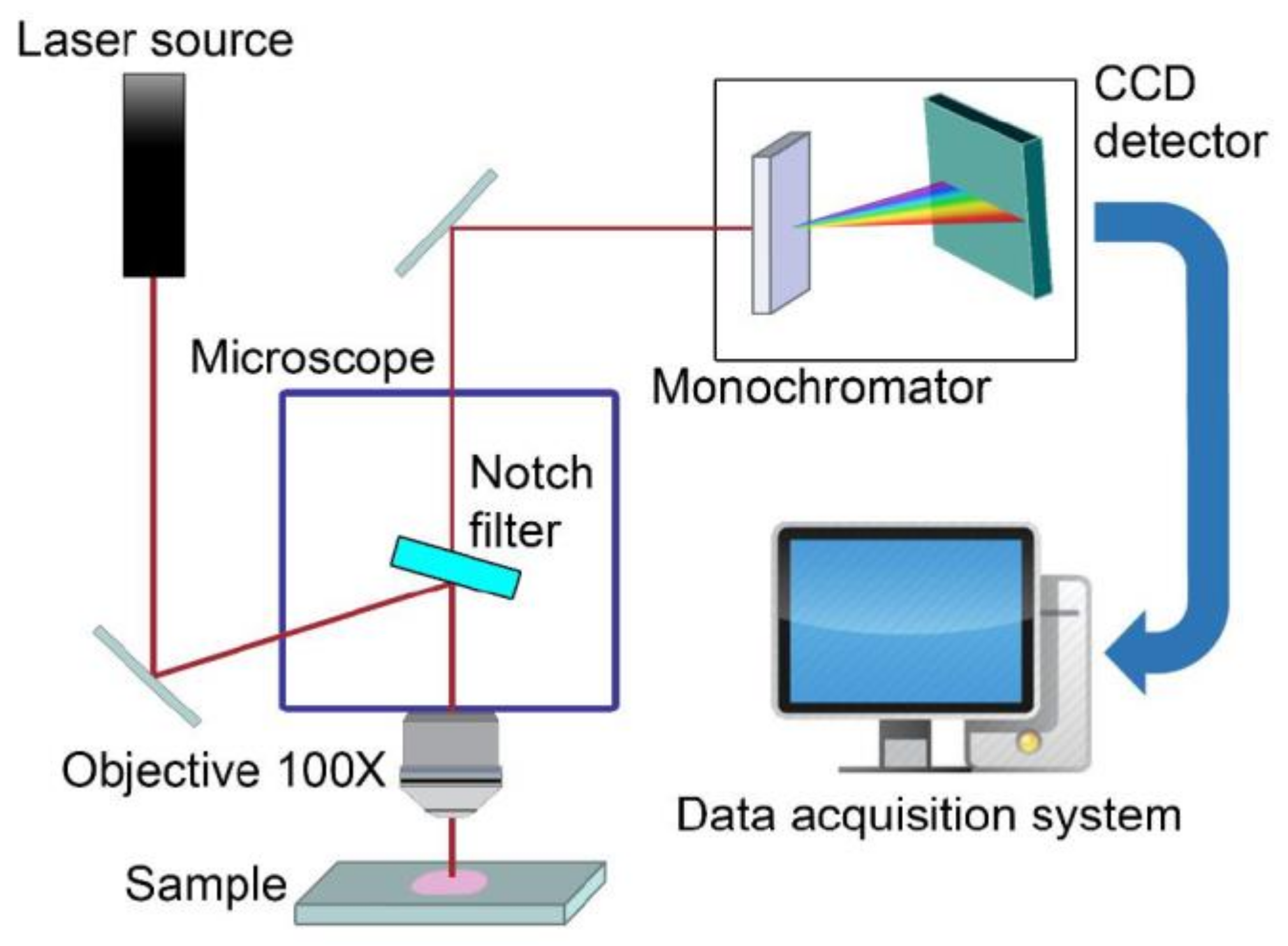
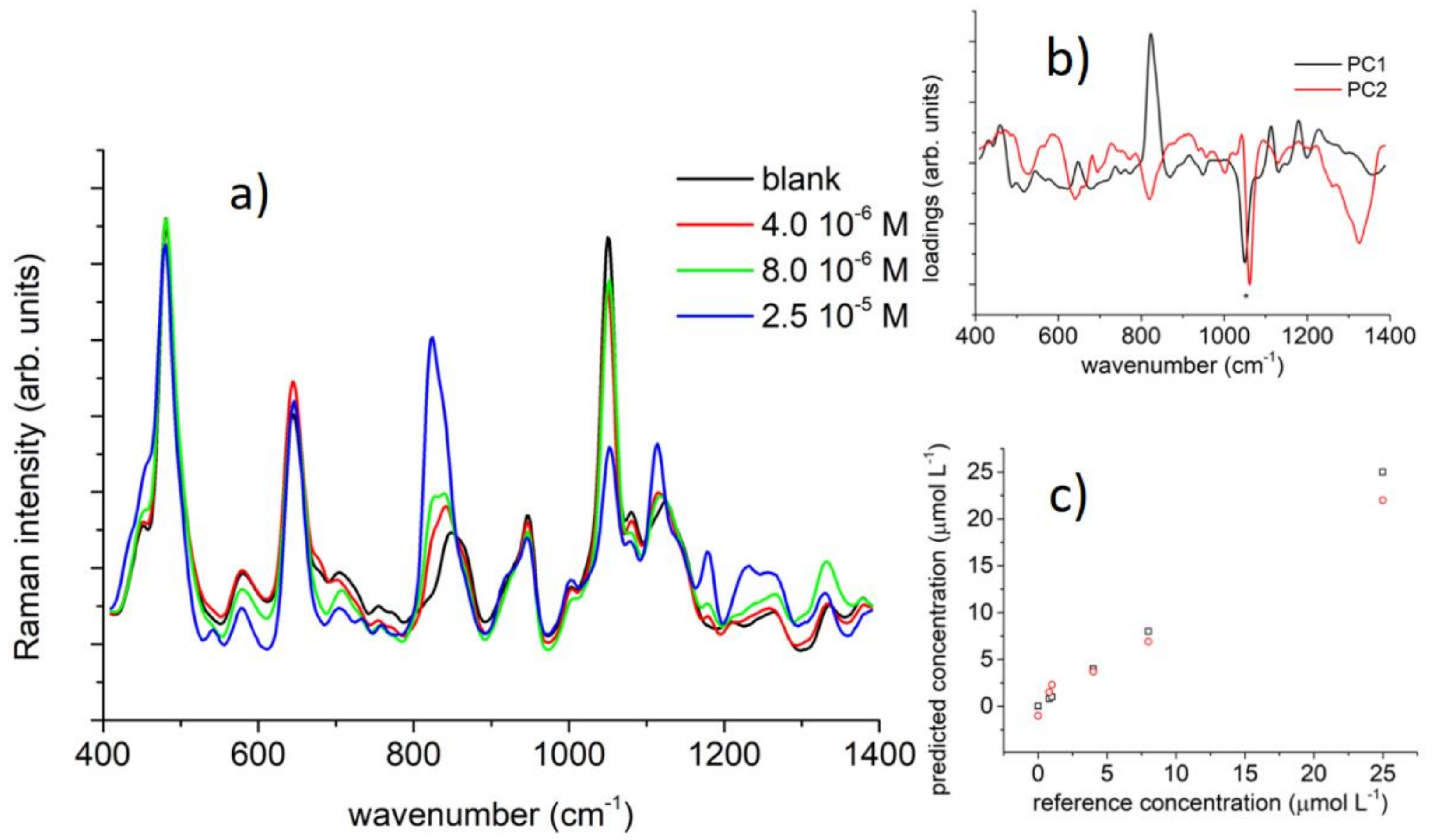
4.4. Sensing Schemes Using Vibrational Spectroscopies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mainali, K. Phenolic compounds contaminants in water: A glance. Curr. Trends Civ. Struct. Eng. 2020, 4, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Wang, Y.; Ni, Y.; Kokot, S. Spectrophotometric analysis of phenols, which involves a hemin–graphene hybrid nanoparticles with peroxidase-like activity. J. Hazard. Mater. 2014, 266, 60–67. [Google Scholar] [CrossRef]
- Appendix A to 40 CFR, Part 423–126 Priority Pollutants. U.S. Environmental Protection Agency. Available online: https://www3.epa.gov/region1/npdes/permits/generic/prioritypollutants.pdf (accessed on 2 November 2021).
- Priority Substances and Certain Other Pollutants according to Annex II of Directive 2008/105/EC. European Commission. Available online: http://data.europa.eu/eli/dir/2008/105/oj (accessed on 2 November 2021).
- Boudet, A.M. Evolution and current status of research in phenolic compounds: Review. Phytochem 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Gami, A.A.; Shukor, M.Y.; Khalil, K.A.; Dahalan, F.A.; Khalid, A.; Ahmad, S.A. Phenol and its toxicity. J. Environ. Microbiol. Toxicol. 2014, 2, 11–24. [Google Scholar]
- Michalowicz, J.; Duda, W. Phenols-sources and toxicity: Review. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Wasi, S.; Tabrez, S.; Ahmad, M. Toxicological effect of major environmental pollutants: An overview. Environ. Monit. Assess. 2013, 185, 2585–2593. [Google Scholar] [CrossRef]
- Frye, C.A.; Bo, E.; Calamandrei, G.; Calzà, L.; Dessì-Fulgheri, F.; Fernández, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012, 24, 144–159. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Reprod. Toxicol. 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-Nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekant, W.; Volkel, W. Human exposure to bisphenol A by biomonitoring: Methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 2008, 228, 114–134. [Google Scholar] [CrossRef]
- Karim, F.; Fakhruddin, A.N.M. Recent advances in the development of biosensor for phenol: A review. Rev. Environ. Sci. Biotechnol. 2012, 11, 261–274. [Google Scholar] [CrossRef]
- Rodionov, P.V.; Veselova, I.A.; Shekhovtsova, T.N. Optical sensors for determining phenolic compounds with different structures. J. Anal. Chem. 2013, 68, 931–941. [Google Scholar] [CrossRef]
- Portaccio, M.; Menale, C.; Diano, N.; Serri, C.; Mita, D.G.; Lepore, M. Monitoring production process of Cisplatin-loaded PLGA nanoparticles by FT-IR microspectroscopy and univariate data analysis. J. Appl. Polym. Sci. 2015, 132, 41305. [Google Scholar] [CrossRef]
