Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Search Strategy and Data Sources
2.3. Inclusion Criteria
2.4. Data Extraction
2.5. Outcomes
2.6. Risk of Bias Assessment and Quality Evidence
2.7. Statistical Analysis
2.8. Sensitivity Analysis
2.9. Subgroup Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of the Studies Included
3.3. Methodological Quality Assessment
3.4. Outcomes Synthesis
3.5. Meta-Analysis Findings
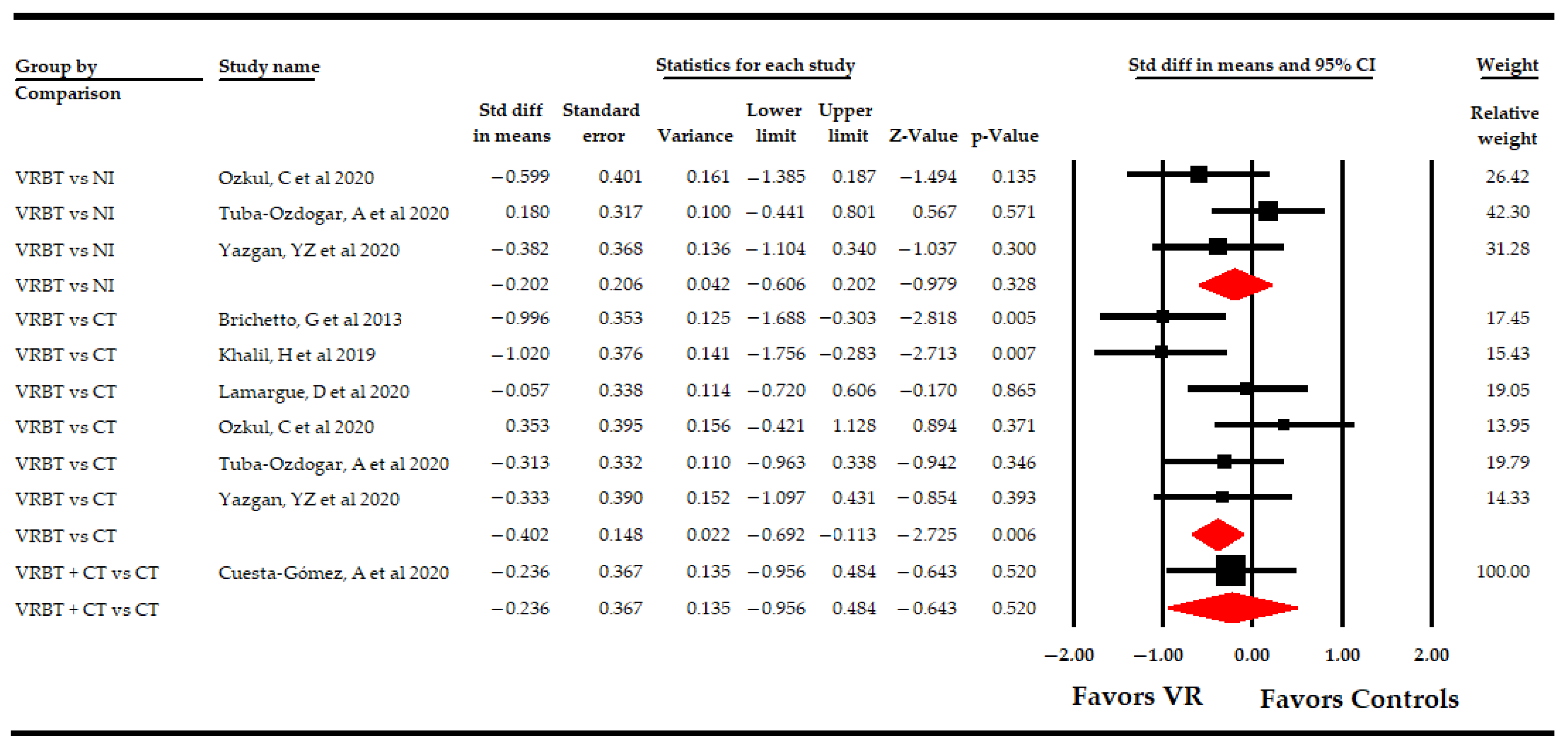
3.5.1. Effect of Virtual Reality on Fatigue
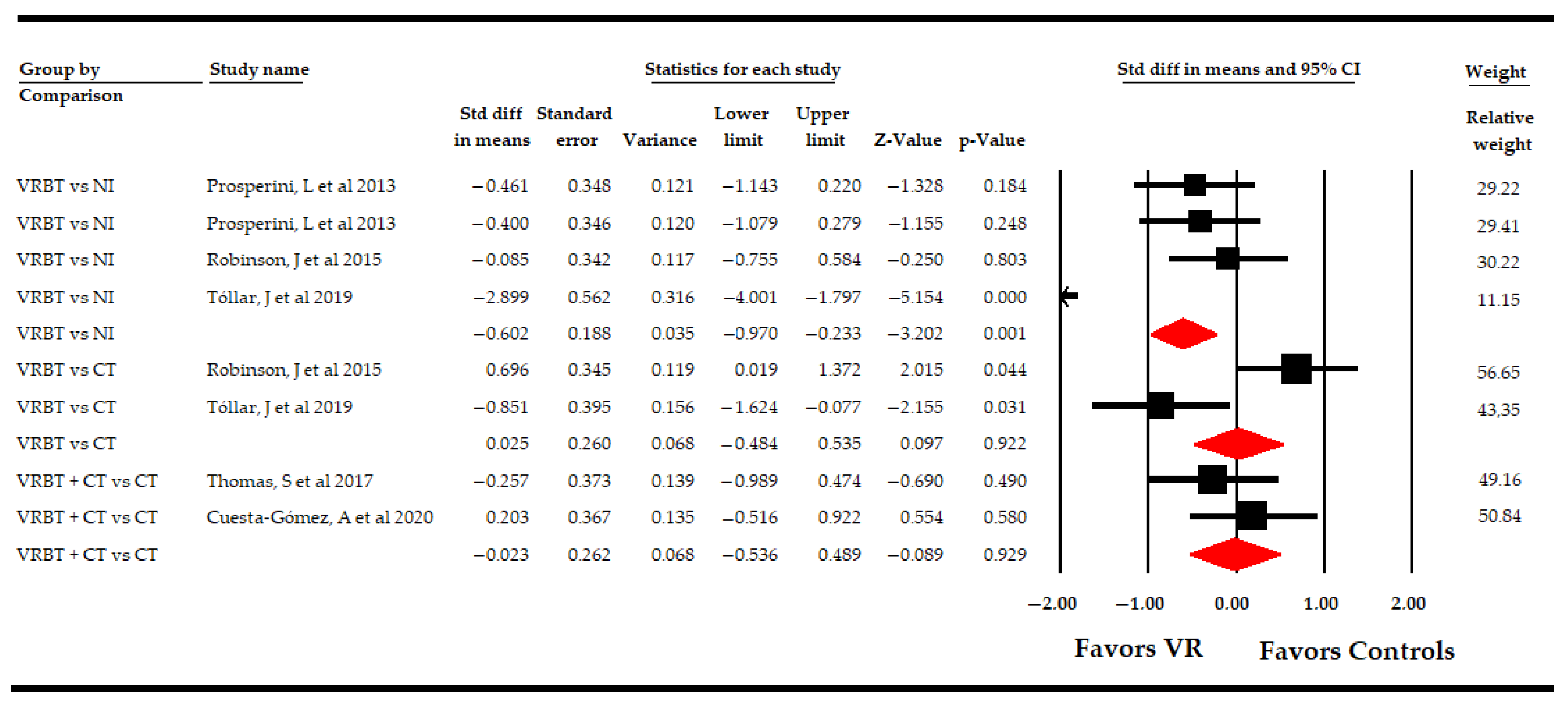
3.5.2. Effect of Virtual Reality-Based Therapy on the Impact of Multiple Sclerosis
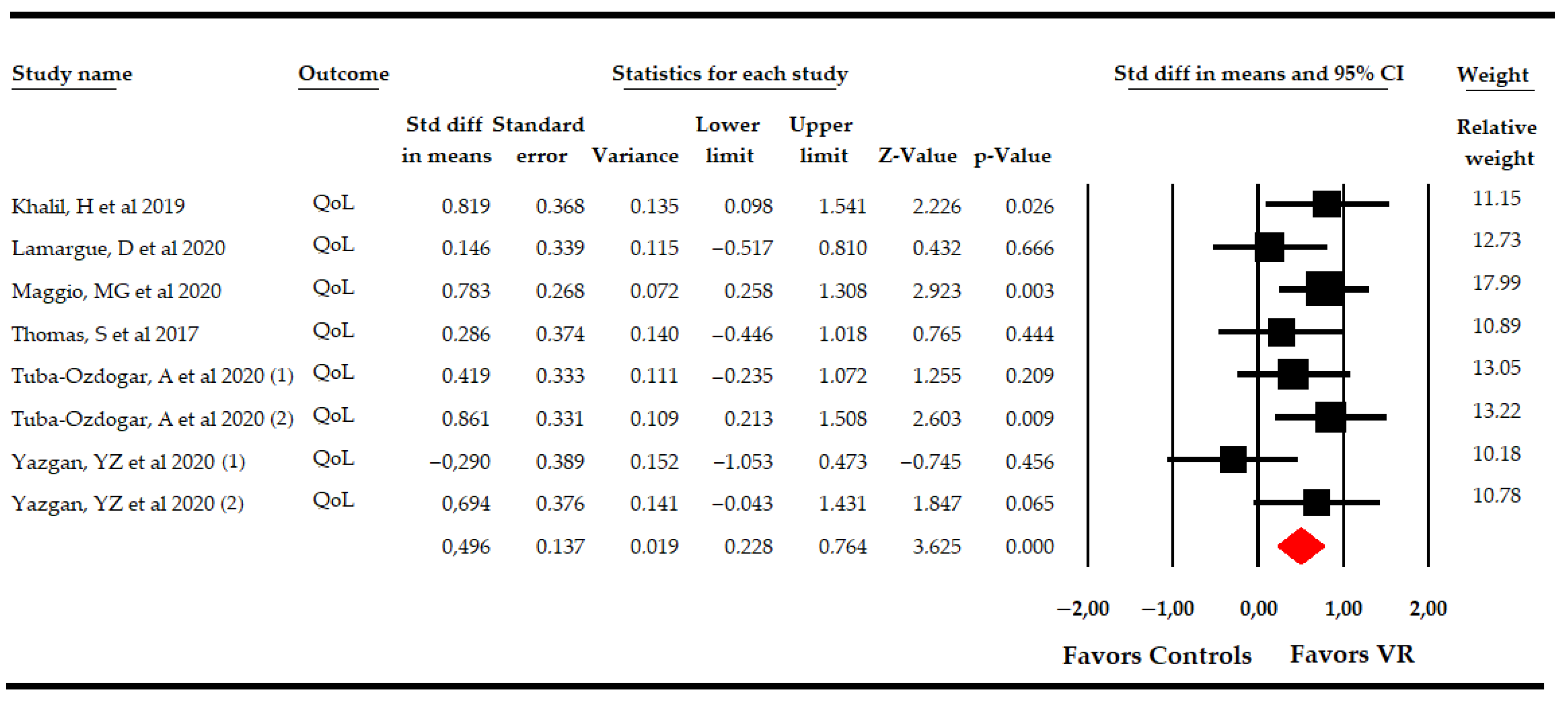
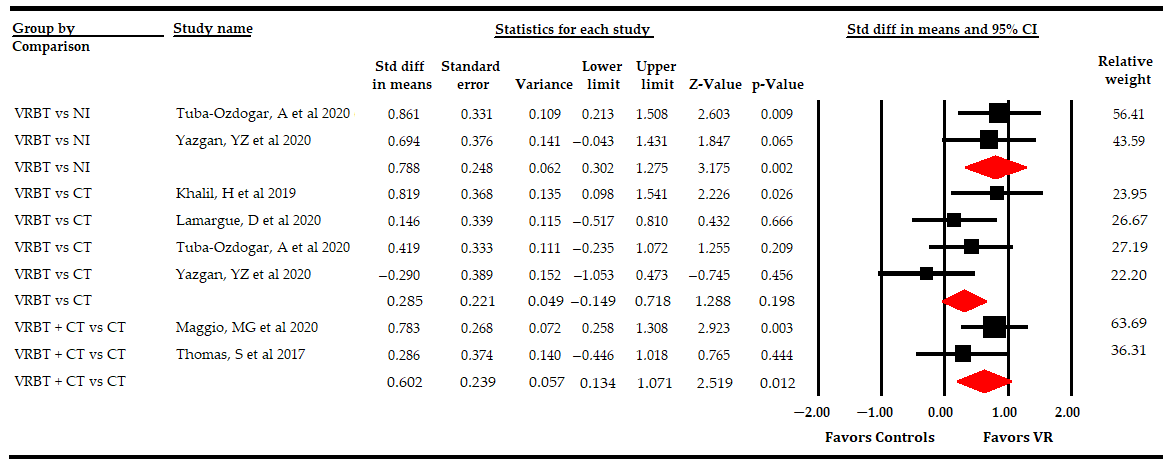
3.5.3. Effect of Virtual Reality-Based Therapy on Overall Quality of Life
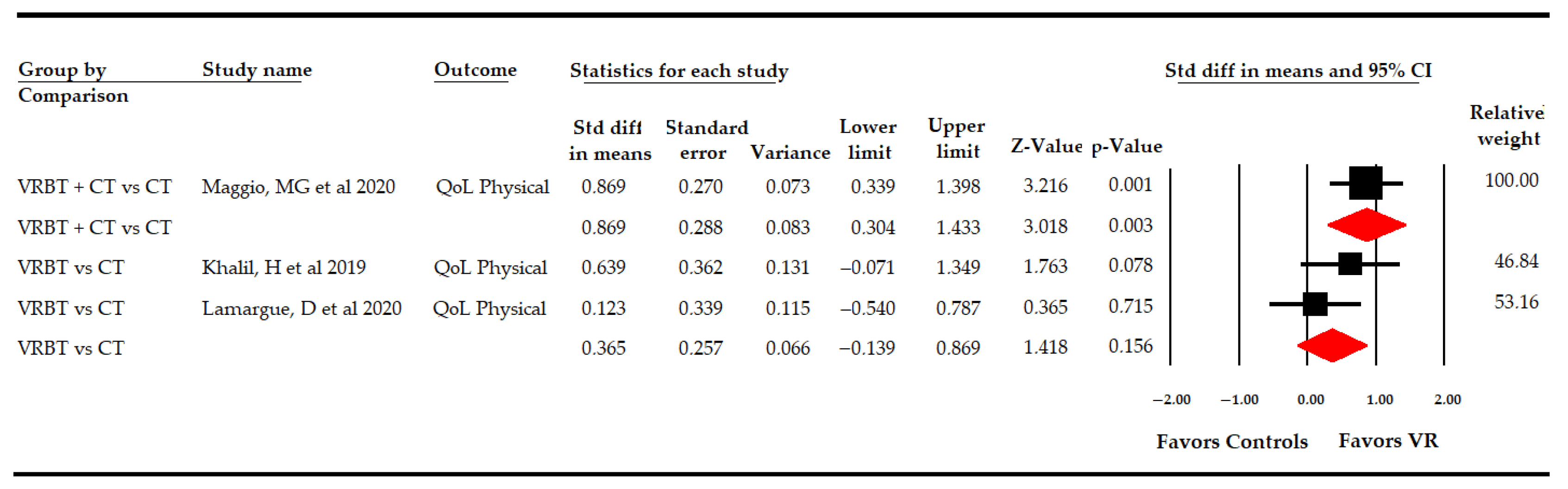
3.5.4 Effect of Virtual Reality-Based Therapy on the Physical Dimension of Quality of Life
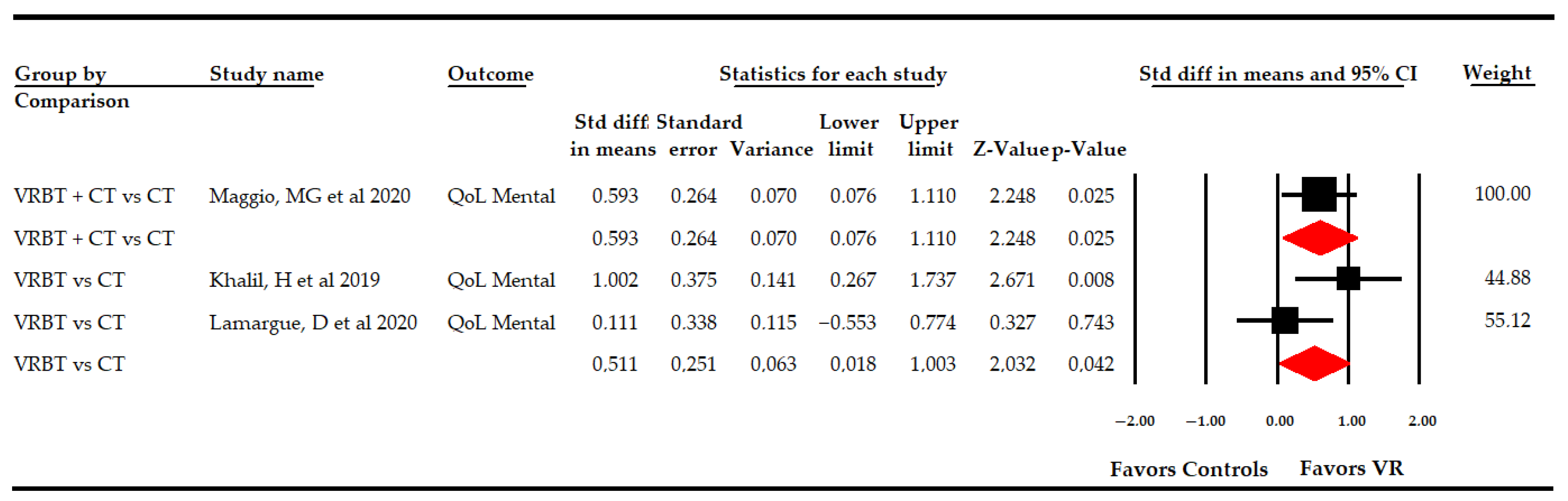
3.5.5 Effect of Virtual Reality-Based Therapy on the Mental Dimension of Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sagawa, Y.; Watelain, E.; Moulin, T.; Decavel, P. Physical Activity during Weekdays and Weekends in Persons with Multiple Sclerosis. Sensors 2021, 21, 3617. [Google Scholar] [CrossRef] [PubMed]
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Ehtesham, N.; Rafie, M.Z.; Mosallaei, M. The global prevalence of familial multiple sclerosis: An updated systematic review and meta-analysis. BMC Neurol. 2021, 21, 246. [Google Scholar] [CrossRef] [PubMed]
- Dahham, J.; Rizk, R.; Kremer, I.; Evers, S.M.A.A.; Hiligsmann, M. Economic Burden of Multiple Sclerosis in Low- and Middle-Income Countries: A Systematic Review. Pharmacoeconomics 2021, 39, 789–807. [Google Scholar] [CrossRef]
- Pugliatti, M.; Rosati, G.; Carton, H.; Riise, T.; Drulovic, J.; Vecsei, L.; Milanov, I. The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 2006, 13, 700–722. [Google Scholar] [CrossRef] [Green Version]
- Angeloni, B.; Bigi, R.; Bellucci, G.; Mechelli, R.; Ballerini, C.; Romano, C.; Morena, E.; Pellicciari, G.; Reniè, R.; Rinaldi, V.; et al. A Case of Double Standard: Sex Differences in Multiple Sclerosis Risk Factors. Int. J. Mol. Sci. 2021, 22, 3696. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.M.; Healy, B.; Augustine, A.; Musallam, A.; Gholipour, T.; Chitnis, T. Effect of gender on late-onset multiple sclerosis. Mult. Scler. J. 2012, 18, 1472–1479. [Google Scholar] [CrossRef]
- Gilli, F.; DiSano, K.D.; Pachner, A.R. SeXX Matters in Multiple Sclerosis. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Krysko, K.M.; Graves, J.S.; Dobson, R.; Altintas, A.; Amato, M.P.; Bernard, J.; Bonavita, S.; Bove, R.; Cavalla, P.; Clerico, M.; et al. Sex effects across the lifespan in women with multiple sclerosis. Adv. Neurol. Disord. 2020, 13, 175628642093616. [Google Scholar] [CrossRef]
- Rodgers, S.; Manjaly, Z.-M.; Calabrese, P.; Steinemann, N.; Kaufmann, M.; Salmen, A.; Chan, A.; Kesselring, J.; Kamm, C.P.; Kuhle, J.; et al. The Effect of Depression on Health-Related Quality of Life Is Mediated by Fatigue in Persons with Multiple Sclerosis. Brain Sci. 2021, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Stolt, M.; Laitinen, A.-M.; Ruutiainen, J.; Leino-Kilpi, H. Research on lower extremity health in patients with multiple sclerosis: A systematic scoping review. J. Foot Ankle Res. 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.; Kishner, S. Modified Ashworth Scale; StatPearls Publishing: Treasure Island, CA, USA, 2020. [Google Scholar]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 276–282. [Google Scholar] [CrossRef] [Green Version]
