Electrogastrography in Autonomous Vehicles—An Objective Method for Assessment of Motion Sickness in Simulated Driving Environments
Abstract
1. Introduction
- it uses the improved (compact printed circuit board) version of the same EGG measuring device;
- it takes place in simulated fully autonomous vehicle (using VR);
- it correlates EGG measurements with subjectively reported sickness (through button presses) and additional physiological responses (galvanic skin response—GSR);
- it investigates also the impact of different driving environments on perceived nausea and sickness
- Does driving environment affect the perceived motion sickness?
- Do EGG measurements correlate with subjectively reported nausea onsets?
- Do two different types of self-reported data correlate (questionnaires vs. frequency of reported nausea onsets through button presses)?
2. Materials and Methods
2.1. Study Design
- EGG:
- ○
- RMS—root mean square value of the signal,
- ○
- MF—median frequency of the signal,
- ○
- MFM—maximum magnitude of power spectrum density,
- ○
- DF—dominant frequency (location of MFM),
- ○
- CF—Crest factor of Power Spectrum Density,
- ○
- FSD—Percentage of PSD that has higher value than MFM/4,
- ○
- amount of time with amplitude increase (Trial 2 only),
- ○
- increase in RMS value of the signal segment with an amplitude increase relative to baseline RMS value (Trial 2 only),
- GSR (galvanic skin response):
- ○
- mean,
- ○
- standard deviation,
- HR (heart rate):
- ○
- mean,
- ○
- standard deviation,
- Subjective assessment methods:
- ○
- number of nausea onsets (by pressing the button, Trial 2 only),
- ○
- SSQ nausea score.
2.2. Driving Environment
- entering a village—from 90 km/h to 50 km/h,
- child running across the road—from 50 km/h to 0 km/h,
- exiting a village—from 50 km/h to 90 km/h.
2.3. Data Acquisition
2.4. Participants
2.5. Tasks
“Although this is a level 5 AV, you should be aware of the driving environment and traffic around you at all times. Therefore, please count how many red vehicles will you see in the simulation, on the road or on the billboards.”
2.6. Experiment Procedure
2.7. Data Processing
3. Results
3.1. Analysis of before, during and after Effects
3.2. Impact of Driving Environment
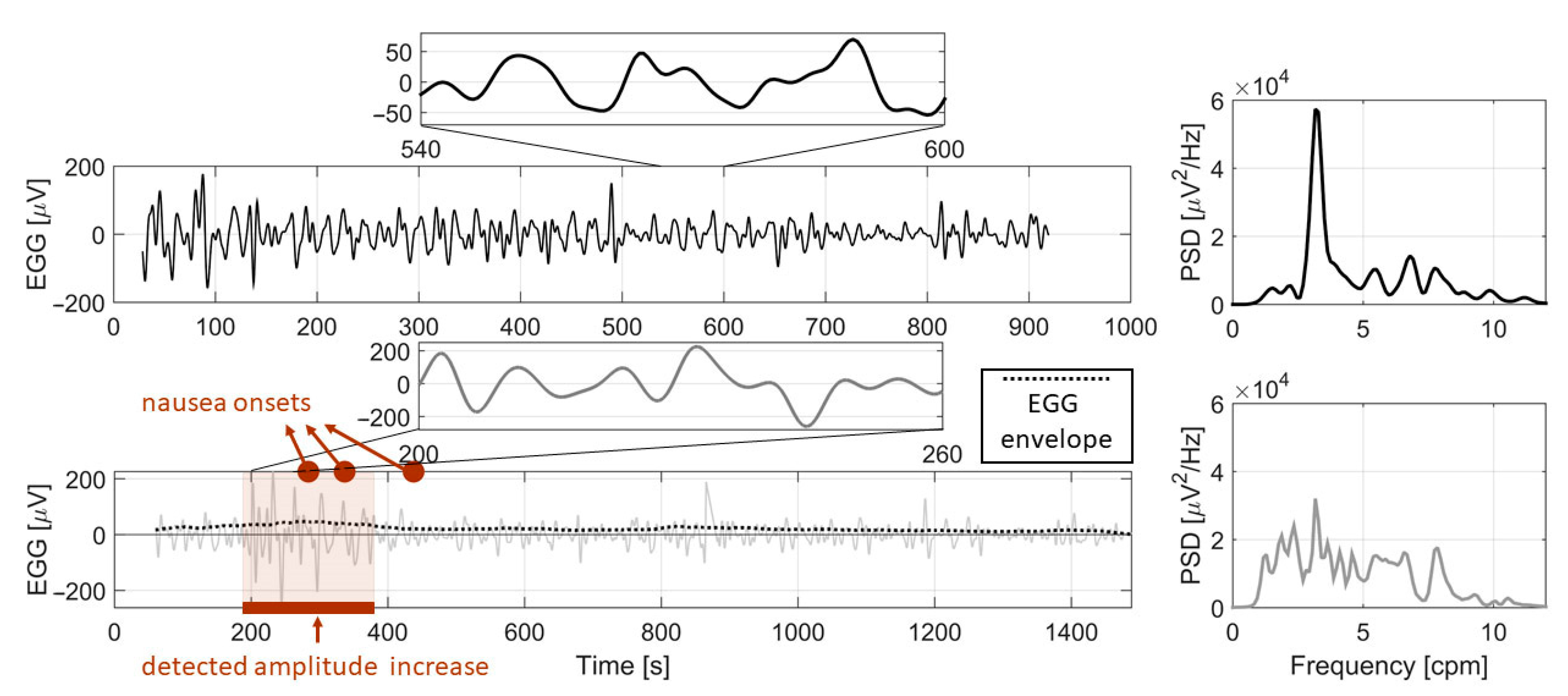
