Clairvoyant Melon Maturity Detection Enabled by Doctor-Blade-Coated Photonic Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solvents
2.2. Colloidal Self-Assembly by Doctor-Blade-Coating
2.3. Preparation of Macroporous Poly(HEMA)/Poly(ETPTA) Photonic Crystals
2.4. Experimental Procedures for Muskmelon Maturity Sensing
2.5. Determination of Brix Values in Muskmelons
2.6. Characterization
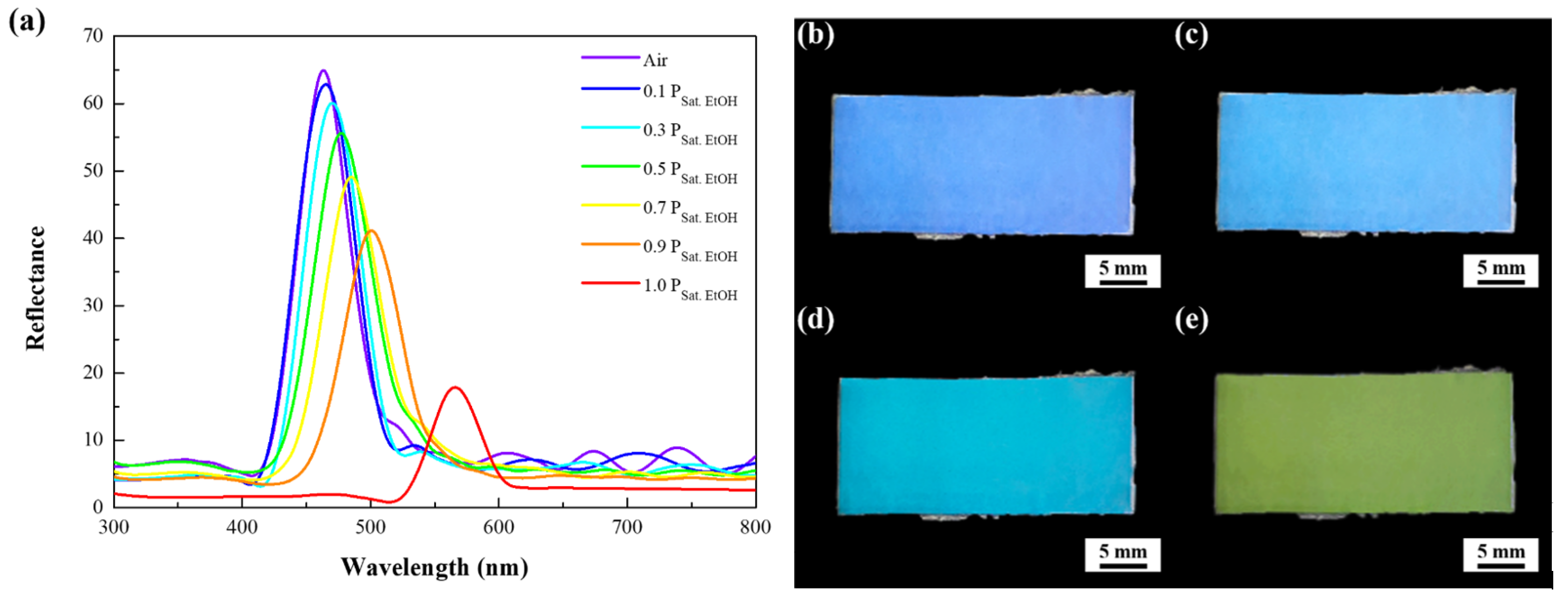
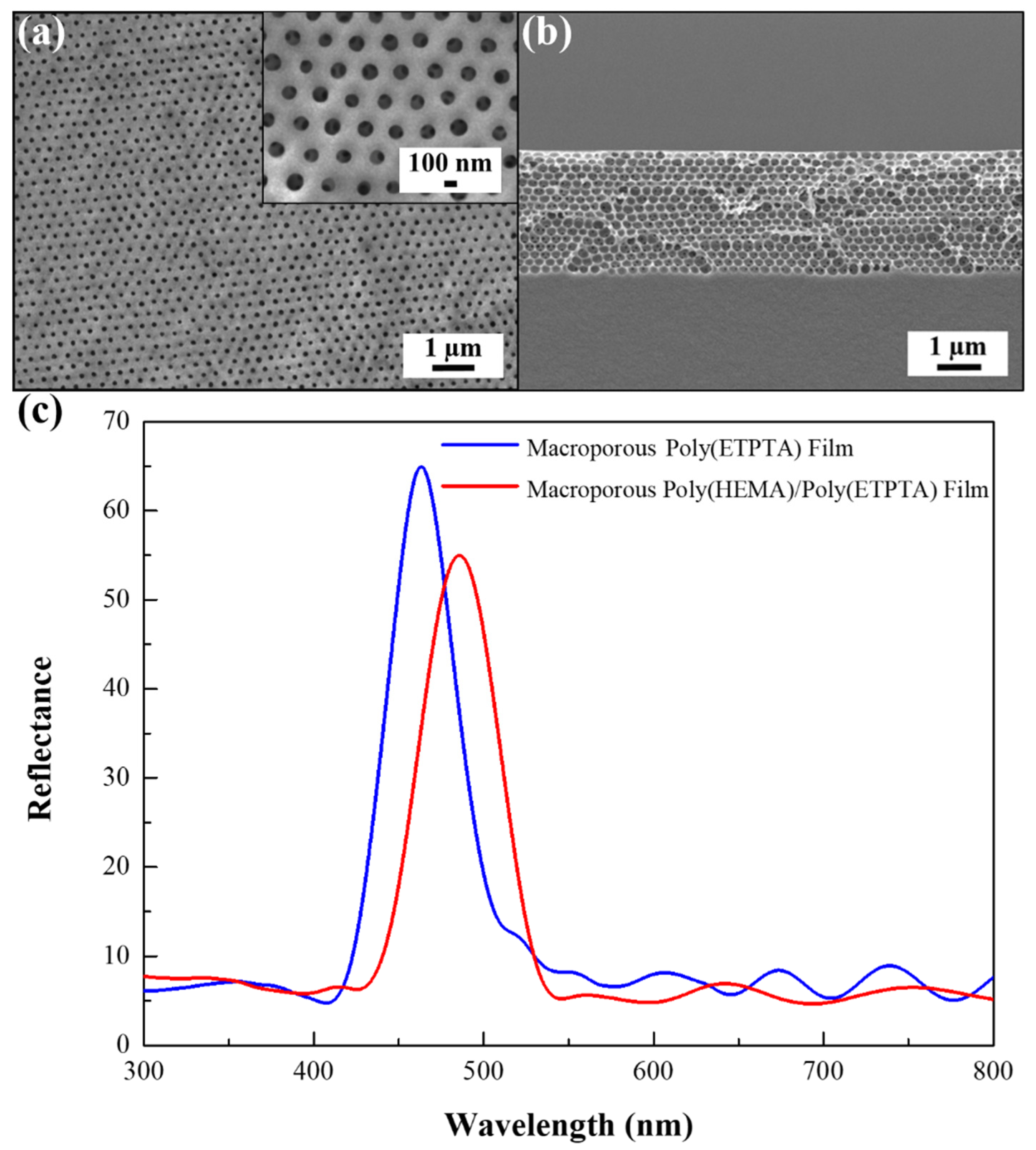
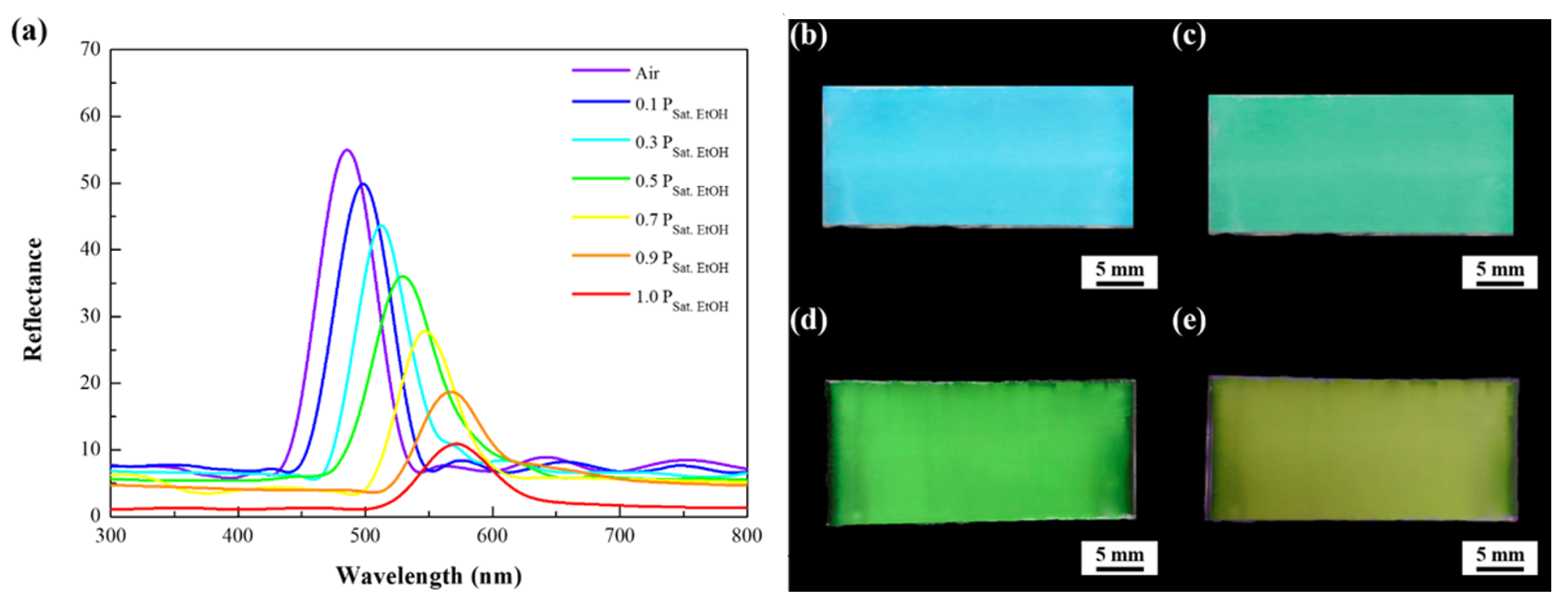
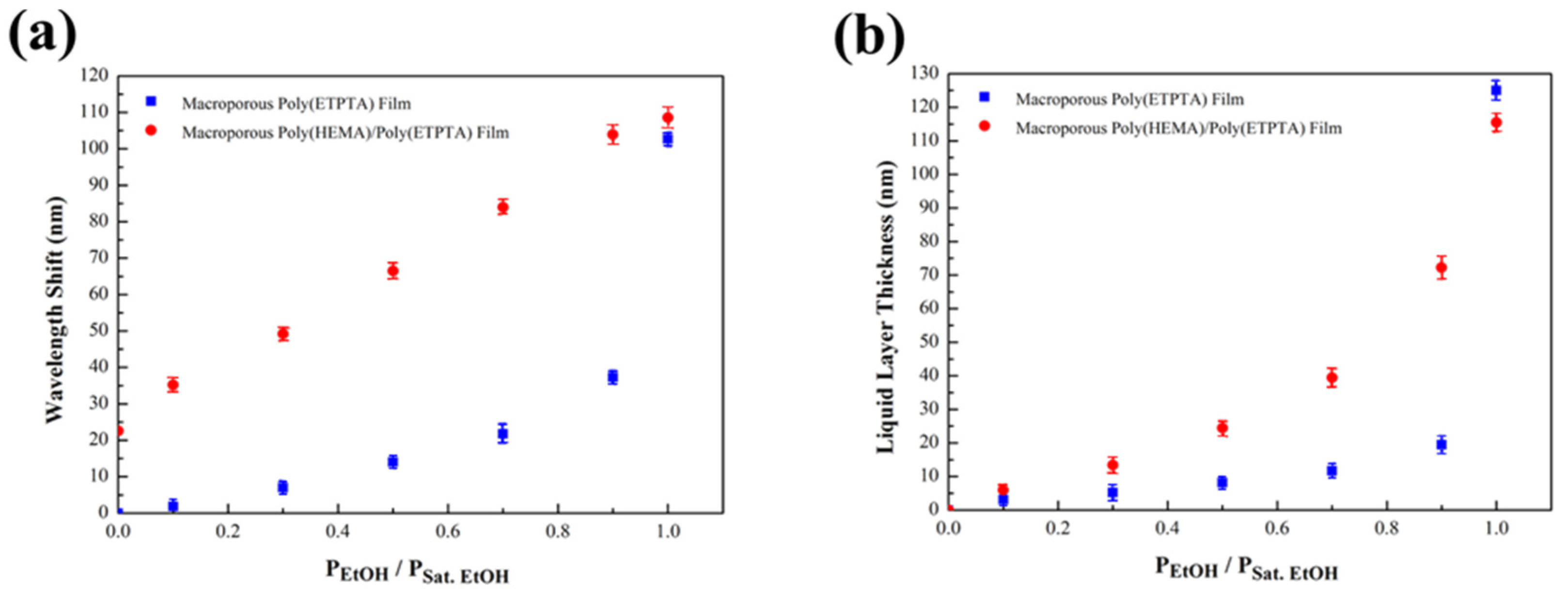
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.Y.; Yermembetova, A.; Washer, B.M.; Jiang, X.; Shuvo, S.N.; Peroulis, D.; Wei, A.; Stanciu, L.A. Selective Detection of Ethylene by MoS2–Carbon Nanotube Networks Coated with Cu (I)–Pincer Complexes. ACS Sens. 2020, 5, 1699–1706. [Google Scholar] [CrossRef]
- Valente, J.; Almeida, R.; Kooistra, L. A Comprehensive Study of the Potential Application of Flying Ethylene-Sensitive Sensors for Ripeness Detection in Apple Orchards. Sensors 2019, 19, 372. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-G.; Jeong, J.; Choi, Y.; Park, J.; Park, E.; Cheon, C.-H.; Kim, N.-K.; Min, B.K.; Kim, W. Synthesis of V-doped In2O3 Nanocrystals via Digestive-Ripening Process and Their Electrocatalytic Properties in CO2 Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 11890–11897. [Google Scholar] [CrossRef]
- Zhou, J.; Yungbluth, D.; Vong, C.N.; Scaboo, A.; Zhou, J. Estimation of the Maturity Date of Soybean Breeding Lines Using UAV-based Multispectral Imagery. Remote Sens. 2019, 11, 2075. [Google Scholar] [CrossRef] [Green Version]