- LaRocca, N.G. Impact of Walking Impairment in Multiple Sclerosis. Patient Patient-Cent. Outcomes Res. 2011, 4, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Bessing, B.; Hussain, M.A.; Claflin, S.B.; Chen, J.; Blizzard, L.; van Dijk, P.; Kirk-Brown, A.; Taylor, B.V.; van der Mei, I. Changes in multiple sclerosis symptoms are associated with changes in work productivity of people living with multiple sclerosis. Mult. Scler. J. 2021, 27, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Is Fatigue Associated with Aerobic Capacity and Muscle Strength in People with Multiple Sclerosis: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 2193–2204. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Montoro-Cárdenas, D.; Lomas-Vega, R.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Leap Motion Controller Video Game-Based Therapy for Upper Extremity Motor Recovery in Patients with Central Nervous System Diseases. A Systematic Review with Meta-Analysis. Sensors 2021, 21, 2065. [Google Scholar] [CrossRef]
- Palacios-Navarro, G.; Hogan, N. Head-Mounted Display-Based Therapies for Adults Post-Stroke: A Systematic Review and Meta-Analysis. Sensors 2021, 21, 1111. [Google Scholar] [CrossRef]
- Obrero-Gaitán, E.; Nieto-Escamez, F.; Zagalaz-Anula, N.; Cortés-Pérez, I. An Innovative Approach for Online Neuroanatomy and Neuropathology Teaching Based on 3D Virtual Anatomical Models Using Leap Motion Controller During COVID-19 Pandemic. Front. Psychol. 2021, 12, 1853. [Google Scholar] [CrossRef]
- Weiss, P.L.; Keshner, E.A.; Levin, M.F. Virtual reality for physical and motor rehabilitation. In Virtual Reality Technologies for Health and Clinical Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Straudi, S.; Basaglia, N. Neuroplasticity-Based Technologies and Interventions for Restoring Motor Functions in Multiple Sclerosis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 171–185. [Google Scholar]
- Montoro-Cárdenas, D.; Cortés-Pérez, I.; Zagalaz-Anula, N.; Osuna-Pérez, M.C.; Obrero-Gaitán, E.; Lomas-Vega, R. Nintendo Wii Balance Board therapy for postural control in children with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2021, 63, 1262–1275. [Google Scholar] [CrossRef]
- An, C.-M.; Park, Y.-H. The effects of semi-immersive virtual reality therapy on standing balance and upright mobility function in individuals with chronic incomplete spinal cord injury: A preliminary study. J. Spinal Cord Med. 2018, 41, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Kern, F.; Gall, D.; Latoschik, M.E.; Pauli, P.; Käthner, I. Immersive virtual reality during gait rehabilitation increases walking speed and motivation: A usability evaluation with healthy participants and patients with multiple sclerosis and stroke. J. Neuroeng. Rehabil. 2021, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, D.; Georgieva, I.; Gong, Z.; Nanjappan, V.; Georgiev, G. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Cornejo Thumm, P.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Tele-Rehabilitation with Virtual Reality: A Case Report on the Simultaneous, Remote Training of Two Patients with Parkinson Disease. Am. J. Phys. Med. Rehabil. 2021, 100, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Manuli, A.; Maggio, M.G.; Tripoli, D.; Gullì, M.; Cannavò, A.; La Rosa, G.; Sciarrone, F.; Avena, G.; Calabrò, R.S. Patients’ perspective and usability of innovation technology in a new rehabilitation pathway: An exploratory study in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102312. [Google Scholar] [CrossRef]
