Single-Use Fluidic Electrochemical Paper-Based Analytical Devices Fabricated by Pen Plotting and Screen-Printing for On-Site Rapid Voltammetric Monitoring of Pb(II) and Cd(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation and Signal Evaluation
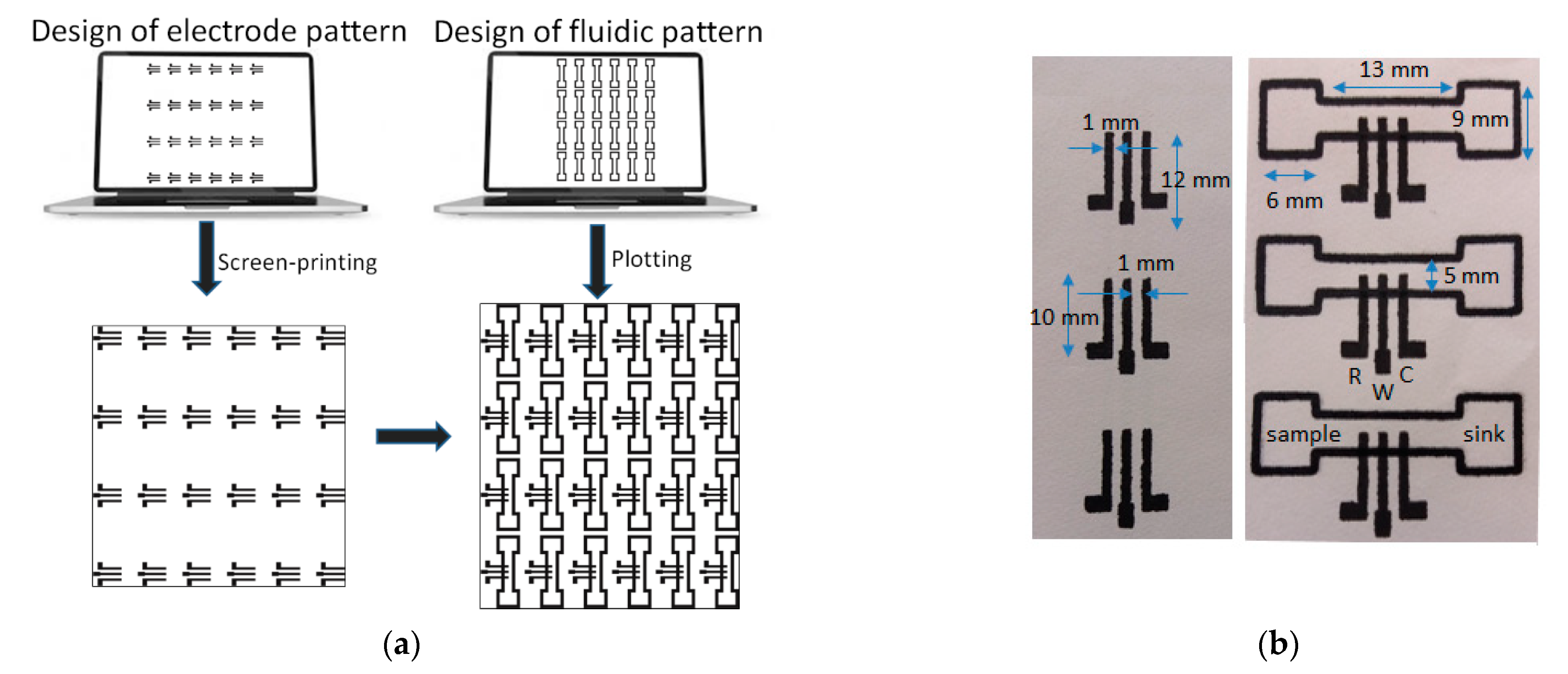
2.3. ePAD Fabrication
- Deposition of the three-electrode planar electrochemical cells. These were printed on paper sheets (Macherey-Nagel chromatography paper MN 261, thickness 180 μm) in 24 (4 × 6) arrays and consisted of graphite ink. Layers based the 423SS ink was cured after printing at 90 °C for 5 min using an infrared curing system (LittleRed-X2, VASTEX, Bethlehem, PA, USA). Layers based on the EDAG 407A ink were cured at 90 °C for 60 min in a conventional oven.