- Quirós Vargas, J.J.; Zhang, C.; Smitchger, J.A.; McGee, R.J.; Sankaran, S. Phenotyping of Plant Biomass and Performance Traits Using Remote Sensing Techniques in Pea (Pisum sativum, L.). Sensors 2019, 19, 2031. [Google Scholar] [CrossRef] [Green Version]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Jaunet, T.; Chi-Ham, C.; Cantu, D.; Bradford, K.J.; Van Deynze, A. Texture Diversity in Melon (Cucumis melo L.): Sensory and Physical Assessments. Postharvest Biol. Technol. 2020, 159, 111024. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Ruggeri, E.; Kim, D.; Cao, Y.; Fare, S.; De Nardo, L.; Marelli, B. A Multilayered Edible Coating to Extend Produce Shelf Life. ACS Sustain. Chem. Eng. 2020, 8, 14312–14321. [Google Scholar] [CrossRef]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the Shelf Life of White Peach Fruit with 1-Methylcyclopropene and Aloe Arborescens Edible Coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Yu, X.; Hu, S.; He, C.; Zhou, J.; Qu, F.; Ai, Z.; Chen, Y.; Ni, D. Chlorophyll Metabolism in Postharvest Tea (Camellia sinensis L.) Leaves: Variations in Color Values, Chlorophyll Derivatives, and Gene Expression Levels under Different Withering Treatments. J. Agric. Food Chem. 2019, 67, 10624–10636. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Oveissi, F.; Chandrawati, R.; Dehghani, F.; Naficy, S. Naked-eye Detection of Ethylene Using Thiol-functionalized Polydiacetylene-based Flexible Sensors. ACS Sens. 2020, 5, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; DeVault, C.; Camayd-Muñoz, S.A.; Liu, Y.; Jia, D.; Du, F.; Mello, O.; Vulis, D.I.; Li, Y.; Mazur, E. Low-Loss Zero-index Materials. Nano Lett. 2021, 21, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Fali, A.; White, S.T.; Folland, T.G.; He, M.; Aghamiri, N.A.; Liu, S.; Edgar, J.H.; Caldwell, J.D.; Haglund, R.F.; Abate, Y. Refractive