Prediction of In Vivo Laser-Induced Thermal Damage with Hyperspectral Imaging Using Deep Learning
Abstract
1. Introduction
2. Materials and Methods
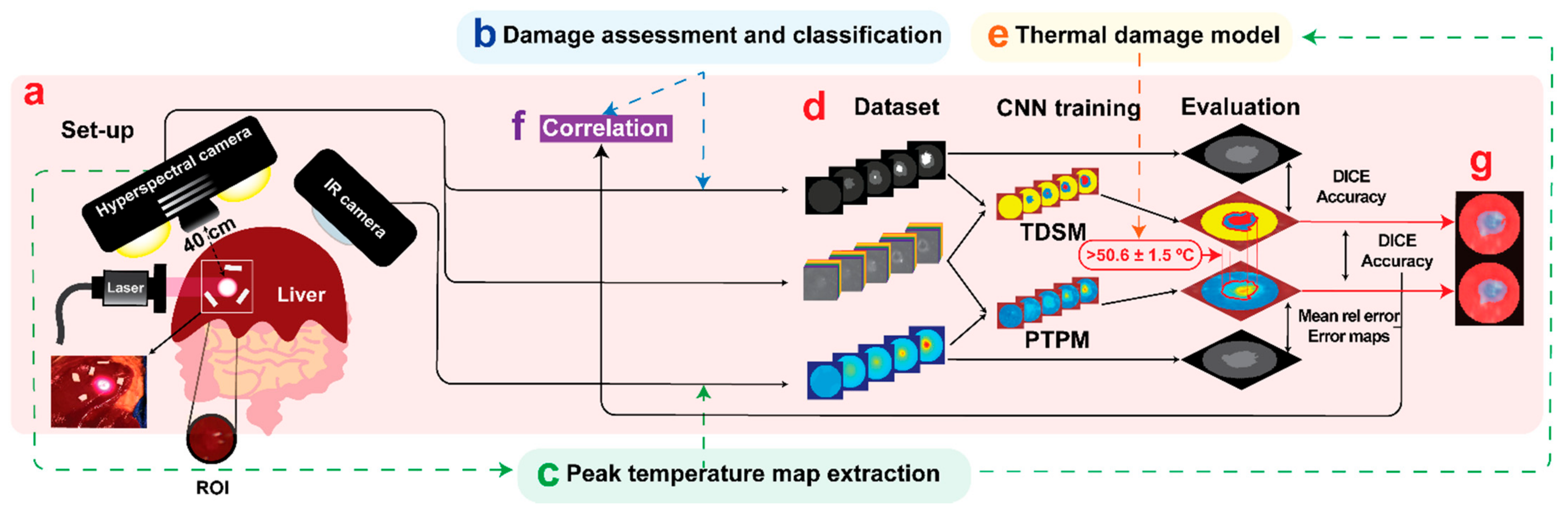
2.1. Study Design
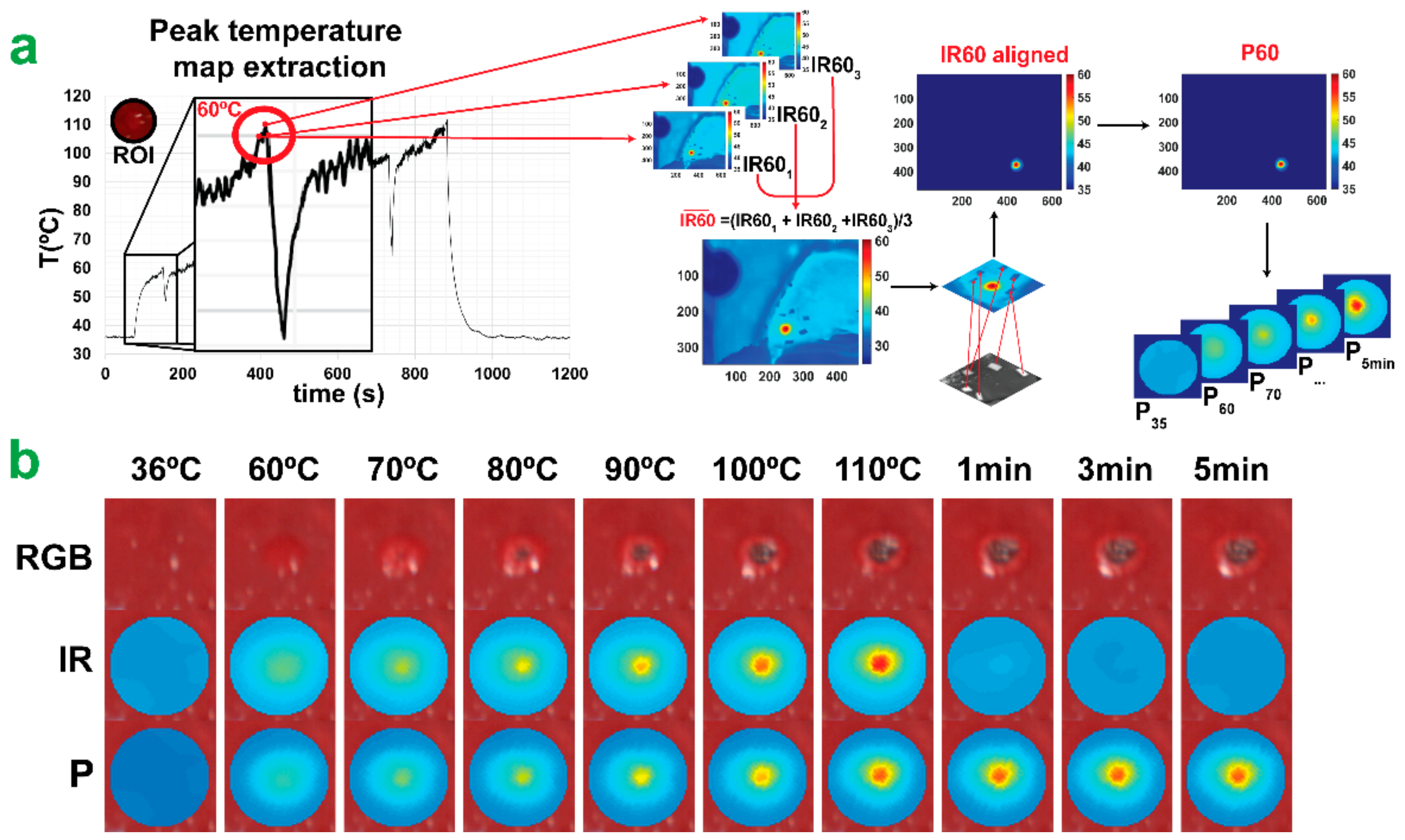
2.2. Experimental Strategy
2.3. Hyperspectral Imaging
2.4. Convolutional Neural Networks
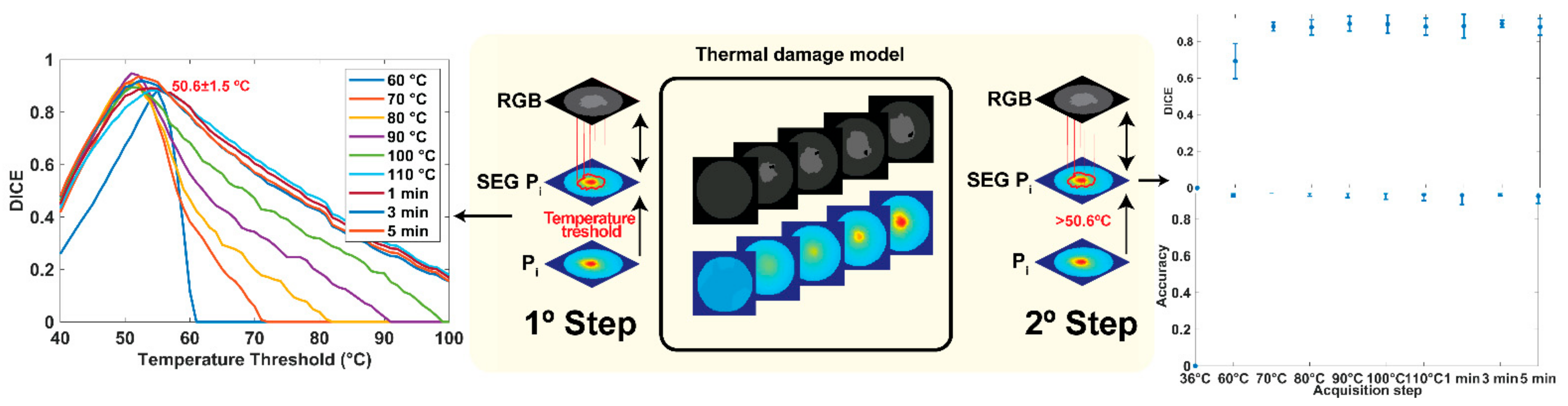
2.4.1. Tissue Damage Segmentation Model (TDSM)
2.4.2. Peak Temperature Prediction Model (PTPM)
2.4.3. CNN Architectures
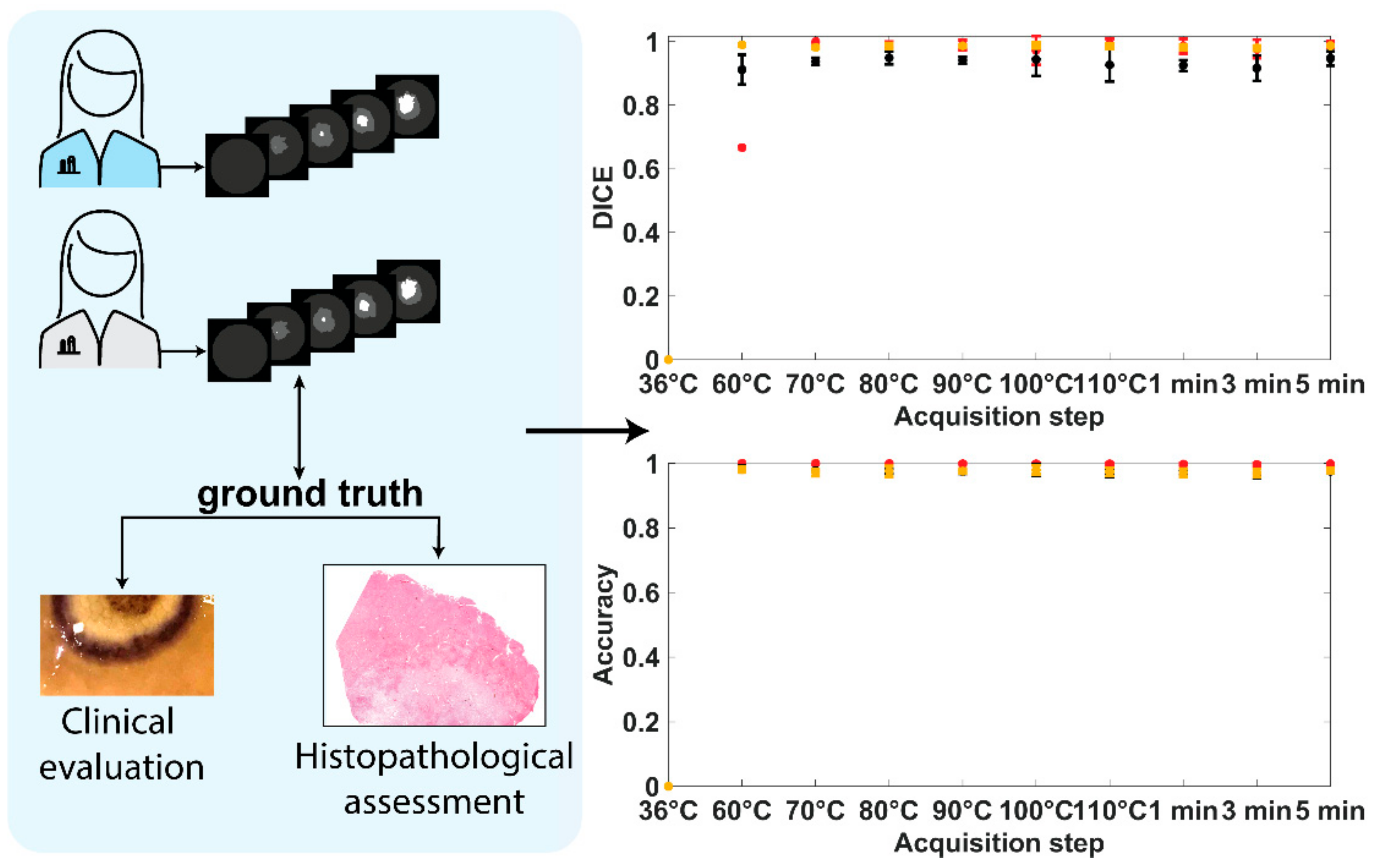
2.5. Histology
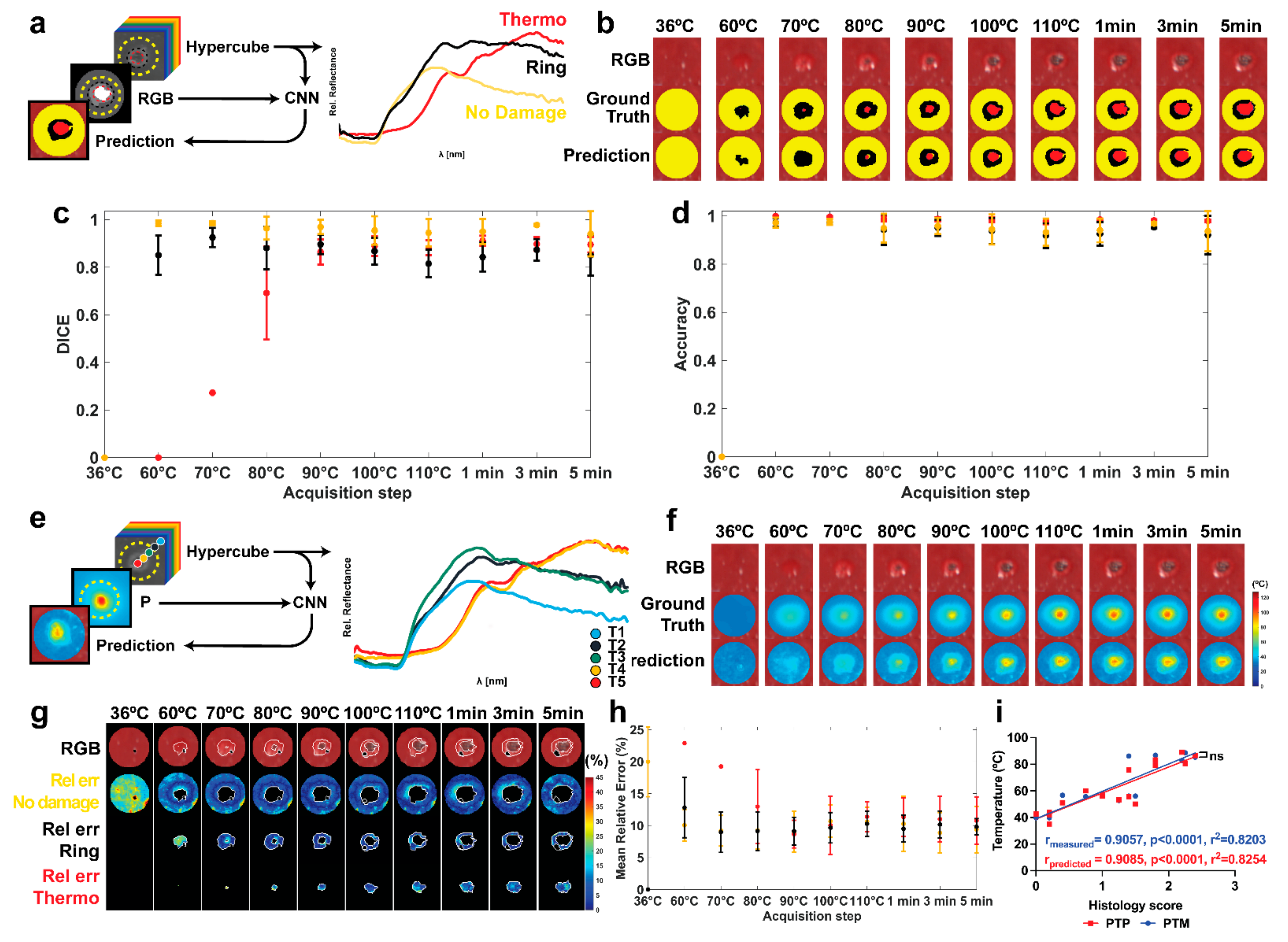
3. Results
3.1. Histological Damage Classification
3.2. Damage Prediction and Margins Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Mullins, F.; Minton, J.P.; Hoye, R.C.; Dearman, J.R.; McKnight, W.B. The effect of high energy laser pulses on the primate liver. Surg. Gynecol. Obstet. 1966, 122, 727–732. [Google Scholar] [PubMed]
- Giorgio, A.; Tarantino, L.; de Stefano, G.; Farella, N.; Catalano, O.; Cusati, B.; Del Viscovo, L.; Alaia, A.; Caturelli, E. Interstitial laser photocoagulation under ultrasound guidance of liver tumors: Results in 104 treated patients. Eur. J. Ultrasound 2000, 11, 181–188. [Google Scholar] [CrossRef]
- Christophi, C.; Muralidharan, V. Treatment of hepatocellular carcinoma by percutaneous laser hyperthermia. J. Gastroenterol. Hepatol. 2001, 16, 548–552. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Tortora, R.; D’Adamo, G.; De Luca, M.; Lampasi, F.; Addario, L.; Galeota Lanza, A.; Picciotto, F.P.; Tartaglione, M.T.; Cordone, G.; et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: A randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 559–565. [Google Scholar] [CrossRef]
