3.1. Characteristics of the Anodization Current–Time Curves
Figure 5 shows the normalized current density curves of the samples SP, SO and SS, anodized in three different electrolytes, namely, sulphuric (21 V), oxalic (40 V) and phosphoric acids (150 V), respectively, which reveals a good similarity with conventional anodization curves conducted in potentiostatic mode, as described by Sulka [
33]. The equations describing the formation of alumina porous structures are invariant with respect to the scale transformation
and
, as reported by Parkhutik et al. [
34]. In this sense, in order to better be able to directly compare the characteristic curves of current density
as a function of time
, a normalization to the curves was performed using
and
. For each electrolyte,
is the maximum value of current density and
is the corresponding time to which the maximum current density occurs, whereas
and
are the dimensionless parameters associated with each of the physical variables.
Concerning the anodizing process conducted in sulphuric acid, the maximum value of its current density is around 36.5 mA/cm
2, reached after about 1.14 min (corresponding to about 1.9% of the total anodizing time), while oxalic acid takes around 0.37 min (only about 0.93% of the total anodizing time) to achieve
. On the other hand, the anodization carried out in phosphoric acid (usually known as hard anodization, HA [
35]) takes about 3.6 s (now corresponding to 12% of its total anodization time, which is 30 s) to reach
meaning that, in terms of relative comparison, it takes much longer than those performed in the other two electrolytes. This behaviour was already expected, because as the structure morphology obtained by anodization in phosphoric acid is characterized by large pores (in this work, diameters of 180 nm were found, compared to 40.6 and 18.7 nm obtained in oxalic and sulfuric acids, respectively, and interpore distances of 264.5 nm, compared to 70.6 and 37.1 nm in oxalic and sulfuric acids, respectively), longer times are needed to complete the self-organization of the nanoporous anodic layer. In addition, from
Figure 5 it is also possible to make other considerations, namely:
(a) Since oxalic acid is an organic compound, its negative ions are probably barely incorporated into the alumina structure. Compared to the other two electrolytes, a lower concentration of ions in the alumina makes the electric field higher than in the other two electrolytes, thus promoting an earlier nucleation of the pores and consequently making the anodization in oxalic acid a faster process; this is verified in this study, because as mentioned above, the time it takes to reach only corresponds to 0.93% of the total anodization time.
(b) The steady-state current density value
is different for the three types of produced samples. The sample SP (fabricated in phosphoric acid electrolyte) present the highest steady-state current density average value (
and the SO sample (obtained from oxalic acid electrolyte) has the smallest, i.e.,
, while the steady-state current density average value for the SS sample (anodized in sulphuric acid) presents the value of
. It is well known that the structure of anodic oxide layer incorporates electrolyte anions (
,
and
) from the electrolyte used in the anodization [
36]. Since
is directly related to the concentration of incorporated electrolyte anions, the results shown in
Figure 5 indicate that the concentration electrolyte anions should higher for the samples that also have a higher steady-state current density value, that is,
. However, the typical content of incorporated anions for some more common electrolytes are of the order of 6–8% in phosphoric acid solution, 10–13% in sulfuric acid and 2–3% in oxalic acid [
36].
For the steady-state growth of porous alumina, the role played by the interaction of water with electrolyte anions is shown schematically in
Figure 6. It is believed that during anodization,
ions are generated in the electrolyte by simple splitting of water, whereas the
ions can be formed at the electrolyte/oxide interface from the water interaction with the adsorbed electrolyte anions [
37].
For the steady-state growth of the anodic layer, the opposite migration of
and
ions is responsible for the oxide formation, which occurs simultaneously at the electrolyte/oxide and oxide/metal interfaces. At the oxide/electrolyte interface, the presence of electrolyte anions as well as
ions affects the sensing characteristics of capacitive-type humidity sensors because they influence the mechanism of water vapour adsorption on the porous oxide layer; therefore, it is expected that a higher concentration of electrolyte anions should lead to a greater change in capacitance values. This is shown and explained later in
Section 3.3.
3.2. Analysis of NP-AAO Surface Morphology
Figure 7 shows the SEM micrographs of bare and gold-coated NP-AAO test specimens. The cross-sectional SEM micrographs of anodized samples in sulphuric, oxalic and phosphoric acid solutions are shown in
Figure 7a–c, respectively, while
Figure 7d–f displays the corresponding surface SEM micrographs, which also include pore diameter information. Additionally,
Figure 7g–i presents the SEM micrographs of gold-coated samples, previously anodized in sulphuric, oxalic and phosphoric acid solution, respectively. Estimates of pore size distribution were determined from the SEM micrographs and are shown in
Figure 7j–m as a size (pore diameter) distribution histogram of samples SS, SO and SP, respectively.
From
Figure 7, it is possible to observe that some structural morphological features (e.g., pore diameter, inter-pore distance, porosity) of the different samples depend on the type of electrolyte used in the anodization process. Nevertheless, the SEM micrographs confirm that all anodized samples present a common denominator, which is their self-aligned cylindrical shape cells (each containing a pore at the centre) with a high aspect ratio (length-to-pore diameter ratio), long-range ordering and the uniformity and size of the pores at the nanometre scale. Even so, and as noted, pore size varies with the type of electrolyte used and increases with increasing applied voltage, e.g., 21 V, 40 V and 150 V for samples SS, SO and SP, respectively. The increase in pore diameter occurs because in the initial phase of pore formation and growth, a higher voltage leads to a strengthening of the electric field and as a result of a stronger electric field (also stronger at the hemispherical barrier oxide layer at the pore base), the dissolution of the oxide layer is increased.