Index-based Control of Hyperbolic Phonon-polariton Propagation. Nano Lett. 2019, 19, 7725–7734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danciu, M.; Alexa-Stratulat, T.; Stefanescu, C.; Dodi, G.; Tamba, B.I.; Mihai, C.T.; Stanciu, G.D.; Luca, A.; Spiridon, I.A.; Ungureanu, L.B. Terahertz Spectroscopy and Imaging: A Cutting-edge Method for Diagnosing Digestive Cancers. Materials 2019, 12, 1519. [Google Scholar] [CrossRef] [Green Version]
- Pech, J.-C.; Bouzayen, M.; Latché, A.J.P.S. Climacteric Fruit Ripening: Ethylene-dependent and Independent Regulation of Ripening Pathways in Melon Fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, M.J.; Tenorio, M.D.; Esteban, M.A.; Mendoza, M.J.F.c. Compositional Changes during Ripening of Two Cultivars of Muskmelon Fruits. Food Chem. 2004, 87, 179–185. [Google Scholar] [CrossRef]
- Shalit, M.; Katzir, N.; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M.J.J.o.A.; et al. Acetyl-CoA: Alcohol Acetyltransferase Activity and Aroma Formation in Ripening Melon Fruits. J. Agric. Food Chem. 2001, 49, 794–799. [Google Scholar] [CrossRef]
- Ritenour, M.; Mangrich, M.; Beaulieu, J.; Rab, A.; Saltveit, M.J.P.B. Ethanol Effects on the Ripening of Climacteric Fruit. Postharvest Biol. Technol. 1997, 12, 35–42. [Google Scholar] [CrossRef]
- Beaulieu, J.; Ingram, D.; Lea, J.; Bett-Garber, K.J.J.O.F.S. Effect of Harvest Maturity on the Sensory Characteristics of Fresh-cut Cantaloupe. J. Food Sci. 2004, 69, 250–258. [Google Scholar] [CrossRef]
- Flores, F.; Ben Amor, M.; Jones, B.; Pech, J.; Bouzayen, M.; Latché, A.; Romojaro, F.J.P.P. The Use of Ethylene-suppressed Lines to Assess Differential Sensitivity to Ethylene of the Various Ripening Pathways in Cantaloupe Melons. Physiol Plant 2001, 113, 128–133. [Google Scholar] [CrossRef]