- Asadi, S.; Bianchi, L.; De Landro, M.; Korganbayev, S.; Schena, E.; Saccomandi, P. Laser-induced optothermal response of gold nanoparticles: From a physical viewpoint to cancer treatment application. J. Biophotonics 2021, 14, e202000161. [Google Scholar] [CrossRef]
- Bull, J.M. A review of immune therapy in cancer and a question: Can thermal therapy increase tumor response? Int. J. Hyperth. 2018, 34, 840–852. [Google Scholar] [CrossRef]
- Paiella, S.; Casetti, L.; Ewald, J.; Marchese, U.; D’Onofrio, M.; Garnier, J.; Landoni, L.; Gilabert, M.; Manzini, G.; Esposito, A.; et al. Laser Treatment of Pancreatic Cancer with Immunostimulating Interstitial Laser Thermotherapy Protocol: Safety and Feasibility Results From Two Phase 2a Studies. J. Surg Res. 2021, 259, 1–7. [Google Scholar] [CrossRef]
- Salem, U.; Kumar, V.A.; Madewell, J.E.; Schomer, D.F.; de Almeida Bastos, D.C.; Zinn, P.O.; Weinberg, J.S.; Rao, G.; Prabhu, S.S.; Colen, R.R. Neurosurgical applications of MRI guided laser interstitial thermal therapy (LITT). Cancer Imaging 2019, 19, 65. [Google Scholar] [CrossRef]
- Kautzner, J.; Albenque, J.P.; Natale, A.; Maddox, W.; Cuoco, F.; Neuzil, P.; Poty, H.; Getman, M.K.; Liu, S.; Starek, Z.; et al. A Novel Temperature-Controlled Radiofrequency Catheter Ablation System Used to Treat Patients With Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 352–363. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Silvestri, S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int J. Hyperth. 2013, 29, 609–619. [Google Scholar] [CrossRef]