- Casuso-Holgado, M.J.; Martín-Valero, R.; Carazo, A.F.; Medrano-Sánchez, E.M.; Cortés-Vega, M.D.; Montero-Bancalero, F.J. Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1220–1234. [Google Scholar] [CrossRef]
- Moreno-Verdu, M.; Ferreira-Sanchez, M.R.; Cano-de-la-Cuerda, R.; Jimenez-Antona, C. Efficacy of virtual reality on balance and gait in multiple sclerosis. Systematic review of randomized controlled trials. Rev. Neurol. 2019, 68, 357–368. [Google Scholar] [CrossRef]
- Webster, A.; Poyade, M.; Rooney, S.; Paul, L. Upper limb rehabilitation interventions using virtual reality for people with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2021, 47, 102610. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Elkins, M.R.; Moseley, A.M.; Sherrington, C.; Herbert, R.D.; Maher, C.G. Growth in the Physiotherapy Evidence Database (PEDro) and use of the PEDro scale. Br. J. Sports Med. 2013, 47, 188–189. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis Software Version 3. Available online: https://www.meta-analysis.com/ (accessed on 1 June 2020).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. The Handbook of Research Synthesis and Meta-Analysis; Russell Sage Foundation: New York, NY, USA, 2009. [Google Scholar]
- Faraone, S.V. Interpreting estimates of treatment effects: Implications for managed care. Pharm. Ther. 2008, 33, 700–711. [Google Scholar]
- Rücker, G.; Schwarzer, G. Beyond the forest plot: The drapery plot. Res. Synth. Methods 2021, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.; Greenland, S.; Lash, T. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brichetto, G.; Spallarossa, P.; de Carvalho, M.L.L.; Battaglia, M.A. The effect of Nintendo® Wii® on balance in people with multiple sclerosis: A pilot randomized control study. Mult. Scler. J. 2013, 19, 1219–1221. [Google Scholar] [CrossRef]
- Khalil, H.; Al-Sharman, A.; El-Salem, K.; Alghwiri, A.A.; Al-Shorafat, D.; Khazaaleh, S.; Abu foul, L. The development and pilot evaluation of virtual reality balance scenarios in people with multiple sclerosis (MS): A feasibility study. NeuroRehabilitation 2019, 43, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Lamargue, D.; Koubiyr, I.; Deloire, M.; Saubusse, A.; Charre-Morin, J.; Moroso, A.; Coupé, P.; Brochet, B.; Ruet, A. Effect of cognitive rehabilitation on neuropsychological and semiecological testing and on daily cognitive functioning in multiple sclerosis: The REACTIV randomized controlled study. J. Neurol. Sci. 2020, 415, 116929. [Google Scholar] [CrossRef] [PubMed]
- Ozdogar, A.T.; Ertekin, O.; Kahraman, T.; Yigit, P.; Ozakbas, S. Effect of video-based exergaming on arm and cognitive function in persons with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 40, 101966. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Guclu-Gunduz, A.; Yazici, G.; Atalay Guzel, N.; Irkec, C. Effect of immersive virtual reality on balance, mobility, and fatigue in patients with multiple sclerosis: A single-blinded randomized controlled trial. Eur. J. Integr. Med. 2020, 35, 101092. [Google Scholar] [CrossRef]
- Yazgan, Y.Z.; Tarakci, E.; Tarakci, D.; Ozdincler, A.R.; Kurtuncu, M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 39, 101902. [Google Scholar] [CrossRef]
- Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Oña-Simbaña, E.D.; Martínez-Medina, A.; Ortiz-Comino, C.; Balaguer-Bernaldo-de-Quirós, C.; Jardón-Huete, A.; Cano-de-la-Cuerda, R. Effects of virtual reality associated with serious games for upper limb rehabilitation in patients with multiple sclerosis: Randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Fortuna, D.; Giannì, C.; Leonardi, L.; Marchetti, M.R.; Pozzilli, C. Home-Based Balance Training Using the Wii Balance Board: A Randomized, Crossover Pilot Study in Multiple Sclerosis. Neurorehabil. Neural Repair 2013, 27, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Dixon, J.; Macsween, A.; van Schaik, P.; Martin, D. The effects of exergaming on balance, gait, technology acceptance and flow experience in people with multiple sclerosis: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2015, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.; Fazakarley, L.; Thomas, P.W.; Collyer, S.; Brenton, S.; Perring, S.; Scott, R.; Thomas, F.; Thomas, C.; Jones, K.; et al. Mii-vitaliSe: A pilot randomised controlled trial of a home gaming system (Nintendo Wii) to increase activity levels, vitality and well-being in people with multiple sclerosis. BMJ Open 2017, 7, e016966. [Google Scholar] [CrossRef]
- Tollár, J.; Nagy, F.; Tóth, B.E.; Török, K.; Szita, K.; Csutorás, B.; Moizs, M.; Hortobagyi, T. Exercise Effects on Multiple Sclerosis Quality of Life and Clinical–Motor Symptoms. Med. Sci. Sport. Exerc. 2020, 52, 1007–1014. [Google Scholar] [CrossRef]
- Maggio, M.G.; De Luca, R.; Manuli, A.; Buda, A.; Foti Cuzzola, M.; Leonardi, S.; D’Aleo, G.; Bramanti, P.; Russo, M.; Calabrò, R.S. Do patients with multiple sclerosis benefit from semi-immersive virtual reality? A randomized clinical trial on cognitive and motor outcomes. Appl. Neuropsychol. Adult 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brenner, P.; Piehl, F. Fatigue and depression in multiple sclerosis: Pharmacological and non-pharmacological interventions. Acta Neurol. Scand. 2016, 134, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B. Rehabilitation in Multiple Sclerosis: A Systematic Review of Systematic Reviews. Arch. Phys. Med. Rehabil. 2017, 98, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Mura, G.; Carta, M.G.; Sancassiani, F.; Machado, S.; Prosperini, L. Active exergames to improve cognitive functioning in neurological disabilities: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.S.; Fagundes, C.V.; dos Santos Mendes, F.A.; Leal, J.C. Effectiveness of Virtual Reality Rehabilitation in Persons with Multiple Sclerosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Mult. Scler. Relat. Disord. 2021, 54, 103128. [Google Scholar] [CrossRef] [PubMed]
- Backus, D.; Manella, C.; Bender, A.; Sweatman, M. Impact of Massage Therapy on Fatigue, Pain, and Spasticity in People with Multiple Sclerosis: A Pilot Study. Int. J. Massage Bodyw. Res. Educ. Pract. 2016, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Razazian, N.; Kazeminia, M.; Moayedi, H.; Daneshkhah, A.; Shohaimi, S.; Mohammadi, M.; Jalali, R.; Salari, N. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: A systematic review and meta-analysis. BMC Neurol. 2020, 20, 93. [Google Scholar] [CrossRef]
- Moradi, M.; Sahraian, M.A.; Aghsaie, A.; Kordi, M.R.; Meysamie, A.; Abolhasani, M. Effects of Eight-week Resistance Training Program in Men with Multiple Sclerosis. Asian J. Sports Med. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; McDonough, D.J.; Gao, Z. The Effectiveness of Virtual Reality Exercise on Individual’s Physiological, Psychological and Rehabilitative Outcomes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4133. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Jeng, M.-C.; Fung, C.-P.; Doong, J.-L.; Chuang, T.-Y. Psychological Benefits of Virtual Reality for Patients in Rehabilitation Therapy. J. Sport Rehabil. 2009, 18, 258–268. [Google Scholar] [CrossRef]