- Camerlingo, C.; Portaccio, M.; Tatè, R.; Lepore, M.; Delfino, I. Fructose and pectin detection in fruit-based food products by surface-enhanced Raman spectroscopy. Sensors 2017, 17, 839. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, V.; Portaccio, M.; Piccolella, S.; Manti, L.; Pacifico, S.; Lepore, M. Study of SH-SY5Y cancer cell response to treatment with polyphenol extracts using FT-IR spectroscopy. Biosensors 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Camerlingo, C.; Verde, A.; Manti, L.; Meschini, R.; Delfino, I.; Lepore, M. Graphene-based Raman spectroscopy for pH sensing of X-rays exposed and unexposed culture media and cells. Sensors 2018, 18, 2242. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, V.; Crescitelli, A.; Moccia, M.; Sandomenico, A.; Portaccio, M.; Lepore, M.; Ruvo, M.; Galdi, V.; Esposito, E. Pixeled metasurface for multiwavelength detection of vitamin D. Nanophotonics 2020, 9, 3921–3930. [Google Scholar] [CrossRef]
- Mazhari, B.B.Z.; Agsar, D. Detection of phenols from industrial effluents using streptomyces mediated gold nanoparticles. Indian J. Mat. Sci. 2016, 2016, 6937489. [Google Scholar] [CrossRef] [Green Version]
- Bayram, A.; Horzum, N.; Metin, A.U.; Kılıç, V.; Solmaz, M.E. Colorimetric bisphenol-A detection with a portable smartphone-based spectrometer. IEEE Sens. J. 2018, 18, 5948–5955. [Google Scholar] [CrossRef]
- Filik, H.; Aksua, D.; Apaka, R.; Sener, I.; Kılıc, E. An optical fibre reflectance sensor for p-aminophenol determination based on tetrahydroxycalix[4]arene as sensing reagent. Sens. Actuat. B 2009, 136, 105–112. [Google Scholar] [CrossRef]
- Griffete, N.; Frederich, H.; Maître, A.; Schwob, C.; Ravaine, S.; Carbonnier, B.; Chehimi, M.M.; Mangeney, C. Introduction of a planar defect in a molecularly imprinted photonic crystal sensor for the detection of bisphenol A. J. Colloid Interface Sci. 2011, 364, 18–23. [Google Scholar] [CrossRef]
- Bishnu, P.; Popma, T. Optical Waveguide Sensors. In Handbook of Optical Sensors; Santos, J.L., Farahi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–43. [Google Scholar]
- Krohn, D.A.; MacDougall, T.; Mendez, A. Fiber Optic Sensors: Fundamentals and Applications; SPIE Press: Bellingham, WA, USA, 2014. [Google Scholar]
- Milvich, J.; Kohler, D.; Freude, W.; Koos, C. Surface sensing with integrated optical waveguides: A design guideline. Opt. Express 2018, 26, 19885–19906. [Google Scholar] [CrossRef] [Green Version]
- Del Villar, I.; Matias, I.R. Optical Fibre Sensors: Fundamentals for Development of Optimized Devices; IEEE Press Series on Sensors; Wiley-IEEE Press: Oxford, UK, 2020. [Google Scholar]
- Floris, I.; Adam, J.M.; Calderón, P.A.; Sales, S. Fiber optic shape sensors: A comprehensive review. Opt. Lasers Eng. 2021, 139, 106508. [Google Scholar] [CrossRef]
- Pospíšilová, M.; Kuncová, G.; Trögl, J. Fiber-optic chemical sensors and fiber-optic biosensors. Sensors 2015, 15, 25208–25259. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2020, 92, 397–430. [Google Scholar] [CrossRef]
- Tow, K.H.; Chow, D.M.; Vollrath, F.; Dicaire, I.; Gheysens, T.; Thevenaz, L. Exploring the use of native spider silk as an optical fiber for chemical sensing. J. Lightwave Technol. 2018, 36, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Campanella, C.E.; Cuccovillo, A.; Campanella, C.; Yurt, A.; Passaro, V.M.N. Fibre bragg grating based strain sensors: Review of Technology and Applications. Sensors 2018, 18, 3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Presti, D. Fiber Bragg gratings for medical applications and future challenges: A Review. IEEE Access 2020, 8, 156863–156888. [Google Scholar] [CrossRef]