- Bianchi, L.; Korganbayev, S.; Orrico, A.; De Landro, M.; Saccomandi, P. Quasi-distributed fiber optic sensor-based control system for interstitial laser ablation of tissue: Theoretical and experimental investigations. Biomed. Opt. Express 2021, 12, 2841–2858. [Google Scholar] [CrossRef]
- De Vita, E.; De Landro, M.; Massaroni, C.; Iadicicco, A.; Saccomandi, P.; Schena, E.; Campopiano, S. Fiber Optic Sensors-Based Thermal Analysis of Perfusion-Mediated Tissue Cooling in Liver Undergoing Laser Ablation. IEEE Trans. Biomed. Eng. 2021, 68, 1066–1073. [Google Scholar] [CrossRef]
- Pearce, J. Mathematical models of laser-induced tissue thermal damage. Int J. Hyperth. 2011, 27, 741–750. [Google Scholar] [CrossRef]
- McNichols, R.J.; Gowda, A.; Kangasniemi, M.; Bankson, J.A.; Price, R.E.; Hazle, J.D. MR thermometry-based feedback control of laser interstitial thermal therapy at 980 nm. Lasers Surg. Med. 2004, 34, 48–55. [Google Scholar] [CrossRef]
- Patel, N.V.; Frenchu, K.; Danish, S.F. Does the Thermal Damage Estimate Correlate With the Magnetic Resonance Imaging Predicted Ablation Size After Laser Interstitial Thermal Therapy? Oper. Neurosurg. 2018, 15, 179–183. [Google Scholar] [CrossRef]
- Feng, Y.; Fuentes, D. Model-based planning and real-time predictive control for laser-induced thermal therapy. Int. J. Hyperth. 2011, 27, 751–761. [Google Scholar] [CrossRef]
- Rieke, V.; Butts Pauly, K. MR thermometry. J. Magn Reson Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef]
- Munier, S.M.; Desai, A.N.; Patel, N.V.; Danish, S.F. Effects of Intraoperative Magnetic Resonance Thermal Imaging Signal Artifact During Laser Interstitial Thermal Therapy on Thermal Damage Estimate and Postoperative Magnetic Resonance Imaging Ablative Area Concordance. Oper. Neurosurg. 2020, 18, 524–530. [Google Scholar] [CrossRef]