- Patterning of the PADs. The paper sheet with the arrays of the three-electrode cells was positioned onto a flat glass surface and aligned with the aid of pre-set alignment marks drawn from the sheets and the glass surface. The marker pen was inserted into the holder of the plotter and the 24 (4 × 6) PADs were drawn on the paper using a plotting speed of 0.76 cm s−1 and left at room temperature for 5 min to allow the solvent to evaporate. The pattern was repeated on the reverse side of the paper after aligning the paper. Finally, the paper was cut using scissors to obtain the individual ePADs which were carefully handled using tweezers. The nominal dimensions of the fluidic ePADs designed and fabricated in this work are shown in Figure 1b.
2.4. Experimental Procedure
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarup, J. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Ali, I.; Aboul-Enein, H.Y. Metal ions speciation in the environment: Distribution, toxicities and analyses. In Developments in Environmental Science; Sarkar, D., Datta, R., Hannigan, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 5. [Google Scholar]
- Masindi, V.; Muedi, K. Environmental contamination by heavy metals. In Heavy Metals; El-Din, H., Saleh, M., Aglan, R.F., Eds.; Intech Open: London, UK, 2018. [Google Scholar]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Fernandez Luqueno, F.; Lopez Valdez, F.; Gamero Melo, P.; Luna, S.; Aguilera Gonzalez, E.N.; Martínez, A.I.; García, M.S.; Hernandez Martínez, G.; Herrera Mendoza, R.; Alvarez, M.A. Heavy metal pollution in drinking water—A global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- World Health Organization. Evaluation of Certain Food Additives and the Contaminats Mercury, Lead, and Cadmium, Sixteenth Report of the Joint FAO/WHO Expert Committee on Food Additive; World Health Organization: Geneva, Switzerland, 1972; pp. 1–33. Available online: https://apps.who.int/iris/handle/10665/40985 (accessed on 10 October 2021).
- Mudgal, V.; Madaan, N.; Mudgal, A.; Singh, R.B.; Mishra, S. Effect of toxic metals on human health. Open Nutraceuticals J. 2014, 3, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog; Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- QA, M.; MS, K. Effect on human health due to drinking water contaminated with heavy metals. J. Pollut. Eff. Control 2016, 5, 10–11. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, B.; Meena, P.; Poonar, N. Effect of heavy metal ions in water on human health. Int. J. Sci. Eng. Res. 2016, 4, 2015–2017. [Google Scholar]
- Bhattacharjee, T.; Goswami, M. Heavy metals (As, Cd & Pb) toxicity & detection of these metals in ground water sample: A review on different techniques. Int. J. Eng. Sci. Invent. 2018, 7, 12–21. [Google Scholar]
- Bulska, E.; Ruszczyńska, A. Analytical techniques for trace element determination. Phys. Sci. Rev. 2017, 2, 20178002. [Google Scholar] [CrossRef]
- Helaluddin AB, M.; Khalid, R.S.; Alaama, M.; Abbas, S.A. Main analytical techniques used for elemental analysis in various matrices. J. Pharm. Res. 2016, 15, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Economou, A.; Kokkinos, C. Electrochemical Strategies in Detection Science; Arrigan, D.W.M., Ed.; The Royal Society of Chemistry: London, UK, 2016; Volume 6, pp. 1–18. [Google Scholar]
- Borrill, A.J.; Reily, N.E.; Macpherson, J.V. Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834–6849. [Google Scholar] [CrossRef]