- Zhong, N.; Chen, M.; Chang, H.; Zhang, T.; Wang, Z.; Xin, X. Optic fiber with Er3+: YAlO3/SiO2/TiO2 coating and polymer membrane for selective detection of phenol in water. Sens. Actuators B Chem. 2018, 273, 1744–1753. [Google Scholar] [CrossRef]
- Zhong, N.; Chen, M.; Wang, Z.; Xin, X.; Li, B. Photochemical device for selective detection of phenol in aqueous solutions. Lab Chip 2018, 18, 1621–1632. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Fauzi, L.N.I.M.; Hashim, H.S.; Ramdzan, N.S.M.; Omar, N.A.S. Recent advances in surface plasmon resonance optical sensors for potential application in environmental monitoring. Sens. Mater. 2020, 32, 4191–4200. [Google Scholar] [CrossRef]
- Yesudasu, V.; Pradhan, H.S.; Pandya, R.J. Recent progress in surface plasmon resonance-based sensors: A comprehensive review. Heliyon 2021, 7, e06321. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M. Vibrational spectroscopic sensors fundamentals, instrumentation and applications. In Optical Chemical Sensor; Baldini, F., Chester, A., Homola, J., Martellucci, S., Eds.; In NATO Science Series II: Mathematics, Physics and Chemistry; Springer: Dordrecht, The Netherlands, 2006; Volume 224, pp. 117–155. [Google Scholar]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [Green Version]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [Green Version]
- Neubrech, F.; Huck, C.; Weber, K.; Pucci, A.; Giessen, H. Surface-Enhanced infrared spectroscopy using resonant nanoantennas. Chem. Rev. 2017, 117, 5110–5145. [Google Scholar] [CrossRef]
- Di Meo, V.; Caporale, A.; Crescitelli, A.; Janneh, M.; Palange, E.; De Marcellis, A.; Portaccio, M.; Lepore, M.; Rendina, I.; Ruvo, M.; et al. Metasurface based on cross-shaped plasmonic nanoantennas as chemical sensor for surface-enhanced infrared absorption spectroscopy. Sens. Actuators B Chem. 2019, 286, 600–607. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Fabris, L. Surface-enhanced raman spectroscopy: Principles, substrates, and applications. In Metal Nanoparticles and Clusters; Deepak, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Borisov, S.M.; Wolfbeis, O.S. Optical biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef]
- Yashas, S.R.; Shivakumara, B.P.; Udayashankara, T.H.; Krishna, B.M. Laccase biosensor: Green technique for quantification of phenols in wastewater (A review). Orient. J. Chem. 2018, 34, 631–637. [Google Scholar]
- Claus, H. Laccases: Structure, reactions, distribution. Micron 2004, 35, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C. Investigating the structure and function of cupredoxins. Coord. Chem. Rev. 2005, 249, 3025–3054. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell. Fact. 2019, 8, 200–232. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.M.; Alemán-Nava, G.S.; Rodríguez-Delgado, J.M.; Dieck-Assad, G.; Martínez-Chapa, S.O.; Barceló, D.; Parra, R. Laccase-based biosensors for detection of phenolic compounds. Trends Anal. Chem. 2015, 74, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, J.; Ahmad, M.; Heng, L.Y.; Karuppiah, N.; Sidek, H. An optical biosensor based on immobilization of laccase and MBTH in stacked films for the detection of catechol. Sensors 2007, 7, 2238–2250. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.; de Marcos, S.; Galbán, J. Autoindicating optical properties of laccase as the base of an optical biosensor film for phenol determination. Anal. Bioanal. Chem. 2012, 404, 351–359. [Google Scholar] [CrossRef]
- Andreu-Navarro, A.; Fernández-Romero, J.M.; Gómez-Hens, A. Determination of polyphenolic content in beverages using laccase, gold nanoparticles and long wavelength fluorimetry. Anal. Chim. Acta 2012, 713, 1–6. [Google Scholar] [CrossRef]
- Cantarella, G.; d’Acunzo, F.; Galli, C. Determination of laccase activity in mixed solvents: Comparison between two chromogens in a spectrophotometric assay. Biotechnol. Bioeng. 2003, 82, 395–398. [Google Scholar] [CrossRef]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef]
- Delfino, I.; Portaccio, M.; Della Ventura, B.; Manzo, G.; Mita, D.G.; Lepore, M. Optical properties of sol-gel immobilized Laccase: A first step for its use in optical biosensing. In Optical Sensing and Detection II; International Society for Optics and Photonics: Washington, DC, USA, 2012; Volume 8439. [Google Scholar]
- Lepore, M.; Portaccio, M. Optical detection of different phenolic compounds by means of a novel biosensor based on sol-gel immobilized laccase. Biotechnol. Appl. Biochem. 2017, 64, 782–792. [Google Scholar] [CrossRef]
- Jȩdrychowska, A.; Malecha, K.; Cabaj, J.; Sołoducho, J. Laccase biosensor based on low temperature co-fired ceramics for the permanent monitoring of water solutions. Electrochim. Acta 2015, 165, 372–382. [Google Scholar] [CrossRef]
- Thelemann, T.; Fischer, M.; Groß, A.; Müller, J. LTCC-based fluidic components for chemical applications. J. Microelectron. Electron. Pack. 2007, 4, 167. [Google Scholar] [CrossRef]
- Baeza, M.; López, C.; Alonso, J.; López-Santin, J.; Álvaro, G. Ceramic microsystem incorporating a microreactor with immobilized biocatalyst for enzymatic spectrophotometric assays. Anal. Chem. 2010, 82, 1006–1011. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Dencheva, N.V.; Braz, J.F.; Malfois, M.; Denchev, Z.Z. Optical biosensor for catechol determination based on laccase-immobilized anionic polyamide 6 microparticles. J. Appl. Polym. Sci. 2020, 137, e49131. [Google Scholar] [CrossRef]
- Gul, I.; Ahmad, M.S.; Naqvi, S.M.S.; Hussain, A.; Wali, R.; Farooqi, A.A.; Ahmed, I. Polyphenol oxidase (PPO) based biosensors for detection of phenolic compounds: A Review. J. App. Biol. Biotechnol. 2017, 5, 72–85. [Google Scholar]
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef]
- Russell, I.M.; Burton, S.G. The development of an immobilized enzyme bioprobe for the detection of phenolic pollutants in water. Anal. Chim. Acta 1999, 389, 161–170. [Google Scholar] [CrossRef]
- Abdullah, J.; Ahmad, M.; Karuppiah, N.; Heng, L.Y.; Sidek, H. Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sens. Actuators B Chem. 2005, 114, 604–609. [Google Scholar] [CrossRef]