- Montana, J.I.; Matamala-Gomez, M.; Maisto, M.; Mavrodiev, P.A.; Cavalera, C.M.; Diana, B.; Mantovani, F.; Realdon, O. The Benefits of emotion Regulation Interventions in Virtual Reality for the Improvement of Wellbeing in Adults and Older Adults: A Systematic Review. J. Clin. Med. 2020, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Jahn, F.S.; Skovbye, M.; Obenhausen, K.; Jespersen, A.E.; Miskowiak, K.W. Cognitive training with fully immersive virtual reality in patients with neurological and psychiatric disorders: A systematic review of randomized controlled trials. Psychiatry Res. 2021, 300, 113928. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Téllez, P.; Moral-Muñoz, J.A.; Salazar, A.; Casado-Fernández, E.; Lucena-Antón, D. Game-Based Virtual Reality Interventions to Improve Upper Limb Motor Function and Quality of Life After Stroke: Systematic Review and Meta-analysis. Games Health J. 2020, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, Y.; Jiang, Y.; Wang, M.; Ang, W.H.D.; Lau, Y. Rehabilitation training based on virtual reality for patients with Parkinson’s disease in improving balance, quality of life, activities of daily living, and depressive symptoms: A systematic review and meta-regression analysis. Clin. Rehabil. 2021, 35, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek-Winiarek, D.J.; Szpakowski, P.; Glabinski, A. Neural Plasticity in Multiple Sclerosis: The Functional and Molecular Background. Neural Plast. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, D.K.; Mangiardi, M.; Xia, X.; López-Valdés, H.E.; Tiwari-Woodruff, S.K. Functional recovery of callosal axons following demyelination: A critical window. Neuroscience 2009, 164, 1407–1421. [Google Scholar] [CrossRef]
- Mezzapesa, D.M.; Rocca, M.A.; Rodegher, M.; Comi, G.; Filippi, M. Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum. Brain Mapp. 2008, 29, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Schoonheim, M.M.; Geurts, J.J.G.; Barkhof, F. The limits of functional reorganization in multiple sclerosis. Neurology 2010, 74, 1246–1247. [Google Scholar] [CrossRef] [PubMed]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012, 36, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata-Bello, J.; Pérez-Martín, Y.; Castañón-Pérez, A.; Modroño, C.; Fariña, H.; Hernández-Martín, E.; González-Platas, M.; Marcano, F.; González–Mora, J.L. The Mirror Neuron System in Relapsing Remitting Multiple Sclerosis Patients with Low Disability. Brain Topogr. 2017, 30, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Calabrò, R.S. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Indovina, P.; Barone, D.; Gallo, L.; Chirico, A.; De Pietro, G.; Giordano, A. Virtual Reality as a Distraction Intervention to Relieve Pain and Distress During Medical Procedures. Clin. J. Pain 2018, 34, 858–877. [Google Scholar] [CrossRef]
- Riva, G.; Mantovani, F.; Capideville, C.S.; Preziosa, A.; Morganti, F.; Villani, D.; Gaggioli, A.; Botella, C.; Alcañiz, M. Affective Interactions Using Virtual Reality: The Link between Presence and Emotions. Cyberpsychol. Behav. 2007, 10, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1996, 351, 1445–1453. [Google Scholar] [CrossRef]
- Deppermann, S.; Notzon, S.; Kroczek, A.; Rosenbaum, D.; Haeussinger, F.B.; Diemer, J.; Domschke, K.; Fallgatter, A.J.; Ehlis, A.-C.; Zwanzger, P. Functional co-activation within the prefrontal cortex supports the maintenance of behavioural performance in fear-relevant situations before an iTBS modulated virtual reality challenge in participants with spider phobia. Behav. Brain Res. 2016, 307, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Maggio, M.G.; Russo, M.; Bramanti, A.; Arcadi, F.A.; Naro, A.; Calabrò, R.S.; De Luca, R. Cognitive recovery in people with relapsing/remitting multiple sclerosis: A randomized clinical trial on virtual reality-based neurorehabilitation. Clin. Neurol. Neurosurg. 2021, 208, 106828. [Google Scholar] [CrossRef]