- Thomas, S.; Ahmadi, M.; Nguyen, T.A.; Afkhami, A.; Madrakian, T. Micro-and Nanotechnology Enabled Applications for Portable Miniaturized Analytical Systems, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Rios, A.; Escarpa, A.; Simonet, B. Miniaturization of Analytical Systems: Principles, Designs and Applications; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Zhang, W.; Wang, R.; Luo, F.; Wang, P.; Lin, Z. Miniaturized electrochemical sensors and their point-of-care applications. Chin. Chem. Lett. 2020, 31, 589–600. [Google Scholar] [CrossRef]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized sensor technologies for personalized and preventive medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef] [PubMed]
- Tribhuwan Singh, A.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-based sensors: Emerging themes and applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [Green Version]
- Kung, C.T.; Hou, C.Y.; Wang, Y.N.; Fu, L.M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sens. Actuat. B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Fu, L.M.; Wang, Y.N. Detection methods and applications of microfluidic paper-based analytical devices. Trends Anal. Chem. TrAC 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Lim, H.; Turab Jafry, A.; Lee, J. Fabrication, flow control, and applications of microfluidic paper-based analytical devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [Green Version]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in paper-based analytical devices. Ann. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [Green Version]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialization. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab. Chip 2020, 20, 9–34. [Google Scholar] [CrossRef]
- Ataide, V.N.; Mendes, L.F.; Gama, L.I.; de Araujo, W.R.; Paixao, T.R. Electrochemical paper-based analytical devices: Ten years of development. Anal. Methods 2020, 12, 1030–1054. [Google Scholar] [CrossRef]
- Mazurkiewicz, W.; Podrażka, M.; Jarosińska, E.; Kappalakandy Valapil, E.; Wiloch, M.; Jönsson-Niedziółka, M.; Witkowska Nery, E. Paper-based electrochemical sensors and how to make them (work). ChemElectroChem 2020, 7, 2939–2956. [Google Scholar] [CrossRef]
- Mettakoonpitak, J.; Boehle, K.; Nantaphol, S.; Teengam, P.; Adkins, J.A.; Srisa-Art, M.; Henry, C.S. Electrochemistry on paper-based analytical devices: A review. Electroanalysis 2016, 28, 1420–1436. [Google Scholar] [CrossRef]
- Dossi, N.; Petrazzi, S.; Toniolo, R.; Tubaro, F.; Terzi, F.; Piccin, E.; Svigelj, R.; Bontempelli, G. Digitally controlled procedure for assembling fully drawn paper-based electroanalytical platforms. Anal. Chem. 2017, 89, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- Ghaderinezhad, F.; Amin, R.; Temirel, M.; Yenilmez, B.; Wentworth, A.; Tasoglu, S. High-throughput rapid-prototyping of low-cost paper-based microfluidics. Sci. Rep. 2017, 7, 3553. [Google Scholar] [CrossRef]
- Amin, R.; Ghaderinezhad, F.; Li, L.; Lepowsky, E.; Yenilmez, B.; Knowlton, S.; Tasoglu, S. Continuous-ink, multiplexed pen-plotter approach for low-cost, high-throughput fabrication of paper-based microfluidics. Anal. Chem 2017, 89, 6351–6357. [Google Scholar] [CrossRef]
- Pagkali, V.; Stavra, E.; Soulis, D.; Ecomomou, A. Development of a high-throughput low-cost approach for fabricating fully drawn paper-based analytical devices using commercial writing tools. Chemosensors 2021, 9, 178. [Google Scholar] [CrossRef]
- Costa-Rama, E.; Fernández-Abedul, M.T. Paper-based screen-printed electrodes: A new generation of low-cost electroanalytical platforms. Biosensors 2021, 11, 51. [Google Scholar] [CrossRef]
- Zhu, C.C.; Bao, N.; Huo, X.L. Paper-based electroanalytical devices for stripping analysis of lead and cadmium in children’s shoes. RSC Adv. 2020, 10, 41482–41487. [Google Scholar] [CrossRef]
- Feng, Q.M.; Zhang, Q.; Shi, C.G.; Xu, J.J.; Bao, N.; Gu, H.Y. Using nanostructured conductive carbon tape modified with bismuth as the disposable working electrode for stripping analysis in paper-based analytical devices. Talanta 2013, 115, 235–240. [Google Scholar] [CrossRef]