Figure 7i also reveals that the deposited gold layer on the SP sample surface (anodized with a phosphoric acid solution) is of suitable thickness, as it does not cover the pores underneath it, thus allowing the water vapour molecules (application as a humidity sensor) or the molecules from the liquid bacterial culture medium (application as a touch sensor) to infiltrate through them more easily. On the other hand, observing
Figure 7g (SS sample, anodized in a sulphuric acid solution), one can infer that the deposition of the gold layer on the surface of the SS sample led to some obstruction of its pores, which can negatively influence the capacitive response of this anodic structure.
For hexagonal cell porous anodic alumina with a pore inside each hexagon, assuming that each pore is a perfect circle, the porosity,
P can be expressed as follows [
33]:
Here,
and
are the pore diameter and the interpore distance (cell diameter) of the alumina nanostructure, respectively, as indicated in
Figure 7a. For the alumina nanostructure with a hexagonal distribution of cells, the pore density,
n, is defined as the total number of pores occupying the surface area of 1 cm
2, expressed as [
33]:
where
is the surface area of a single hexagonal cell (in nm
2) and
is expressed in nm.
Table 4 presents the structural parameters for the as-prepared NP-AAO samples in which the tabulated values result from the analysis of SEM micrographs shown in
Figure 7 by using the image-processing program, ImageJ.
From
Table 4 it is possible to verify that the values of the structural parameters of the NP-AAO samples contrast significantly because the samples were anodized with different electrolytes and applied voltages. For example, comparing the SS sample (anodized with a H
2SO
4 acid solution and an applied voltage of 21 V) with the sample SP (anodized with a H
3PO
4 acid solution and an applied voltage of 150 V), it is observed that their structural parameters are quite different, as for the SP sample the values of
Dp,
Di and
P are about 9.63, 7.13 and 1.83 times higher than those of the SS sample. Therefore, the SS sample presents a much higher pore density (about 49 times higher) than the SP sample. However, the difference in structural parameters is much smaller when comparing these values for the SS and SO samples. For the SO sample (anodized with an oxalic acid solution and an applied voltage of 40 V) their structural parameters, namely,
Dp,
Di and
P, are about 2.17, 1.90 and 1.30 times higher, respectively, than the ones obtained for the sample SS; thus, the pore density of the SS sample is only 3.65 times greater than that of the SO sample. In this sense, it is expected that variations of the morphological parameters of nanoporous alumina are responsible for the response of a metal–insulator–metal-based sensor device, in capacitive mode.
3.3. Structure and Electrical Equivalent Circuit of a Capacitive-Type Sensor
The pore’s morphology of NP-AAO structures have an important contribution on their capacitive response, because the wetting and formation of a physisorbed layer of water on the pore’s wall surfaces causes a variation in the dielectric constant and consequently, a change in the response of capacitive-type sensors (humidity sensors or touch sensors). Since the very high permittivity exhibited by water is due to the polar structure of its H2O molecule, the permittivity of porous dielectric materials is greatly increased with the adsorption of water because the air in the pores is gradually replaced by adsorbed water vapour as the humidity level in the environment increases. In the following, a qualitative model is presented to estimate and explain the sensing behaviour of the fabricated sensor devices, operating in capacitive mode.
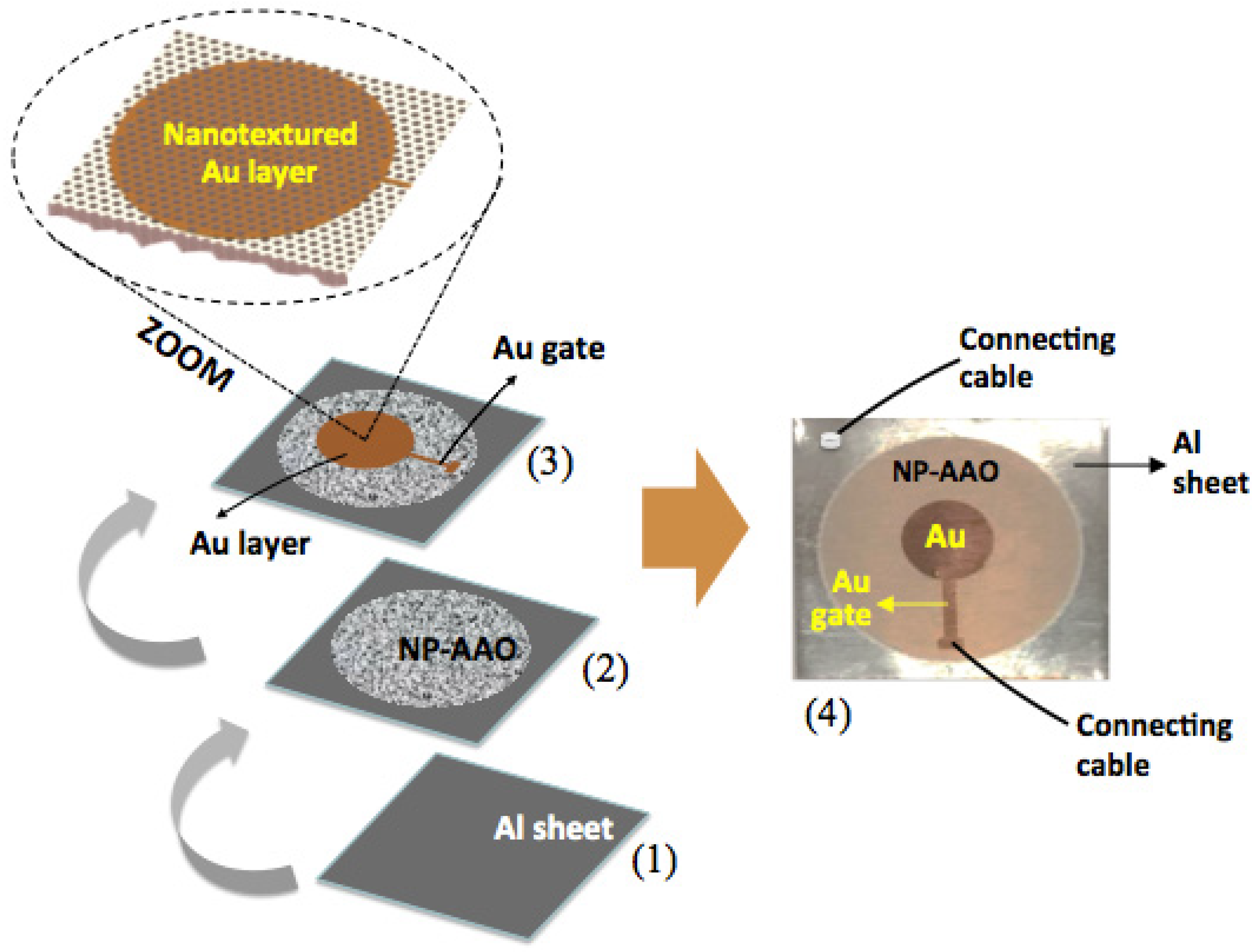
The nanoporous alumina is a metal–dielectric–metal (MIM) capacitive-type sensing device, as it comprises a cylindrical-shape Al
2O
3 porous layer grown on a barrier layer and inserted between two metal electrodes, namely the bottom Al substrate and the top Au thin layer. The top and cross-sectional views of nanoporous alumina humidity sensor are schematically represented in