- King, E.S.; Chapman, D.M.; Luo, K.; Ferris, S.; Huang, G.; Mitchell, A.E. Defining the Sensory Profiles of Raw Almond (Prunus dulcis) Varieties and the Contribution of Key Chemical Compounds and Physical Properties. J. Agric. Food Chem. 2019, 67, 3229–3241. [Google Scholar] [CrossRef] [Green Version]
- Serrano, M.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martinez-Romero, D.; Valverde, J.M.; Valero, D. Maturity Stage at Harvest Determines the Fruit Quality and Antioxidant Potential After Storage of Sweet Cherry Cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef] [PubMed]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D.; Boldingh, H. Fruit Maturation and the Soluble Solids Harvest Index for ‘Hayward’Kiwifruit. Sci. Hortic. 2016, 213, 193–198. [Google Scholar] [CrossRef]
- Han, J.; Su, H.; Song, F.; Gu, J.; Zhang, D.; Jiang, L. Novel Photonic Crystals: Incorporation of Nano-CdS into the Natural Photonic Crystals within Peacock Feathers. Langmuir 2009, 25, 3207–3211. [Google Scholar] [CrossRef]
- Xu, D.; Yu, H.; Xu, Q.; Xu, G.; Wang, K. Thermoresponsive Photonic Crystal: Synergistic Effect of Poly (N-isopropylacrylamide)-co-acrylic Acid and Morpho Butterfly Wing. ACS Appl. Mater. Interfaces 2015, 7, 8750–8756. [Google Scholar] [CrossRef]
- Kim, G.H.; An, T.; Lim, G. Bioinspired Structural Colors Fabricated with ZnO Quasi-ordered Nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 19057–19062. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, S.; Slocik, J.M.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Bio-optics and Bio-inspired Optical Materials. Chem. Rev. 2017, 117, 12705–12763. [Google Scholar] [CrossRef]