- Bi, X.M.; Wang, H.R.; Ge, L.Q.; Zhou, D.M.; Xu, J.Z.; Gu, H.Y.; Bao, N. Gold-coated nanostructured carbon tape for rapid electrochemical detection of cadmium in rice with in situ electrodeposition of bismuthin paper-based analytical devices. Sens. Actuat. B 2018, 260, 475–479. [Google Scholar] [CrossRef]
- Pokpas, K.; Jahed, N.; Iwuoha, E. Tuneable, pre-stored paper-based electrochemical cells (μPECs): An adsorptive stripping voltammetric approach to metal analysis. Electrocatalysis 2019, 10, 352–364. [Google Scholar] [CrossRef]
- Nunez-Bajo, E.; Blanco-Lopez, M.C.; Costa-García, A.; Fernandez-Abedul, M.T. Electrogeneration of gold nanoparticles on porous-carbon paper-based electrodes and application to inorganic arsenic analysis in white wines by chronoamperometric stripping. Anal. Chem. 2017, 89, 6415–6423. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Calvo, A.; Blanco-López, M.C.; Costa-García, A. Paper-based working electrodes coated with mercury or bismuth films for heavy metals determination. Biosensors 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Ninwong, B.; Ratnarathorn, N.; Henry, C.S.; Mace, C.R.; Dungchai, W. Dual sample preconcentration for simultaneous quantification of metal ions using electrochemical and colorimetric assays. ACS Sens. 2020, 5, 3999–4008. [Google Scholar] [CrossRef] [PubMed]
- Thangphatthanarungruang, J.; Lomae, A.; Chailapakul, O.; Chaiyo, S.; Siangproh, W. A low-cost paper-based diamond electrode for trace copper analysis at on-site environmental area. Electroanalysis 2021, 33, 226–232. [Google Scholar] [CrossRef]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef]
- Nurak, T.; Praphairaksit, N.; Chailapakul, O. Fabrication of paper-baseddevices by lacquer spraying method for the determination of nickel (II) ion in wastewater. Talanta 2013, 114, 291–296. [Google Scholar] [CrossRef]
- Mettakoonpitak, J.; Volckens, J.; Henry, C.S. Janus electrochemical paper-based analytical devices for metals detection in aerosol samples. Anal. Chem. 2020, 92, 1439–1446. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Tong, J.; Guan, X.; Bian, C.; Xia, S. Paper-based sensor chip for heavy metal ion detection by SWSV. Micromachines 2018, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Pungjunun, K.; Nantaphol, S.; Praphairaksit, N.; Siangproh, W.; Chaiyo, S.; Chailapakul, O. Enhanced sensitivity and separation for simultaneous determination of tin and lead using paper-based sensors combined with a portable potentiostat. Sens. Actuat. B Chem. 2020, 318, 128241. [Google Scholar] [CrossRef]
- Cinti, S.; De Lellis, B.; Moscon, D.; Arduini, F. Sustainable monitoring of Zn (II) in biological fluids using office paper. Sens. Actuat. B 2017, 253, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Pokpas, K.; Jahed, N.; McDonald, E.; Bezuidenhout, P.; Smith, S.; Land, K.; Iwuoha, I. Graphene-AuNP enhanced inkjet-printed silver nanoparticle paper electrodes for the detection of nickel (II)-Dimethylglyoxime [Ni (DMGH2)] complexes by adsorptive cathodic stripping voltammetry (AdCSV). Electroanalysis 2020, 32, 3017–3031. [Google Scholar] [CrossRef]
- Ariño, C.; Serrano, N.; Díaz-Cruz, J.M.; Esteban, M. Voltammetric determination of metal ions beyond mercury electrodes. A review. Anal. Chim. Acta 2017, 990, 11–53. [Google Scholar] [CrossRef] [Green Version]
- Czop, E.; Economou, A.; Bobrowski, A. A study of in situ plated tin-film electrodes for the determination of trace metals by means of square-wave anodic stripping voltammetry. Electrochim. Acta 2011, 56, 2206–2212. [Google Scholar] [CrossRef]
- The Drinking Water Directive. Available online: https://ec.europa.eu/environment/water/water-drink/legislation_en.html (accessed on 10 October 2021).