- Ritz, J.P.; Roggan, A.; Isbert, C.; Muller, G.; Buhr, H.J.; Germer, C.T. Optical properties of native and coagulated porcine liver tissue between 400 and 2400 nm. Lasers Surg. Med. 2001, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.M.; Sturesson, C.; Liu, D.L.; Andersson-Engels, S. Changes in spectral shape of tissue optical properties in conjunction with laser-induced thermotherapy. Appl. Opt. 1998, 37, 1256–1267. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Ward, J.M.; Yu, B. Association of Liver Tissue Optical Properties and Thermal Damage. Lasers Surg. Med. 2020, 52, 779–787. [Google Scholar] [CrossRef]
- Tanis, E.; Spliethoff, J.W.; Evers, D.J.; Langhout, G.C.; Snaebjornsson, P.; Prevoo, W.; Hendriks, B.H.; Ruers, T.J. Real-time in vivo assessment of radiofrequency ablation of human colorectal liver metastases using diffuse reflectance spectroscopy. Eur. J. Surg. Oncol. 2016, 42, 251–259. [Google Scholar] [CrossRef]
- Buttemere, C.R.; Chari, R.S.; Anderson, C.D.; Washington, M.K.; Mahadevan-Jansen, A.; Lin, W.C. In vivo assessment of thermal damage in the liver using optical spectroscopy. J. Biomed. Opt. 2004, 9, 1018–1027. [Google Scholar] [CrossRef][Green Version]
- Gil, D.A.; Swift, L.M.; Asfour, H.; Muselimyan, N.; Mercader, M.A.; Sarvazyan, N.A. Autofluorescence hyperspectral imaging of radiofrequency ablation lesions in porcine cardiac tissue. J. Biophotonics 2017, 10, 1008–1017. [Google Scholar] [CrossRef]
- Hsu, C.P.; Razavi, M.K.; So, S.K.; Parachikov, I.H.; Benaron, D.A. Liver tumor gross margin identification and ablation monitoring during liver radiofrequency treatment. J. Vasc. Interv. Radiol. 2005, 16, 1473–1478. [Google Scholar] [CrossRef][Green Version]