- Busch, K.; Von Freymann, G.; Linden, S.; Mingaleev, S.; Tkeshelashvili, L.; Wegener, M. Periodic Nanostructures for Photonics. Phys. Rep. 2007, 444, 101–202. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Li, Y.; Wang, C.T.; Jau, H.C.; Chen, C.W.; Li, C.C.; Bisoyi, H.K.; Bunning, T.J.; Li, Q. Red, Green and Blue Reflections Enabled in an Optically Tunable Self-organized 3D Cubic Nanostructured Thin Film. Adv. Mater. 2013, 25, 5050–5054. [Google Scholar] [CrossRef]
- Choi, S.Y.; Mamak, M.; Von Freymann, G.; Chopra, N.; Ozin, G.A. Mesoporous Bragg Stack Color Tunable Sensors. Nano Lett. 2006, 6, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Guo, Y.; Ye, J.Y.; Divin, C.; Huang, B.; Thomas, T.P.; Baker, J.; James, R.; Norris, T.B. Real-time Biomolecular Binding Detection Using a Sensitive Photonic Crystal Biosensor. Anal. Chem. 2010, 82, 5211–5218. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Li, G.; Wu, Y.; Chen, Q.; Wu, Z.; Yu, R. Label-free Photonic Crystal-based β-lactamase Biosensor for β-lactam Antibiotic and β-lactamase Inhibitor. Anal. Chem. 2016, 88, 9207–9212. [Google Scholar] [CrossRef]
- Moon, J.H.; Yang, S. Chemical Aspects of Three-dimensional Photonic Crystals. Chem. Rev. 2010, 110, 547–574. [Google Scholar] [CrossRef]
- Yu, S.-P.; Muniz, J.A.; Hung, C.-L.; Kimble, H. Two-dimensional Photonic Crystals for Engineering Atom–light Interactions. Proc. Natl. Acad. Sci. USA 2019, 116, 12743–12751. [Google Scholar] [CrossRef] [Green Version]