Figure 8a,b, respectively.
Figure 8a depicts the top view geometrical model of the porous alumina comprising a closed-packed array of hexagonally arranged cells containing pores at each cell centre. Meanwhile, the cross-sectional view of the anodized layer is depicted in
Figure 8b, where the cylindrical-shape parallel channels are sandwiched between the top and bottom electrodes. In order to take the effect of the humidity level on the sensor capacitance into account, a qualitative model was developed, in which an annular region (top view) is considered, where each pore is partially filled with a thin layer of water vapour molecules of thickness
. The main parameters of the porous alumina nanostructures are also indicated, namely, the pore radius in the presence of humidity
, the pore diameter without humidity
, the interpore distance
, the wall thickness
and the length of the fine parallel channels
. In this model,
varies between 0 (no humidity; in this case
) and
(pore completely filled with water vapour; in this case,
). The capacitance resulting only from the contribution of the Al
2O
3 anodic layer
, can be expressed as:
where
is the dielectric permittivity at 0% RH (i.e., dry environment),
[
38] and
are the dielectric constant and depth of the NP-AAO layer, respectively, and
is the area of gold film capped on the surface of the NP-AAO layer, which is related to the porosity of the NP-AAO layer according to the equation:
Here,
is the circular area of the gold thin film on flat surface without nanopores and
is the porosity (no humidity) previously defined by Equation (1). Next, one has to consider the effect of the humidity level on the capacitance of the NP-AAO structure. According to
Figure 8a, the hexagonal cell geometric parameters
and
can be expressed as
and
, respectively. In this sense, an area fraction
should be defined, which for a hexagonal cell with an annular region within each hexagon (see
Figure 8a) corresponds to the ratio of the annular region area to the area of the hexagon:
Since
and
, then the area fraction
can be expressed as:
Considering the area of a single hexagon under which a single AAO cylindrical-shape channel resides, and also assuming that the depth of the water vapour layer is around the NP-AAO layer, then the capacitance due to the contribution of humidity
can be expressed by the equation:
where
[
39] is the dielectric constant of water.
Figure 8c represents the equivalent electrical circuit of the NP-AAO humidity sensor device. The sensor’s electrical circuit has two components, namely the NP-AAO dry pore wall capacitance
and an additional capacitor that depends on the surrounding humidity level
. Since both the capacitors (i.e.,
and
) are electrically connected in parallel (see
Figure 8c), the effective capacitance
of the NP-AAO sensor device in the presence of humidity can be expressed as:
As an analytical example, it is interesting to analyse two extreme and opposite conditions. In the first condition (dry state), there is no water vapour inside the pores (in this case,
,
and
). In the second condition, the pores are fully filled with condensed water, thus
,
and
.
Table 5 shows the estimated capacitance values (calculated from Equation (8)) for the two extreme conditions invoked above, which refer to the three different types of NP-AAO fabricated by the one-step anodization technique.
It is interesting to analyse the results shown in
Table 5. At the beginning (i.e., the first condition where there is no humidity), it is observed that the capacitance is greater for samples with lower porosity values, i.e.,
. This behaviour was already predicted, because in this condition, the effective capacitance only results from the contribution of the AAO dielectric material; therefore, capacitance is evaluated only by applying Equation (3), whose numerator presents higher values for samples with lower porosity. In other words, the area
of the gold film capped on the surface of the NP-AAO layer is higher when the porosity is lower. However, the situation changes when considering the contribution of humidity (second condition).