- Perrochon, A.; Borel, B.; Istrate, D.; Compagnat, M.; Daviet, J.-C. Exercise-based games interventions at home in individuals with a neurological disease: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Zasadzka, E.; Trzmiel, T.; Pieczyńska, A.; Hojan, K. Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19. Medicina 2021, 57, 549. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, P.M.; Śliwiński, M.; Studnicki, R.; Hansdorfer-Korzon, R. Telerehabilitation of Post-Stroke Patients as a Therapeutic Solution in the Era of the Covid-19 Pandemic. Healthcare 2021, 9, 654. [Google Scholar] [CrossRef]
- Russo, M.; Dattola, V.; De Cola, M.C.; Logiudice, A.L.; Porcari, B.; Cannavò, A.; Sciarrone, F.; De Luca, R.; Molonia, F.; Sessa, E.; et al. The role of robotic gait training coupled with virtual reality in boosting the rehabilitative outcomes in patients with multiple sclerosis. Int. J. Rehabil. Res. 2018, 41, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Dorsey, E.R.; Okun, M.S. The Coronavirus Disease 2019 Crisis as Catalyst for Telemedicine for Chronic Neurological Disorders. JAMA Neurol. 2020, 77, 927. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zeng, N.; Pope, Z.C.; McDonough, D.J.; Gao, Z. Acute Effects of Immersive Virtual Reality Exercise on Young Adults’ Situational Motivation. J. Clin. Med. 2019, 8, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Database | Search Strategy |
|---|---|
| PubMed Medline | (multiple sclerosis[mh] or multiple sclerosis[tiab] or “multiple sclerosis”[tiab]) AND (virtual reality[mh] OR virtual reality[tiab] OR virtual reality exposure therapy[mh] OR virtual reality exposure therapy[tiab] OR “virtual reality”[tiab] OR videogames[tiab] OR exergames[tiab] OR serious games[tiab]) AND (fatigue[mh] OR “fatigue”[tiab] OR muscle fatigue[mh] OR muscle fatigue[tiab] OR quality of life[mh] OR quality of life[tiab]) |
| SCOPUS | (TITLE-ABS-KEY (“multiple sclerosis” OR “esclerosis múltiple”) AND TITLE-ABS-KEY (“virtual reality” OR “videogames” OR “exergames” OR “serious games” OR “games”) AND TITLE-ABS-KEY (“fatigue” OR “quality of life”)) |
| Web of Science | (*multiple sclerosis* OR *esclerosis múltiple*) (Topic) and (*virtual reality* OR *exergames* OR * videogames* OR *serious games* OR *games*) (Topic) and (*fatigue* OR *quality of life*) (Topic) |
| PEDro | (multiple sclerosis) and (virtual reality) |
| VR Group | Control Group | Outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Design | Country | Funding | K | N | Age | F:M | Intervention | N | Age | F:M | Intervention | Outcomes | Test |
| Brichetto, G. et al., 2013 [48] | Pilot RCT | Italy | No | 1 | 18 | 40.7 | 10:8 | 12 sessions of Nintendo® Wii Balance Board® during 4 weeks, 3 sessions per week. Each sessions lasted 60 min | 18 | 43.2 | 12:6 | CT (Static and dynamic exercises in single and double leg stance) | Fatigue | M-FIS |
| Cuesta-Gómez, A. et al., 2020 [54] | RCT | Spain | Yes | 2 | 16 | 49.8 | 9:7 | 20 sessions using serious games with LMC, during 10 weeks, 2 sessions per week of 60 min. Conventional Therapy, for 60 min, in upper extremity was added | 14 | 42.6 | 9:5 | CT (Physiotherapy motor rehabilitation of upper extremity joints using mobilizations, stretching and functional tasks) | Fatigue | FSS |
| Impact | MSIS-29 | |||||||||||||
| Khalil, H. et al., 2019 [49] | Pilot RCT | Jordan | Yes | 2 | 16 | 39.8 | 12:4 | Exercises in different niVR scenarios (Nintendo® Wii Balance Board®), during 6 weeks, 2 sessions per week | 16 | 34.8 | 10:6 | CT (Balance exercises) | Fatigue | M-FIS |
| QoL | SF-36 | |||||||||||||