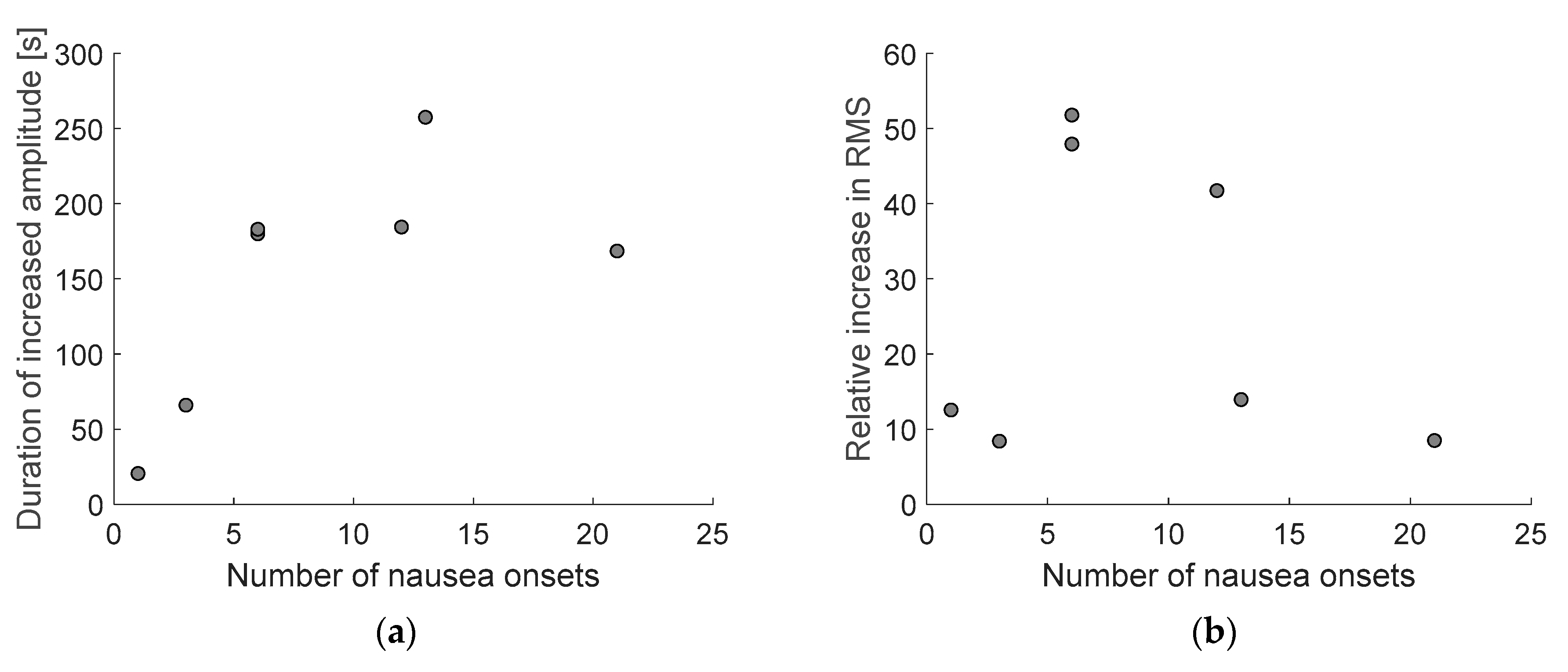
3.3. Nausea Onset vs. Amplitude Increase Analysis
3.4. Subjective Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Probst, T.; Krafczyk, S.; Brandt, T. Visual prevention from motion sickness in cars. Arch. Psychiatr. Nervenkr. 1982, 231, 409–421. [Google Scholar] [CrossRef]
- Guedry, F. Factors Influencing Susceptibility: Individual Differences and Human Factors. In Motion Sickness: Significance in Aerospace Operations and Prophylaxis; AGARD: Neuilly Sur Seine, France, 1991; p. 18. [Google Scholar]
- Sivak, M.; Schoettle, B. Motion Sickness in Self-Driving Vehicles; University of Michigan, Ann Arbor, Transportation Research Institute: Ann Arbor, MI, USA, 2015. [Google Scholar]
- Iskander, J.; Attia, M.; Saleh, K.; Nahavandi, D.; Abobakr, A.; Mohamed, S.; Asadi, H.; Hossny, M.; Lim, C.P.; Hossny, M. From car sickness to autonomous car sickness: A review. Transp. Res. Part F Traffic Psychol. Behav. 2019, 62, 716–726. [Google Scholar] [CrossRef]
- Mühlbacher, D.; Tomzig, M.; Reinmueller, K.; Rittger, L. Methodological Considerations Concerning Motion Sickness Investigations during Automated Driving. Information 2020, 11, 265. [Google Scholar] [CrossRef]
- De Winkel, K.N.; Pretto, P.; Nooij, S.A.; Cohen, I.; Bülthoff, H.H. Efficacy of augmented visual environments for reducing sickness in autonomous vehicles. Appl. Ergon. 2021, 90, 103282. [Google Scholar] [CrossRef]
- Diels, C.; Bos, J.E. Self-driving carsickness. Appl. Ergon. 2016, 53, 374–382. [Google Scholar] [CrossRef]
- Berman, B. Avoiding Carsickness When the Cars Drive Themselves. The New York Times, 21 January 2020. [Google Scholar]
- Saruchi, S.A.; Ariff, M.H.M.; Zamzuri, H.; Amer, N.H.; Wahid, N.; Hassan, N.; Kadir, Z.A. Lateral control strategy based on head movement responses for motion sickness mitigation in autonomous vehicle. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Saruchi, S.A.; Ariff, M.H.M.; Zamzuri, H.; Amer, N.H.; Wahid, N.; Hassan, N.; Abu Kassim, K.A. Novel Motion Sickness Minimization Control via Fuzzy-PID Controller for Autonomous Vehicle. Appl. Sci. 2020, 10, 4769. [Google Scholar] [CrossRef]
- Reason, J.T. Motion Sickness Adaptation: A Neural Mismatch Model. J. R. Soc. Med. 1978, 71, 819–829. [Google Scholar] [CrossRef]