In the presence of humidity, Equation (8) is applied and as the humidity level increases (increase in thickness
), the annular area
(see
Figure 4) increases (and consequently also increases the area fraction,
), because pores are progressively covered by a layer of water vapour molecules, thus causing a change in the original capacitance. As a comparative analysis, let us first consider the SS sample which is the one with the smallest pores and the lowest porosity value,
. Taking its extreme condition, in which the pores are completely filled with water vapour, the annular area
becomes equal to the pore circular area
. Therefore, the area fraction
reaches a maximum and equals the original porosity. Under this extreme condition, the capacitance for the SS sample reaches the maximum and even if the humidity level increases, the capacitance does not increase anymore because its pores are already saturated (i.e., fully filled with water vapour). However, for the other two anodized samples (SO and SP) that present a larger pore size and porosity, their capacitance still has room to increase if the humidity level also increases, because their pores are only partially filled with water vapour (i.e., there is still an annular water vapour area).
Now, let us compare the behaviour of the SS sample with the SP sample, which has a larger pore size and porosity. For the extreme condition of the SS sample, where the pores are completely filled with water vapour (i.e.,
), it is verified that
and
. However, for the same water vapour layer with thickness
, the SP sample displays an area fraction
(well below that of the SS sample), which is still far from its maximum of 0.42 (equal to its porosity value). Therefore, when compared to the SS sample, the capacitance of the SP sample still presents a relatively low value (0.55 nF, as calculated from Equation (8)), as it corresponds to about half of the value displayed by the SS sample. One the other hand, if the humidity level increases again, the area fraction
of the SP sample still has enough room to increase (as well as its capacitance) until it eventually reaches its maximum, which corresponds to a porosity value of 0.42. However, the SS sample can no longer keep up with this increase because its pores are already saturated and therefore its capacitance should remain unchanged even though the humidity level is increasing. In this extreme condition, the capacitance of the SP sample (1.814 nF) already exceeds the value displayed by the SS sample by about 1.5 times, as calculated from Equation (8).
Figure 9 compares the capacitive response of the produced samples, as estimated by Equation (8). In order to better perform a direct comparison of the estimated capacitive response for the different samples, the corresponding capacitance values are plotted as a function of the percentage ratio of the adsorbed water layer thickness
and the pore radius
.
From
Figure 9, it is observed that as the humidity condensation inside the pores increases, that is, for increasing values of the percentage ratio
, the capacitance values also increase for all samples. Furthermore, it is also perceived that as one progresses towards pore saturation, which occurs for increasing humidity levels (here simulated by high values for
and for the area fraction
), the capacitance values of samples that present a high porosity (yet having low pore density) surpass the capacitance values of the samples that have a low porosity but high pore density. In other words, for the extreme condition (i.e., the second condition indicated in
Table 5), there is a change in the order of capacitance values, that is,
. It is clear that the progressive filling (with water vapour) of the area of the originally void regions (air) is the key determining condition for the resulting capacitance of the device.
3.4. NP-AAO Humidity Sensor Performance
General speaking, the sensing mechanism underlying humidity sensors is related to the change in capacitance due to the change in the dielectric after adsorption of water vapour.
In most bibliographic references of published works, the sensitivity
of a sensor is defined as the slope of its response curve (electrical output signal) as a function of an input stimulus. For a capacitive-type humidity sensor, the slope of the response curve is the ratio of the incremental change in capacitance,
(sensor output signal), to the incremental change in relative humidity,
(physical property at the input), being expressed by the following equation [
40]:
where
denotes the capacitance at the relative humidity of
RH and
is the capacitance at the relative humidity of 23% RH, which in this study is also the lower limit of the relative humidity operating range (
).
For a more general response, the sensor’s sensitivity can also be expressed in percentage (
S%), which is given by the equation [
41,
42]:
Here, the subscripts u and l represent the values at the upper and lower limits of the operating range, respectively.
In the present work, the sensitivity parameters of the developed sensors were evaluated through Equation (9) because its application provides a more useful understanding of the sensor’s performance, since it carries information about the input and output parameters instead of Equation (10), which only provides output parameters, and therefore, the reader does not have a clear idea about the type of input parameter and its range. It has been reported that the capacitive response arises from the contribution of two main aspects, namely the pore diameter and the presence of anion species, such as
and
, and electrolyte-driven anions, such as
,
and
generated within the pores of the anodic aluminium oxide layer [
16]. In an attempt to concisely explain the response of nanoporous alumina exposed to different humidity levels, it was suggested that the electrolyte anions act as a source of high charge density [
43], which helps the physisorption of water molecules and leads to the formation of a liquid-like arrangement within the pores, thus changing the dielectric material surrounding the air pore and, consequently, the effective capacitance.