- Fiorentino, D.; Gallone, A.; Fiocco, D.; Palazzo, G.; Mallardi, A. Mushroom tyrosinase in polyelectrolyte multilayers as an optical biosensor for o-diphenols. Biosens. Bioelectron. 2010, 25, 2033–2037. [Google Scholar] [CrossRef]
- Alkasir, R.S.; Ornatska, M.; Andreescu, S. Colorimetric paper bioassay for the detection of phenolic compounds. Anal. Chem. 2012, 84, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Son, K.J.; Kim, B.; Koh, W.G. Phenol biosensor based on hydrogel microarrays entrapping tyrosinase and quantum dots. Analyst 2010, 135, 2871–2878. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, S.K.; Gupta, B.D. SPR based fibre optic biosensor for phenolic compounds using immobilization of tyrosinase in polyacrylamide gel. Sens. Actuators B Chem. 2013, 186, 388–395. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Saleviter, S.; Abdullah, J. Structural, optical and potential sensing properties of tyrosinase immobilized graphene oxide thin film on gold surface. Optik 2020, 212, 164786–164796. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Sheh Omar, N.A.; Abdullah, J.; Daniyal, W.M.E.M.M.; Saleviter, S. Detection of phenol by incorporation of gold modified-enzyme based graphene oxide thin film with surface plasmon resonance technique. Opt. Express 2020, 28, 9738–9752. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; de, A. Falcão, I.R.; da S. Souza, J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of nanomaterials-based enzymatic biosensors: Synthesis, properties, and applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Shankaran, D.R.; Gobi, K.V.; Miura, N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sens. Actuators B Chem. 2007, 121, 158–177. [Google Scholar] [CrossRef]
- Dostálek, J.; Přibyl, J.; Homola, J.; Skládal, P. Multichannel SPR biosensor for detection of endocrine-disrupting compounds. Anal. Bioanal. Chem. 2007, 389, 1841–1847. [Google Scholar] [CrossRef]
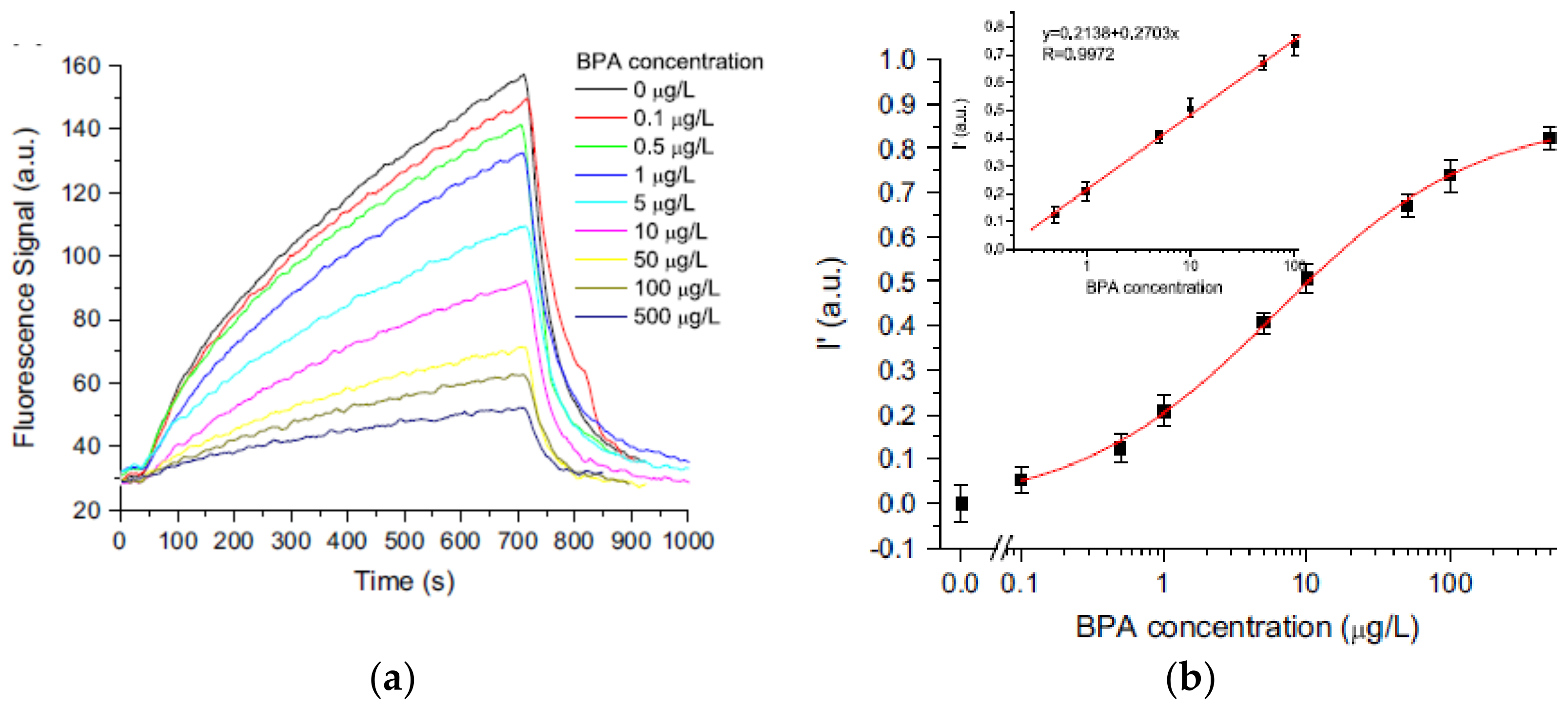
- Long, F.; Zhu, A.; Zhou, X.; Wang, H.; Zhao, Z.; Liu, L.; Shi, H. Highly sensitive and selective optofluidics-based immunosensor for rapid assessment of Bisphenol A leaching risk. Biosens. Bioelectron. 2014, 55, 19–25. [Google Scholar] [CrossRef]
- Li, X.; Ying, G.G.; Su, H.C.; Yang, X.B.; Wang, L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010, 36, 557–562. [Google Scholar] [CrossRef]
- Wright, J.D.; Oliver, J.V.; Nolte, R.J.M.; Holder, S.J.; Sommerdijk, N.A.J.M.; Nikitin, P.I. The detection of phenols in water using a surface Plasmon resonance system with specific receptors. Sens. Actuators B Chem. 1998, 51, 305–310. [Google Scholar] [CrossRef]
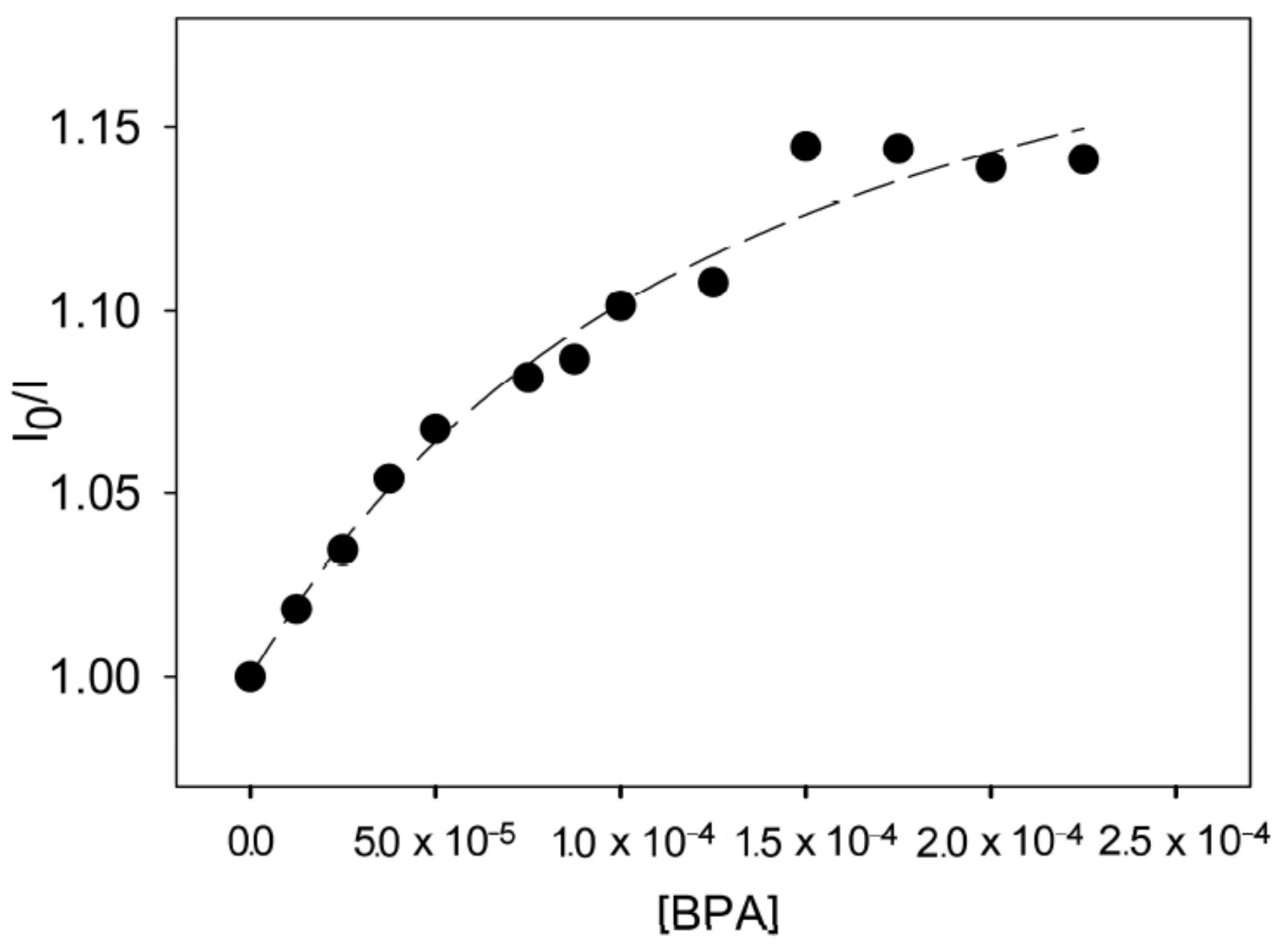
- Conti, L.; Mummolo, L.; Romano, G.M.; Giorgi, C.; Giacomazzo, G.E.; Prodi, L.; Bencini, A. Exploring the ability of luminescent metal assemblies to bind and sense anionic or ionizable analytes a Ru(phen)2bipy-based dizinc complex for bisphenol A (BPA) recognition. Molecules 2021, 26, 527. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.; Long, F.; He, M.; Shid, H.C.; Gu, A.Z. A portable optic fiber aptasensor for sensitive, specific and rapid detection of bisphenol-A in water samples. Environ. Sci. Process Impacts 2014, 16, 1379–1386. [Google Scholar] [CrossRef]