- Koohestani, A.; Nahavandi, D.; Asadi, H.; Kebria, P.M.; Khosravi, A.; Alizadehsani, R.; Nahavandi, S. A Knowledge Discovery in Motion Sickness: A Comprehensive Literature Review. IEEE Access 2019, 7, 85755–85770. [Google Scholar] [CrossRef]
- Laviola, J.J. A discussion of cybersickness in virtual environments. ACM SIGCHI Bull. 2000, 32, 47–56. [Google Scholar] [CrossRef]
- Crampton, G.H. Motion and Space Sickness; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-0-8493-4703-0. [Google Scholar]
- Golding, J.F. Motion sickness. In Handbook of Clinical Neurology; Furman, J.M., Lempert, T., Eds.; Neuro-Otology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 371–390. [Google Scholar]
- Kennedy, R.S.; Graybiel, A. The Dial Test: A Standardized Procedure for the Experimental Production of Canal Sickness Symptomatology in a Rotating Environment; U.S. Naval School of Aviation Medicine, U.S. Naval Aviation Medical Center: Pensacola, FL, USA, 1965. [Google Scholar]
- Kennedy, R.S. Motion sickness questionnaire and field independence scores as predictors of success in naval aviation training. Aviat. Space Environ. Med. 1975, 46, 1349–1352. [Google Scholar]
- Kennedy, R.S.; Lane, N.E.; Berbaum, K.S.; Lilienthal, M.G. Simulator Sickness Questionnaire: An Enhanced Method for Quantifying Simulator Sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Keshavarz, B.; Hecht, H. Validating an Efficient Method to Quantify Motion Sickness. Hum. Factors J. Hum. Factors Ergon. Soc. 2011, 53, 415–426. [Google Scholar] [CrossRef]
- Golding, J.F. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res. Bull. 1998, 47, 507–516. [Google Scholar] [CrossRef]
- Gable, T.M.; Walker, B.N. Georgia Tech Simulator Sickness Screening Protocol; Georgia Institute of Technology: Atlanta, GA, USA, 2013. [Google Scholar]
- Doweck, I.; Gordon, C.R.; Shlitner, A.; Spitzer, O.; Gonen, A.; Binah, O.; Melamed, Y.; Shupak, A. Alterations in R–R variability associated with experimental motion sickness. J. Auton. Nerv. Syst. 1997, 67, 31–37. [Google Scholar] [CrossRef]
- Yokota, Y.; Aoki, M.; Mizuta, K.; Ito, Y.; Isu, N. Motion sickness susceptibility associated with visually induced postural instability and cardiac autonomic responses in healthy subjects. Acta Oto-Laryngol. 2005, 125, 280–285. [Google Scholar] [CrossRef]