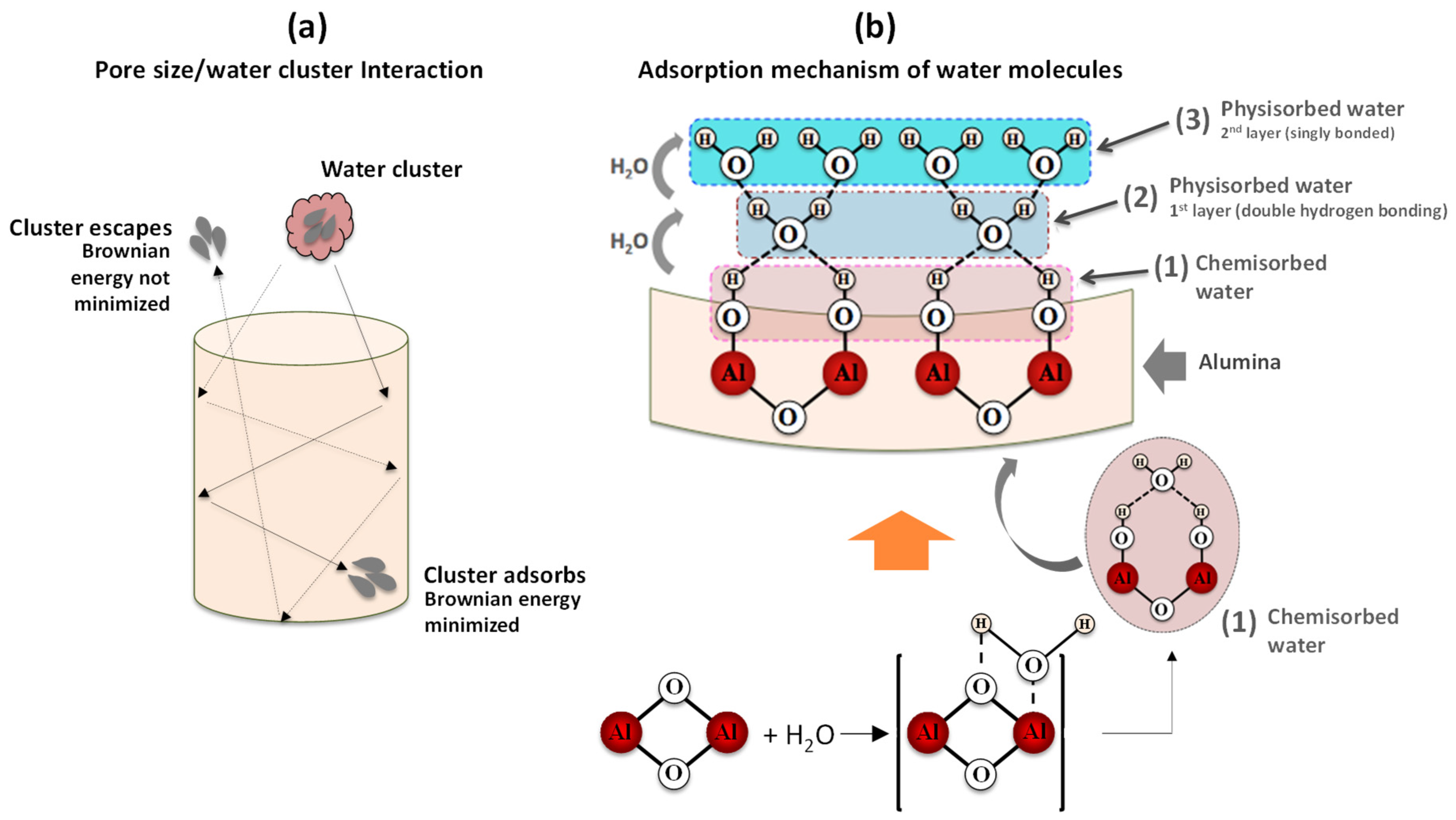
Figure 10 is a schematic representation of the effect of pore diameter as well as the mechanism underlying water adsorption by oxide surfaces.
Regarding the pore size contribution, the process involves three main steps (
i) the entry of water molecules through the pores; (
ii) Brownian motion with loss of kinetic energy due to the inelastic collisions of the water molecules with the pore walls or with other molecules, and (
iii) the adsorption of water molecules on the surface of the pore wall (or eventual exit through the entry door, depending on the molecular Brownian energy). With respect to adsorption, water molecules undergo different chemical and physical processes. If porous alumina is brought into contact with humid air, the water molecules (present at the surface of the base material) are firstly chemisorbed on an activated site of the alumina surface, forming an adsorption complex where the hydroxyl group adsorbs on metal cations present in the oxide surface layer and the proton interacts with an adjacent surface
group to form a second
group (1) [
44]; an increase in humidity level enables another water molecule to be physisorbed (via a double hydrogen bonding of a single water molecule) on the two neighbouring hydroxyl groups, thus forming the first monolayer of physisorbed water (2); as the humidity level continues to rise, the single-layer physisorption changes to multilayer physisorption, where each water molecule will now be singly bonded to a hydroxyl group to form a liquid-like arrangement (3) [
44]. This is a necessary condition to enable a change in the capacitive response, since singly bonded water molecules form electrical dipoles that can be freely reoriented under an external applied electrical field, thus resulting in an increase of the dielectric constant.
Figure 11 shows the variation of capacitance as a function of relative humidity (%RH) for the different fabricated capacitive-type sensors. The inset included in
Figure 11 refers to the capacitive response of the SS sample to facilitate its comparative analysis with the other samples.
From
Figure 11, it can be observed that as the humidity level increases, the capacitance value of the sensors also increases, but for the same humidity range (23–75% RH), the change in capacitance for SS, SO and SP samples, is significantly different. The differences in capacitive responses are due to their different pore morphology because it plays an important role in the adsorption/condensation of water vapours in the porous structure of alumina.
The SP capacitive sensor (with the largest pore diameter) is quite sensitive in the range of 23–55% RH and becomes much less sensitive above 55% RH because its capacitance values increase rapidly with relative humidity up to 55% RH, and afterwards the increase becomes much slower (with a tendency to remain unchanged). This behaviour is directly related to the large pore diameter. We suggest that in the range of 23–75% RH, both small and big water clusters can get easy access into the pores. If only small water clusters existed, their average free path would be high, as they would move in large pores; therefore, they would suffer less inelastic scattering with other clusters, which would result in small losses of Brownian energy and consequently, they would easily escape through the initial entry. However, for this range of humidity values, there should be a strong probability of the presence of a high number of big clusters, so that the mean free path is smaller. Under this condition, the inelastic scattering with other clusters and with the pore walls becomes high, the reduction of Brownian energy is much higher, and the cluster escape is much more difficult. As a result, the cluster adsorption mechanism (in these large-volume pores) is promoted, which causes an increase in the dielectric constant. As the humidity level increases, more and more clusters join to form bigger clusters, more layers of adsorbed water are formed until the saturation condition is reached (when the pores become almost filled), where the dielectric constant no longer changes and therefore, the capacitance also tends to remain unchanged, which seems to happen from 55% RH.