- Safe Drinking Water Act. Available online: https://www.epa.gov/sdwa (accessed on 10 October 2021).
- Kokkinos, C.; Raptis, I.; Economou, A.; Speliotis, T. Determination of trace Tl (I) by anodic stripping voltammetry on novel disposable microfabricated bismuth-film sensors. Electroanalysis 2010, 22, 2359–2365. [Google Scholar] [CrossRef]





| Title 1 | Cd(II) | Pb(II) | ||
|---|---|---|---|---|
| DP | SW | DP | SW | |
| Slope ± SD (μA μg−1 L) | 1.13 × 10−2 ± 2.3 × 10−4 | 1.96 × 10−2 ± 4.3 × 10−4 | 6.1 × 10−3 ± 2.3 × 10−4 | 1.63 × 10−2 ± 6.4 × 10−4 |
| Intercept ± SD (μA) | 2.0 × 10−2 ± 8.1 × 10−3 | 4.2 × 10−2 ± 1.6 × 10−2 | 5.5 × 10−3 ± 8.3 × 10−3 | 3.5 × 10−2 ± 2.1 × 10−2 |
| R2 | 0.996 | 0.996 | 0.994 | 0.997 |
| LOD (μg L−1) 1 | 2.4 | 2.7 | 4.5 | 4.2 |
| LOQ (μg L−1) 2 | 7.1 | 8.1 | 13.5 | 12.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soulis, D.; Trachioti, M.; Kokkinos, C.; Economou, A.; Prodromidis, M. Single-Use Fluidic Electrochemical Paper-Based Analytical Devices Fabricated by Pen Plotting and Screen-Printing for On-Site Rapid Voltammetric Monitoring of Pb(II) and Cd(II). Sensors 2021, 21, 6908. https://doi.org/10.3390/s21206908
Soulis D, Trachioti M, Kokkinos C, Economou A, Prodromidis M. Single-Use Fluidic Electrochemical Paper-Based Analytical Devices Fabricated by Pen Plotting and Screen-Printing for On-Site Rapid Voltammetric Monitoring of Pb(II) and Cd(II). Sensors. 2021; 21(20):6908. https://doi.org/10.3390/s21206908
Chicago/Turabian StyleSoulis, Dionysios, Maria Trachioti, Christos Kokkinos, Anastasios Economou, and Mamas Prodromidis. 2021. "Single-Use Fluidic Electrochemical Paper-Based Analytical Devices Fabricated by Pen Plotting and Screen-Printing for On-Site Rapid Voltammetric Monitoring of Pb(II) and Cd(II)" Sensors 21, no. 20: 6908. https://doi.org/10.3390/s21206908
APA StyleSoulis, D., Trachioti, M., Kokkinos, C., Economou, A., & Prodromidis, M. (2021). Single-Use Fluidic Electrochemical Paper-Based Analytical Devices Fabricated by Pen Plotting and Screen-Printing for On-Site Rapid Voltammetric Monitoring of Pb(II) and Cd(II). Sensors, 21(20), 6908. https://doi.org/10.3390/s21206908