- Felli, E.; Al-Taher, M.; Collins, T.; Baiocchini, A.; Felli, E.; Barberio, M.; Ettorre, G.M.; Mutter, D.; Lindner, V.; Hostettler, A.; et al. Hyperspectral evaluation of hepatic oxygenation in a model of total vs. arterial liver ischaemia. Sci. Rep. 2020, 10, 15441. [Google Scholar] [CrossRef]
- Urade, T.; Felli, E.; Barberio, M.; Al-Taher, M.; Felli, E.; Goffin, L.; Agnus, V.; Ettorre, G.M.; Marescaux, J.; Mutter, D.; et al. Hyperspectral enhanced reality (HYPER) for anatomical; liver resection. Surg. Endosc. 2020, 7, 464. [Google Scholar] [CrossRef]
- De Landro, M.; Espiritu, G.-M.I.; Barberio, M.; Felli, E.; Agnus, V.; Pizzicannella, M.; Diana, M.; Zappa, E.; Saccomandi, P. Hyperspectral Imagery for Assessing Laser-Induced Thermal State Change in Liver. Sensors 2021, 21, 643. [Google Scholar] [CrossRef]
- Abdlaty, R.; Abbass, M.A.; Awadallah, A.M. High Precision Monitoring of Radiofrequency Ablation for Liver Using Hyperspectral Imaging. Ann. Biomed. Eng. 2021, 15, 1–11. [Google Scholar]
- Aref, M.H.; Aboughaleb, I.H.; Youssef, A.M.; El-Sharkawy, Y.H. Hyperspectral image-based analysis of thermal damage for ex-vivo bovine liver utilizing radiofrequency ablation. Surg. Oncol. 2021, 38, 101564. [Google Scholar] [CrossRef] [PubMed]
- Felli, E.; Al-Taher, M.; Collins, T.; Nkusi, R.; Felli, E.; Baiocchini, A.; Lindner, V.; Vincent, C.; Barberio, M.; Geny, B.; et al. Automatic Liver Viability Scoring with Deep Learning and Hyperspectral Imaging. Diagnostics 2021, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Barberio, M.; Collins, T.; Bencteux, V.; Nkusi, R.; Felli, E.; Viola, M.G.; Marescaux, J.; Hostettler, A.; Diana, M. Deep Learning Analysis of In Vivo Hyperspectral Images for Automated Intraoperative Nerve Detection. Diagnostics 2021, 11, 1508. [Google Scholar] [CrossRef]