- Lim, H.J.; Chu, B.; Son, A. Detection of bisphenol A using palm-size NanoAptamer analyzer. Biosens. Bioelectron. 2017, 94, 10–18. [Google Scholar] [CrossRef]
- Kim, G.Y.; Wang, X.; Ahn, H.; Son, A. Gene quantification by the NanoGene assay is resistant to inhibition by humic acids. Environ. Sci. Technol. 2011, 45, 8873–8880. [Google Scholar] [CrossRef] [PubMed]
- Allsop, T.D.P.; Neal, R.; Wang, C.; Nagel, D.A.; Hine, A.V.; Culverhouse, P.; Castañón, J.D.A.; Webb, D.J.; Scarano, S.; Minunni, M. An ultra-sensitive aptasensor on optical fibre for the direct detection of bisphenol A. Biosens. Bioelectron. 2019, 135, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mazhari, B.B.Z.; Agsar, D.; Prasad, M.V.N.A. Development of paper biosensor for the detection of phenol from industrial effluents using bioconjugate of Tyr-AuNps mediated by novel isolate Streptomyces tuirus DBZ39. J. Nanomat. 2017, 2017, 1352134. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, Y.; Takano, E.; Takeuchi, T. SPR sensing of bisphenol A using molecularly imprinted nanoparticles immobilized on slab optical waveguide with consecutive parallel Au and Ag deposition bands coexistent with bisphenol A-immobilized Au nanoparticles. Langmuir 2012, 28, 7083–7088. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, Z.; Xu, J.; Liu, Y.; Zhang, H. A microvolume molecularly imprinted polymer modified fiber-optic evanescent wave sensor for bisphenol A determination. Anal. Bioanal. Chem. 2014, 406, 2411–2420. [Google Scholar] [CrossRef]
- Qiao, P.; Wang, X.H.; Gao, S.; Yin, X.; Wang, Y.; Wang, P. Integration of black phosphorus and hollow-core anti-resonant fiber enables two-order magnitude enhancement of sensitivity for bisphenol A detection. Biosens. Bioelectron. 2020, 149, 111821–111829. [Google Scholar] [CrossRef]
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913–112926. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of fluorescent probes based on protection-deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, S.H.; Wu, L.; Yang, W.C.; Yang, G.F. A highly sensitive and selective fluorescent probe for thiophenol designed via a twist-blockage strategy. Anal. Chem. 2016, 88, 2266–2272. [Google Scholar] [CrossRef]
- Li, Y.; Su, W.; Zhou, Z.; Huang, Z.; Wu, C.; Yin, P.; Li, H.; Zhang, Y. A dual-response near-infrared fluorescent probe for rapid detecting thiophenol and its application in water samples and bio-imaging. Talanta 2019, 199, 355–360. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, N.; Chen, M.; Chang, H.; Zhong, D.; Wu, Y.; Liu, H.; Xin, X.; Zhao, M.; Tang, B.; et al. Photochemical reflective optical fiber sensor for selective detection of phenol in aqueous solutions. Appl. Opt. 2019, 58, 2091–2099. [Google Scholar] [CrossRef]