Concerning the SS sensor, the response characteristic was found to be almost linear, but its capacitance maximum value was remarkably lower than that of the SP sensor (only about 30%) and also very far from the saturation value estimated by Equation (8) (about half). The SS sensor holds the smallest pore diameter, so it acts as a filter that screens for smaller cluster sizes, comparable to its pore diameter. In this sense, irrespective of the humidity level, larger clusters are prevented from entering and do not contribute to changing the dielectric constant. For the low humidity levels (even starting at 23% RH), it can be inferred that fewer clusters are adsorbed because water vapours do not continuously cover the surface. However, as the humidity level increases, more layers of water are successively formed, and the result is a progressive increase in capacitance. We believe that a possible explanation for the low capacitance values recorded for the SS sensor could be related to the inefficient nanotexturing of the gold top electrode; the gold film may have been deposited too thick so that many pores may have been obstructed, as suggested by the SEM micrograph analysis of
Figure 7b.
Regarding the SO sensor, and unlike the SP sensor, it is noted that in the range of 23–55% RH the increase in its capacitance is much smaller, while above 55% RH its capacitive response is much more expressive because its capacitance increases significantly (a kind of exponential increase). Compared to the SP and SS sensors, the SO sensor presents an in-between pore diameter; therefore, it is suggested that the SO sensor’s pore morphology is acting as a filter that constrains the entry of the larger molecular clusters into the pores, while smaller ones easily enter and escape through their initial entry without contributing to the adsorption of water vapour molecules. In this sense, it appears that only the statistical population of water clusters that show molecular size compatibility with the pore size of the SO sensor is available to promote a change in dielectric constant upon adsorption, thus leading to a change in its capacitive response, which is much more significant above 55% RH. We assume that above this humidity level, the number of compatible clusters also will increase and become bigger and bulky in weight, thus enabling the coalescence of water vapour molecules on the pore wall surface and subsequent condensation, resulting in a rapid change in capacitive response. Another observation is related to the effect of the incorporation of electrolyte anions in the SO nanoporous oxide structure. Since oxalic acid is an organic acid, it is more than likely that its negative ions are barely incorporated into the alumina layer, because as shown in
Figure 5, it exhibits a lower steady-state current density (lower charge density), which does not contribute to potentiate changes in capacitance.
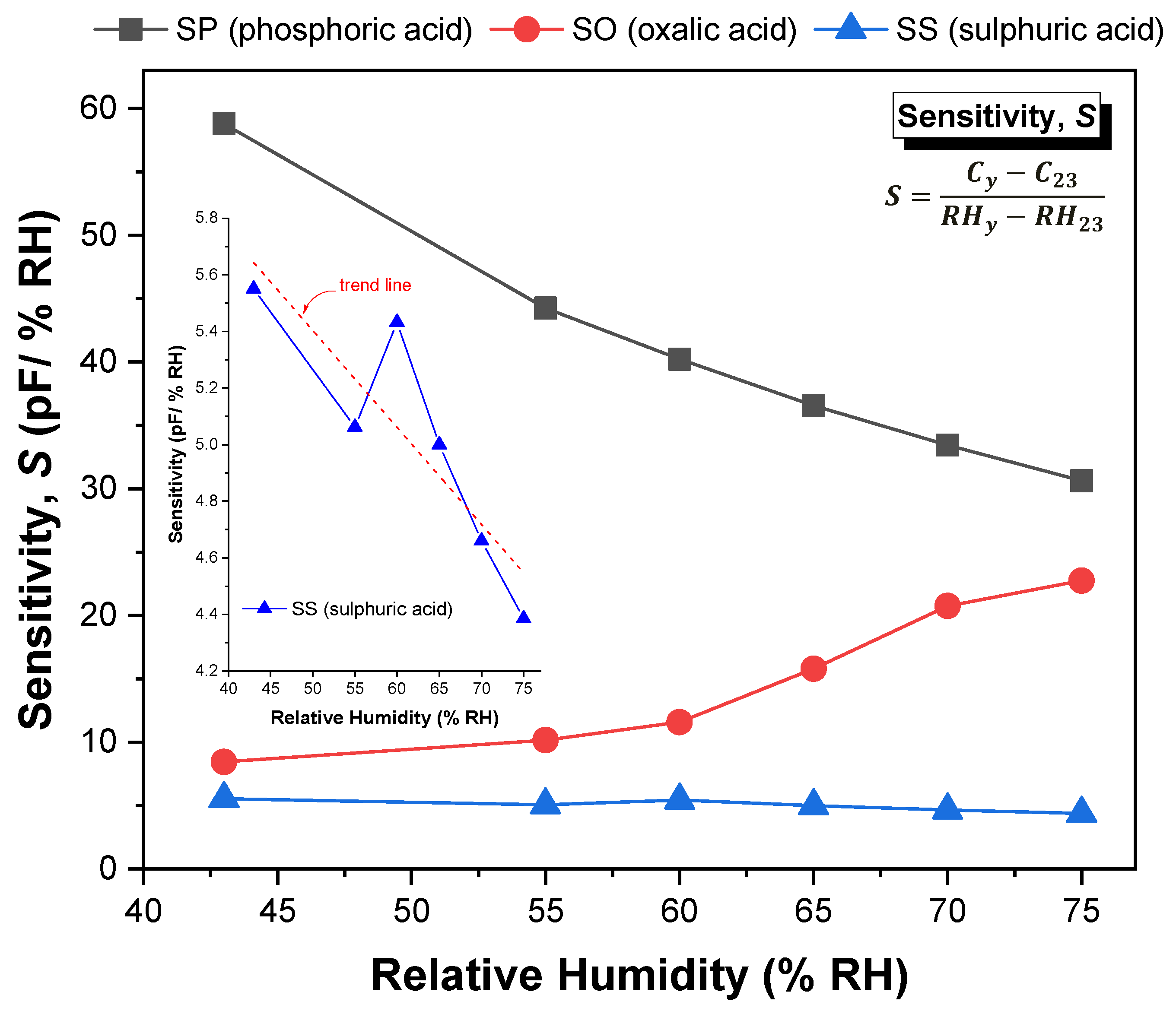
Figure 12 shows the sensitivity values of the SP, SO and SS sensors, determined by Equation (9) in the range of 23–75% RH.