- Guan, S.; Asfour, H.; Sarvazyan, N.; Loew, M. Application of unsupervised learning to hyperspectral imaging of cardiac ablation lesions. J. Med. Imaging 2018, 5, 046003. [Google Scholar] [CrossRef]
- Hardy, J.D. The radiation of heat from the human body: III. The Human Skin as a Black-Body Radiator. J. Clin. Investig. 1934, 13, 615–620. [Google Scholar] [CrossRef]
- Kulcke, A.; Holmer, A.; Wahl, P.; Siemers, F.; Wild, T.; Daeschlein, G. A compact hyperspectral camera for measurement of perfusion parameters in medicine. Biomed. Tech. 2018, 63, 519–527. [Google Scholar] [CrossRef]
- Hamida, A.B.; Benoit, A.; Lambert, P.; Amar, C.B. 3-D Deep Learning Approach for Remote Sensing Image Classification. IEEE Trans. Geosci. Remote. Sens. 2018, 56, 4420–4434. [Google Scholar] [CrossRef]
- Yu, S.; Jia, S.; Xu, C. Convolutional neural networks for hyperspectral image classification. Neurocomputing 2017, 219, 88–98. [Google Scholar] [CrossRef]
- Cetas, T.C.; Connor, W.G. Thermometry considerations in localized hyperthermia. Med. Phys. 1978, 5, 79–91. [Google Scholar] [CrossRef]
- Thomsen, S.P.J.A. Thermal Damage and Rate Processes in Biologic Tissues. In Optical-Thermal Response of Laser-Irradiated Tissue; Springer: Dordrecht, The Netherlands, 2010; pp. 487–549. [Google Scholar] [CrossRef]
- Liu, C.H.; Arellano, R.S.; Uppot, R.N.; Samir, A.E.; Gervais, D.A.; Mueller, P.R. Radiofrequency ablation of hepatic tumours: Effect of post-ablation margin on local tumour progression. Eur. Radiol. 2010, 20, 877–885. [Google Scholar] [CrossRef]