| Lamargue, D et al., 2020 [50] | RCT | France | Yes | 2 | 18 | 43.8 | 12:6 | REACTIV program based niVR, during 4 months, 3 sessions per week. Each session lasted 45 min. | 17 | 38.3 | 14:3 | CT (Physical activity and global cognitive stimulation) | Fatigue | M-FIS |
| QoL | SF-36 | |||||||||||||
| Maggio, M.G. et al., 2020 [59] | RCT | Italy | NR | 1 | 30 | 51.9 | 12:18 | Semi-immersive VR using BTS-Nirvana for a total of 24 sessions using niVR, during 8 weeks, 3 sessions per week. Each session lasted 60 min. In addition, CT program was added | 30 | 48.2 | 17:13 | CT (General conditioning exercises of strengthening, gait and postural control) | QoL | MS-QoL |
| Ozkul, C. et al., 2020 [52] | RCT | Turkey | No | 2 | 13 | 29 | 9:4 | Exercises using augmented and iVR (RAGU system) during 8 weeks, 2 sessions per week. A total of 16 sessions, 20 min each. | 13 | 34 | 11:2 | CT (balance training trough exercises with ball) | Fatigue | FSS |
| 13 | 34 | 10:3 | NI (Simple observation) | |||||||||||
| Prosperini, L. et al., 2013 [55] | RCT | Italy | No | 2 | 18 | 35.3 | 13:5 | Home-based training with Nintendo® Wii Balance Board® during 12 weeks, 5 sessions per week. Sessions lasted 30 min. | 18 | 37.1 | 12:6 | NI (Simple observation) | Impact | MSIS-29 |
| 18 | 37.1 | 12.6 | 18 | 35.3 | 13:5 | NI (Simple observation) | ||||||||
| Robinson, J. et al., 2015 [56] | RCT | UK | Yes | 2 | 20 | 52.6 | 14:6 | 8 sessions of exercises using Nintendo® Wii Fit videogames during 4 weeks, 2 sessions per week. Sessions lasted 40–60 min | 16 | 53.9 | 12:4 | CT (Balance training) | Impact | WHODAS 2.0 |
| 15 | 51.9 | 12:3 | NI (Simple observation) | |||||||||||
| Thomas, S. et al., 2017 [57] | RCT | UK | Yes | 2 | 15 | 50.9 | 14:1 | Home-based training and personalized Nintendo® Wii Balance Board® using Mii-vitaleSe, in addition to other therapies, including CT, as medical treatment if patients require it. | 15 | 47.6 | 13:2 | CT (usual care, physical, medicine and education support) | Impact | MSIS-29 |
| QoL | SF-36 | |||||||||||||
| Tollár, J. et al., 2019 [58] | RCT | Hungary | No | 2 | 14 | 48.2 | 12:2 | 25 sessions using Xbox 360 and Kinect sensor, during 5 weeks, 5 sessions per week. Each session lasted 1 h. | 14 | 46.9 | 12:2 | CT (Dynamic and static balance exercises | Impact | MSIS-29 |
| 12 | 47 | 11:1 | NI (Simple observation) | |||||||||||
| Tuba-Ozdogar, A. et al., 2020 [51] | RCT | Turkey | Yes | 4 | 20 | 39.2 | 16:4 | 8 sessions using Microsoft Xbox One and Kinect motion sensor, during 8 weeks, 1 session per week (45 min per session) | 17 | 43.6 | 12:5 | CT (Balance, stretching and core stability exercises) | Fatigue | M-FIS |
| 20 | 37.9 | 15:5 | NI (Simple observation) | QoL | MS-QoL | |||||||||
| Yazgan, Y.Z. et al., 2020 [53] | RCT | Turkey | Yes | 4 | 15 | 47.4 | 13:2 | 16 sessions of exercises using Nintendo® WiFit videogames during 8 weeks, 2 sessions per week. Sessions lasted 60 min | 12 | 43.1 | 12 | CT (Balance training exercises) | Fatigue | FSS |
| 15 | 40.6 | 13:2 | NI (Simple observation) | QoL | MS-QoL | |||||||||
| Study | i1 | i2 | i3 | i4 | i5 | i6 | i7 | i8 | i9 | i10 | i11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brichetto, G. et al., 2013 [48] | Yes | Yes | No | Yes | No | No | Yes | No | No | Yes | Yes | 5/10 |
| Cuesta-Gómez, A. et al., 2020 [54] | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6/10 |
| Khalil, H. et al., 2019 [49] | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes | Yes | 6/10 |
| Lamargue, D. et al., 2020 [50] | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6/10 |
| Maggio, M.G. et al., 2020 [59] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7/10 |