It is understandable that the sensitivity values reflect the behaviour of the capacitive response with the increment in humidity level, as explained in the previous paragraphs. It is observed that for the SP and SS sensors, the sensitivity decreases continuously as the humidity level increases (although SS sensor exhibits a linear-like decrease trend over the entire relative humidity range), while for the SO sensor the sensitivity increases with the increment in the humidity level (a more substantial increase for RH > 60%). The insert included in
Figure 12 refers to the sensitivity of the SS sample to facilitate its comparative analysis with the other samples. The average sensitivity value of the SP sensor reaches up to 39 (pF/% RH), while in the case of the SO and SS sensors, the average sensitivity is around 14.5 and 4.8 (pF/% RH), respectively. These values are of the order of magnitude of those reported in [
45], except for the value of the SP sample, which is relatively higher (about twice). The reason for this difference may be related with the structural differences or with a higher change of the dielectric constant due to water adsorption.
3.5. Bacterial Sensing Performance of NP-AAO Touch Sensors
For absorbance measurements, OD is a logarithmic measurement of the percentage transmission (%T) and it can be represented by the equation,
This means that a sample with 1 OD allows 10% of light to be transmitted through the sample.
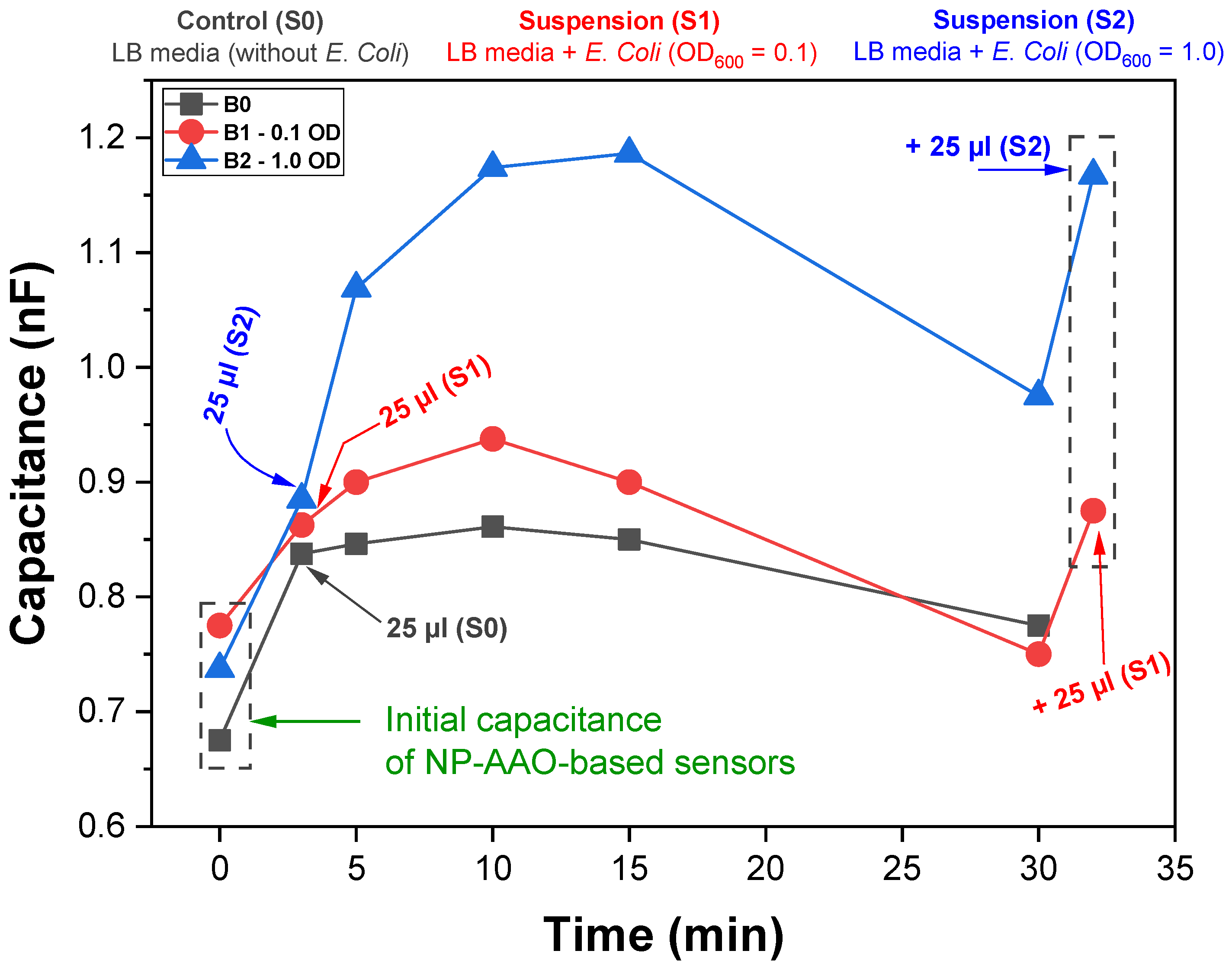
Figure 13 shows the variation of sensors capacitive response along time for samples B0 (control), B1 (
E. Coli OD
600nm = 0.1) and B2 (
E. Coli OD
600nm = 1.0).