- Armstrong, K.; Larson, C.; Asfour, H.; Ransbury, T.; Sarvazyan, N. A Percutaneous Catheter for In Vivo Hyperspectral Imaging of Cardiac Tissue: Challenges, Solutions and Future Directions. Cardiovasc. Eng. Technol. 2020, 11, 560–575. [Google Scholar] [CrossRef]
- Lim, H.T.; Murukeshan, V.M. A four-dimensional snapshot hyperspectral video-endoscope for bio-imaging applications. Sci. Rep. 2016, 6, 24044. [Google Scholar] [CrossRef]
- Cha, J.; Shademan, A.; Le, H.N.; Decker, R.; Kim, P.C.; Kang, J.U.; Krieger, A. Multispectral tissue characterization for intestinal anastomosis optimization. J. Biomed. Opt. 2015, 20, 106001. [Google Scholar] [CrossRef]
- Primavesi, F.; Swierczynski, S.; Klieser, E.; Kiesslich, T.; Jager, T.; Urbas, R.; Hutter, J.; Neureiter, D.; Ofner, D.; Stattner, S. Thermographic real-time-monitoring of surgical radiofrequency and microwave ablation in a perfused porcine liver model. Oncol. Lett. 2018, 15, 2913–2920. [Google Scholar] [CrossRef]
- Brzezinski, R.Y.; Levin-Kotler, L.; Rabin, N.; Ovadia-Blechman, Z.; Zimmer, Y.; Sternfeld, A.; Finchelman, J.M.; Unis, R.; Lewis, N.; Tepper-Shaihov, O.; et al. Automated thermal imaging for the detection of fatty liver disease. Sci. Rep. 2020, 10, 15532. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]



| Layer | Kernel Shape | Number of Output Channels | Stride | Number of Trainable Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Conv1 | (3,3,3) | 20 | (1,1,1) | 560 | ||||
| ReLU | / | / | / | / | ||||
| Pool1 | (3,1,1) | 20 | (2,1,1) | 1220 | ||||
| Conv2 | (3,3,3) | 35 | (1,1,1) | 18,935 | ||||