| Ozkul, C. et al., 2020 [52] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5/10 |
| Prosperini, L. et al., 2013 [55] | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6/10 |
| Robinson, J. et al., 2015 [56] | Yes | Yes | No | Yes | No | No | No | No | Yes | Yes | Yes | 5/10 |
| Thomas, S. et al., 2017 [57] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7/10 |
| Tollár, J. et al., 2019 [58] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7/10 |
| Tuba-Ozdogar, A. et al., 2020 [51] | No | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5/10 |
| Yazgan, Y.Z. et al., 2020 [53] | Yes | Yes | No | Yes | No | No | No | Yes | No | Yes | Yes | 5/10 |
| Effect Size | Publication Bias | Heterogeneity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Groups | K | N | Ns | SMD | 95% CI | p | Funnel Plot | Trim-and-Fill | Q-test | I2 | p | ||
| Adjusted SMD | % of Var. | |||||||||||||
| Fatigue | Overall Analysis | 10 | 319 | 31.9 | −0.33 | [−0.61, −0.06] | 0.02 | Symmetric | −0.33 | 0% | 9 | 0% | 0.44 | |
| Specific comparison subgroups | VR vs NI | 3 | 96 | 33 | −0.2 | [−0.61, 0.2] | 0.33 | Symmetric | −0.22 | 0% | 1.9 | 0% | 0.37 | |
| VR vs CT | 6 | 193 | 32.2 | −0.4 | [−0.7, −0.11] | 0.006 | Asymmetric | −0.51 | 27% | 5.1 | 1.9% | 0.41 | ||
| VR + CT vs CT | 1 | 30 | 30 | −0.23 | [−0.95, 0.48] | 0.52 | NP | NP | NP | NP | NP | NP | ||
| Impact of Multiple Sclerosis | Overall Analysis | 8 | 287 | 31.8 | −0.3 | [−0.55, −0.04] | 0.02 | Asymmetric | −0.61 | 50% | 8.9 | 21% | 0.26 | |
| Specific comparison subgroups | VR vs NI | 4 | 103 | 25.8 | −0.61 | [−0.97, −0.23] | 0.001 | Asymmetric | −0.74 | 23% | 5.5 | 27.7% | 0.23 | |
| VR vs CT | 2 | 64 | 32 | −0.03 | [−0.48, 0.53] | 0.92 | NP | NP | NP | 0.98 | 0% | 0.32 | ||
| VR + CT vs CT | 2 | 90 | 45 | −0.02 | [−0.54, 0.49] | 0.93 | NP | NP | NP | 0.3 | 0% | 0.6 | ||
| Overall Quality of Life | Overall Analysis | 8 | 291 | 36.3 | 0.5 | [0.23, 0.76] | <0.001 | Symmetric | 0.5 | 0% | 7.1 | 2% | 0.21 | |
| Specific comparison subgroups | VR vs NI | 2 | 99 | 49.5 | 0.79 | [0.3, 1.28] | 0.002 | NP | NP | NP | 0.1 | 0% | 0.75 | |
| VR vs CT | 4 | 166 | 41.5 | 0.29 | [−0.15, 0.72] | 0.2 | Asymmetric | 0.44 | 57% | 3.1 | 3.5% | 0.21 | ||
| VR + CT vs CT | 2 | 90 | 45 | 0.6 | [0.13, 1.07] | 0.012 | NP | NP | NP | 1 | 0% | 0.32 | ||
| Physical Quality of Life | Overall Analysis | 3 | 127 | 42.3 | 0.58 | [0.13, 1.02] | 0.011 | Symmetric | 0.58 | 0% | 1.9 | 0% | 0.38 | |
| Specific comparison subgroups | VR vs CT | 2 | 67 | 33.5 | 0.37 | [−0.14, 0.87] | 0.16 | NP | NP | NP | 1 | 0% | 0.32 | |
| VR + CT vs CT | 1 | 60 | 60 | 0.87 | [0.3, 1.43] | 0.003 | NP | NP | NP | NP | NP | NP | ||
| Mental Quality of Life | Overall Analysis | 3 | 127 | 42.3 | 0.55 | [0.09, 1.01] | 0.018 | Asymmetric | 0.38 | 31% | 2.1 | 6.2% | 0.35 | |
| Specific comparison subgroups | VR vs CT | 67 | 33.5 | 33.5 | 0.51 | [0.02, 1] | 0.042 | NP | NP | NP | 1 | 0% | 0.32 | |
| VR + CT vs CT | 60 | 60 | 60 | 0.6 | [0.08, 1.11] | 0.025 | NP | NP | NP | NP | NP | NP | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. https://doi.org/10.3390/s21217389
Cortés-Pérez I, Sánchez-Alcalá M, Nieto-Escámez FA, Castellote-Caballero Y, Obrero-Gaitán E, Osuna-Pérez MC. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors. 2021; 21(21):7389. https://doi.org/10.3390/s21217389
Chicago/Turabian StyleCortés-Pérez, Irene, Marcelina Sánchez-Alcalá, Francisco Antonio Nieto-Escámez, Yolanda Castellote-Caballero, Esteban Obrero-Gaitán, and María Catalina Osuna-Pérez. 2021. "Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis" Sensors 21, no. 21: 7389. https://doi.org/10.3390/s21217389