Regardless of the presence/absence of
E. Coli, an increase of the capacitance was observed after the addition of the suspensions to the surface of the AAO sensors for all the tested conditions. However, this capacitive response was differentiated according to the amount of
E. Coli present in the bacterial suspension, with the highest capacitance values being recorded for the suspension with the highest amount of
E. Coli (S2). Although the increase of the capacitive response varied according to the amount of
E. Coli on the surface of the touch sensors, there was a slow and continuous decrease of the capacitance values after 15 min for all the samples. This phenomenon was most likely related with the evaporation of water from the suspensions. It is noteworthy that the capacitive response was recovered after addition of an equal amount of bacterial suspension to sensors B1 and B2, pointing out the influence of the presence of water on the sensors’ capacitive response (water has a high dielectric constant). For touch sensor applications, it is common to define the touch sensor sensitivity
as the ratio of the percentage change in capacitance and the area
A of the gold top electrode (i.e., the gold circular area of the flat surface without nanopores) [
23]. Thus, using Equation (10) and dividing it by the area
A of the top electrode, the sensitivity of the touch sensor is given by the expression:
where the percentage change in capacitance is expressed as
Here,
is the capacitance of the touch sensor at a particular time instant and
is its initial capacitance, i.e., the touch sensor capacitance measured before adding the bacterial suspension. The measurement results presented in
Figure 13 show that the B2 touch sensor has a capacitance change of 42.8% (on average), while the B1 touch sensor presents a capacitance change of about 16.1%. This demonstrates that the B2 touch sensor with the addition of the S2 bacterial suspension, is more sensitive
than the B1 sensor
. These values are aligned with those reported by Hong et al. [
23], who obtained a touch sensor with a sensitivity of about
.
The increase in capacitance after the addition of suspensions to the NP-AAO sensors’ surface can be attributed to a change on the effective surface area of the nanotextured top electrode. Actually, considering that bacteria can play the role of an electrically conductive object, it is expected that as they come into contact with the top nanotextured surface of the gold electrode, (air-containing) alumina nanopores will be covered and this causes a change in capacitance. The area change will be
and therefore the change in capacitance can be determined as:
However, bacteria (objects) are added to the gold nanotextured surface through a culture medium that essentially consists of a suspension of water containing the nutrients and salts necessary to keep the bacteria alive. In this sense, the increase in capacitance recorded in sensors B1 and B2 is probably associated with the liquid infiltrated into the nanoporous channels of the NP-AAO sensors structure, which should partially replace the initial air. As the exposure time increases, the
E. Coli bacteria deposit on the nanotextured Au surface and cover some nanopores containing the culture medium. The presence of bacteria on the surface of the gold top electrode, blocking the pores, will contribute to the appearance of an increased number of new capacitors. Therefore, each individual pore should act as two series-connected capacitors, where the total change in effective capacitance has the contribution of the LB culture medium and the air, as shown schematically in
Figure 14.
The sensor circuit has three components: the NP-AAO dry pore wall capacitance
and two additional capacitors (series-connected) causing the change in capacitance due to the presence of two different physical media that are distinguished by different dielectric constants, namely, the dielectric constant of the culture medium
and the air
. The effective capacitance
of the NP-AAO touch sensor can be expressed as:
Here, where and . These new capacitors will increase the capacitance performance of the AAO sensors. The differences observed on the capacitive response for sensor B2 and sensor B1 are thus related with the number of E. Coli capacitors on the surface of the sensor. The increase in capacitive response of sensor B2 is greater than that recorded for sensor B1, since the number of bacteria present in bacterial suspension 2 (OD600nm = 1) is much higher than that in bacterial suspension 1 (OD600nm = 0.1).
In order to justify this statement and to get an idea about the difference in the number of bacteria present on the surface of each sensor, it is possible to perform a simple exercise where the following conditions are assumed:
- (a)
For each sensor, every active pore is assumed to be blocked by bacteria and fully filled with LB culture medium (i.e., the pores no longer contain air inside);
- (b)
For each sensor, the time instant in which the effective maximum capacitance value is registered, that is, 0.938 nF and 1.18 nF for sensors B1 and B2, respectively, is considered.
Under these conditions, the change in capacitance is only due to the infiltration effect of the culture medium into the pores. Therefore, , where is total area of the pores covered by bacteria and fully filled with LB culture medium. In this sense, for each sensor, it is possible to estimate the percentage fraction of the electrode area that is responsible for the increase in the sensor’s capacitance, which is about 4.4% and 30.2% for sensors B1 and B2, respectively, clearly demonstrating that the number of bacteria on the surface of sensor B2 is much higher than on sensor B1.
Figure 15 is the SEM micrograph of the B1 touch sensor covered by the
Escherichia Coli population for the exposure time (10 min) at which the maximum capacitance value was recorded.
The SEM micrograph clearly shows that there are large areas on the surface of the B1 touch sensor that are not covered by the bacterial population, which obviously limits the capacitance value measured for this sensor. The inset included in
Figure 15 is the cross-sectional view of the SEM micrograph in which the coverage of a certain number of pores by the bacterial population is clearly observed.