- Yethiraj, A.; van Blaaderen, A. A Colloidal Model System with an Interaction Tunable from Hard Sphere to Soft and Dipolar. Nature 2003, 421, 513–517. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.; Hwang, K.S.; Colvin, V. Single-crystal Colloidal Multilayers of Controlled Thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Brozell, A.M.; Muha, M.A.; Parikh, A.N. Formation of Spatially Patterned Colloidal Photonic Crystals through the Control of Capillary Forces and Template Recognition. Langmuir 2005, 21, 11588–11591. [Google Scholar] [CrossRef]
- Mathur, A.; Brown, A.-D.; Erlebacher, J. Self-ordering of Colloidal Particles in Shallow Nanoscale Surface Corrugations. Langmuir 2006, 22, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Josephson, D.P.; Stein, A. Colloidal Assembly: The Road from Particles to Colloidal Molecules and Crystals. Angew. Chem. Int. Ed. 2011, 50, 360–388. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Pope, C.G. X-ray Diffraction and the Bragg Equation. J. Chem. Educ. 1997, 74, 129. [Google Scholar] [CrossRef]
- Mansoori, G.A. A Perturbation Correction of the Flory-Huggins Polymer Solution Theory, Condens. Matter Phys. 2005, 8, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Aubert, C.; Bourger, N.J.J.O.A. Investigation of Volatiles in Charentais Cantaloupe Melons (Cucumis melo Var. cantalupensis). Characterization of Aroma Constituents in Some Cultivars. J. Agric. Food Chem. 2004, 52, 4522–4528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.F.; Li, Z.L.J.F.c. A Comparison of Sugar-accumulating Patterns and Relative Compositions in Developing Fruits of Two Oriental Melon Varieties as Determined by HPLC. Food Chem. 2005, 90, 785–790. [Google Scholar] [CrossRef]
- Lignou, S.; Parker, J.K.; Baxter, C.; Mottram, D.S.J.F.c. Sensory and Instrumental Analysis of Medium and Long Shelf-life Charentais Cantaloupe Melons (Cucumis melo, L.) Harvested at Different Maturities. Food Chem. 2014, 148, 218–229. [Google Scholar] [CrossRef] [Green Version]