- Ganesan, M.; Nagaraaj, P. Quantum dots as nanosensors for detection of toxics: A literature review. Anal. Methods 2020, 12, 4254–4275. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, M.R.H.; Alimohammadi, M.; Tashkhourian, J.; Razavian, S.M. Optical detection of phenolic compounds based on the surface plasmon resonance band of Au nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; You, F.; Promthaveepong, K.; Li, N. Ultrasensitive bisphenol A sensing based on responsive plasmonic nanoparticles. Sens. Actuators B Chem. 2017, 245, 369–374. [Google Scholar] [CrossRef]
- Frasco, M.F.; Chaniotakis, N. Semiconductor quantum dots in chemical sensors and biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesiak, A.; Drzozga, K.; Cabaj, J.; Bański, M.; Malecha, K.; Podhorodecki, A. Optical sensors based on II-VI quantum dots. Nanomaterials 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Hu, T.; Wang, L.; Zhang, L.; Su, X. Near-infrared fluorescence nanoprobe for enzyme-substrate system sensing and in vitro imaging. Biosens. Bioelectron. 2016, 79, 922–929. [Google Scholar] [CrossRef]
- Nayak, R.; Ali, F.A.; Mishra, D.K.; Ray, D.; Aswald, V.K.; Sahoo, S.K.; Nanda, B. Fabrication of CuO nanoparticle: An efficient catalyst utilized for sensing and degradation of Phenol. J. Mater. Res. Technol. 2020, 9, 11045–11059. [Google Scholar] [CrossRef]
- Jaiswal, A.; Kumar, A.; Prakash, R. Facile synthesis of doped CxNy QDs as photoluminescent matrix for direct detection of hydroquinone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119019–119027. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, R.; Chauhan, M.S.; Al-Hadeethi, Y. ZnO–SnO2 nanocubes for fluorescence sensing and dye degradation applications. Ceram. Int. 2021, 47, 6201–6210. [Google Scholar] [CrossRef]
- Ponnusamy, R.; Venkatesan, R.; Samal, R.; Kandasamy, M.; Gandhiraj, V.; Chakraborty, B.; Rout, C.S. Bifunctional WO3 microrods decorated RGO composite as catechol sensor and optical limiter. Appl. Surf. Sci. 2021, 536, 147669. [Google Scholar] [CrossRef]
- Frazão, O.; Santos, J.L.; Araũjo, F.M.; Ferreira, L.A. Optical sensing with photonic crystal fibers. Laser Photon. Rev. 2008, 2, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Niger, M.; Hasin, T.F. Detection of harmful chemical compounds in plastics with highly sensitive photonic crystal fiber with higher nonlinear coefficient. In Proceedings of the 2019 IEEE International Conference on Signal Processing, Information, Communication & Systems (SPICSCON), Dhaka, Bangladesh, 28–30 November 2019. [Google Scholar]
- Marley, N.A.; Mann, C.K.; Vickers, T.J. Determination of phenols in water using Raman spectroscopy. Appl. Spectrosc. 1984, 380, 540–543. [Google Scholar] [CrossRef]
- Marks, H.L.; Pishko, M.V.; Jackson, G.W.; Coté, G.L. Rational design of a bisphenol a aptamer selective surface-enhanced Raman scattering nanoprobe. Anal. Chem. 2014, 86, 11614–11619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakar, N.A.; Salleh, M.M.; Umar, A.A.; Shapter, J.G. Design and measurement technique of surface-enhanced Raman scattering for detection of bisphenol A. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025008–025012. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, Q.; Zeng, X.; Liu, Q.; Hou, X.; Tian, Y.; Wu, L. Sensitive detection of bisphenol A by coupling solid phase microextraction based on monolayer graphene-coated Ag nanoparticles on Si fibers to surface enhanced Raman spectroscopy. Talanta 2018, 187, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zeng, Y.; Shen, A.G.; Hu, J.M. A highly sensitive SERS probe for bisphenol A detection based on functionalized Au@Ag nanoparticles. Anal. Methods 2018, 10, 5622–5628. [Google Scholar] [CrossRef]
- Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.C.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-enhanced Raman spectroscopy for bisphenols detection: Toward a better understanding of the analyte–nanosystem interactions. Nanomaterials 2021, 11, 881. [Google Scholar] [CrossRef]
- Li, D.; Li, D.W.; Fossey, J.S.; Long, Y.T. Portable surface-enhanced Raman scattering sensor for rapid detection of aniline and phenol derivatives by on-site electrostatic preconcentration. Anal. Chem. 2010, 82, 9299–9305. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Pienpinijtham, P.; Zhao, B.; Ozaki, Y. Coupling reaction-based ultrasensitive detection of phenolic estrogens using surface-enhanced resonance Raman scattering. Anal. Chem. 2011, 83, 8582–8588. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Tang, D.; Liu, L.; Wang, Y.; Zhou, Q.; Su, S.; Hu, D.; Han, B.; Jin, M.; Ao, X.; et al. Highly reproducible surface-enhanced Raman scattering substrate for detection of phenolic pollutants. Nanotechnology 2016, 27, 455301–455319. [Google Scholar] [CrossRef]
- Pompeu, D.R.; Larondelle, Y.; Rogez, H.; Abbas, O.; Pierna, J.A.F.; Baeten, V. Characterization and discrimination of phenolic compounds using Fourier transform Raman spectroscopy and chemometric tools. Biotechnol. Agron. Soc. Environ. 2018, 22, 13–28. [Google Scholar]
- Carreira-Casais, A.; Montes-García, V.; Pastoriza-Santos, I.; Prieto, M.Á.; Simal-Gandara, J.; Pérez-Juste, J. Multiple SERS detection of phenol derivatives in tap water. Proceedings 2021, 70, 88. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Biswas, S.; Zhu, F.; Zhan, J.; Wang, G.; Tung, C.H.; Wang, Y. Surface-enhanced Raman scattering of phenols and catechols by a molecular analogue of titanium dioxide. Anal. Chem. 2020, 92, 5929–5936. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delfino, I.; Diano, N.; Lepore, M. Advanced Optical Sensing of Phenolic Compounds for Environmental Applications. Sensors 2021, 21, 7563. https://doi.org/10.3390/s21227563
Delfino I, Diano N, Lepore M. Advanced Optical Sensing of Phenolic Compounds for Environmental Applications. Sensors. 2021; 21(22):7563. https://doi.org/10.3390/s21227563
Chicago/Turabian StyleDelfino, Ines, Nadia Diano, and Maria Lepore. 2021. "Advanced Optical Sensing of Phenolic Compounds for Environmental Applications" Sensors 21, no. 22: 7563. https://doi.org/10.3390/s21227563
APA StyleDelfino, I., Diano, N., & Lepore, M. (2021). Advanced Optical Sensing of Phenolic Compounds for Environmental Applications. Sensors, 21(22), 7563. https://doi.org/10.3390/s21227563