| ReLU | / | / | / | / | ||||
| Pool2 | (3,1,1) | 35 | (2,1,1) | 3710 | ||||
| Conv3 | (3,1,1) | 35 | (1,1,1) | 3710 | ||||
| ReLU | / | / | / | / | ||||
| Pool3 | (2,1,1) | 35 | (2,1,1) | 2485 | ||||
| ReLU | / | / | / | / | ||||
| FC | TDSM | PTPM | TDSM | PTPM | TDSM | PTPM | TDSM | PTPM |
| (455,3) | (455,1) | 3 | 1 | / | / | 1368 | 456 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Landro, M.; Felli, E.; Collins, T.; Nkusi, R.; Baiocchini, A.; Barberio, M.; Orrico, A.; Pizzicannella, M.; Hostettler, A.; Diana, M.; et al. Prediction of In Vivo Laser-Induced Thermal Damage with Hyperspectral Imaging Using Deep Learning. Sensors 2021, 21, 6934. https://doi.org/10.3390/s21206934
De Landro M, Felli E, Collins T, Nkusi R, Baiocchini A, Barberio M, Orrico A, Pizzicannella M, Hostettler A, Diana M, et al. Prediction of In Vivo Laser-Induced Thermal Damage with Hyperspectral Imaging Using Deep Learning. Sensors. 2021; 21(20):6934. https://doi.org/10.3390/s21206934
Chicago/Turabian StyleDe Landro, Martina, Eric Felli, Toby Collins, Richard Nkusi, Andrea Baiocchini, Manuel Barberio, Annalisa Orrico, Margherita Pizzicannella, Alexandre Hostettler, Michele Diana, and et al. 2021. "Prediction of In Vivo Laser-Induced Thermal Damage with Hyperspectral Imaging Using Deep Learning" Sensors 21, no. 20: 6934. https://doi.org/10.3390/s21206934
APA StyleDe Landro, M., Felli, E., Collins, T., Nkusi, R., Baiocchini, A., Barberio, M., Orrico, A., Pizzicannella, M., Hostettler, A., Diana, M., & Saccomandi, P. (2021). Prediction of In Vivo Laser-Induced Thermal Damage with Hyperspectral Imaging Using Deep Learning. Sensors, 21(20), 6934. https://doi.org/10.3390/s21206934