| Detection Method | Instruments | Sensitivity | Reference |
|---|---|---|---|
| Regulation of Ethylene | MCP Treatments | 2.5–5 (μL/L) | [16] |
| Brix Analysis | Refractometer | 0–15.5 (°Brix) | [45] |
| Aroma Analysis | GC-MS/GC-FID | 0–89/0–97 (%) | [45] |
| Brix Analysis | Refractometer | 0–14.6 (°Brix) | [17] |
| Sugar Contents Analysis | HPLC | 0–20 (mg/g) | [46] |
| Volatile Compounds Analysis | GC-MS | 0–88.8 (%) | [18] |
| Volatile Compounds Analysis | GC–MS/GC-O-MS | 0–93/0–77 (%) | [47] |
| Firmness Analysis | Firmness Tester | 0–70 (N) | [19] |
| Texture Analysis | Texture Analyzer | 0–16 (N) | [6] |
| Texture Evaluation | Texture Analyzer | 0–1.0 (N) | [20] |
| Rind Color Analysis | Reflectance Meter | 0.1–5.0 | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.-C.; Pan, L.-C.; Lei, Y.-W.; Lin, K.-Y.A.; Yang, H. Clairvoyant Melon Maturity Detection Enabled by Doctor-Blade-Coated Photonic Crystals. Sensors 2021, 21, 7046. https://doi.org/10.3390/s21217046
Lu Y-C, Pan L-C, Lei Y-W, Lin K-YA, Yang H. Clairvoyant Melon Maturity Detection Enabled by Doctor-Blade-Coated Photonic Crystals. Sensors. 2021; 21(21):7046. https://doi.org/10.3390/s21217046
Chicago/Turabian StyleLu, Yi-Cheng, Liang-Cheng Pan, Yao-Wei Lei, Kun-Yi Andrew Lin, and Hongta Yang. 2021. "Clairvoyant Melon Maturity Detection Enabled by Doctor-Blade-Coated Photonic Crystals" Sensors 21, no. 21: 7046. https://doi.org/10.3390/s21217046
APA StyleLu, Y.-C., Pan, L.-C., Lei, Y.-W., Lin, K.-Y. A., & Yang, H. (2021). Clairvoyant Melon Maturity Detection Enabled by Doctor-Blade-Coated Photonic Crystals. Sensors, 21(21), 7046. https://doi.org/10.3390/s21217046




_Lin.png)