- Cevette, M.J.; Pradhan, G.N.; Cocco, D.; Crowell, M.D.; Galea, A.M.; Bartlett, J.; Stepanek, J. Electrogastrographic and autonomic responses during oculovestibular recoupling in flight simulation. Aviat. Space Environ. Med. 2014, 85, 15–24. [Google Scholar] [CrossRef]
- Nalivaiko, E.; Rudd, J.A.; So, R.H.Y. Motion sickness, nausea and thermoregulation: The “toxic” hypothesis. Temperature 2014, 1, 164–171. [Google Scholar] [CrossRef]
- Golding, J.F. Phasic skin conductance activity and motion sickness. Aviat. Space Environ. Med. 1992, 63, 165–171. [Google Scholar] [PubMed]
- Hu, S.; Grant, W.F.; Stern, R.M.; Koch, K.L. Motion sickness severity and physiological correlates during repeated exposures to a rotating optokinetic drum. Aviat. Space Environ. Med. 1991, 62, 308–314. [Google Scholar]
- Stern, R.M.; Koch, K.L.; Stewart, W.R.; Lindblad, I.M. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology 1987, 92, 92–97. [Google Scholar] [CrossRef]
- Money, K.E. Motion sickness. Physiol. Rev. 1970, 50, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Harm, D.L. Physiology of Motion Sickness Symptoms. In Motion and Space Sickness; CRC Press: Boca Raton, FL, USA, 1990; pp. 154–177. [Google Scholar]
- Cheung, B.; Vaitkus, P. Perspectives of electrogastrography and motion sickness. Brain Res. Bull. 1998, 47, 421–431. [Google Scholar] [CrossRef]
- Popović, N.; Miljković, N.; Stojmenova, K.; Jakus, G.; Prodanov, M.; Sodnik, J. Lessons Learned: Gastric Motility Assessment During Driving Simulation. Sensors 2019, 19, 3175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; McCallum, R.W. Electrogastrography: Measuremnt, analysis and prospective applications. Med Biol. Eng. Comput. 1991, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Lerman, I.; Bu, T.; Huang, M.; Wu, V.; Coleman, T. Non-invasive Targeted Gastric Vagal Complex Stimulation: Preliminary in Human Cutaneous Measures of Gastric Slow Wave Function. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.D.Z. Electrogastrography: Methodology, Validation and Applications. J. Neurogastroenterol. Motil. 2013, 19, 5–17. [Google Scholar] [CrossRef]
- Alvarez, W.C. The Electrogastrogram and What It Shows. JAMA 1922, 78, 1116–1119. [Google Scholar]
- Chen, J.D.; McCallum, R.W. Clinical applications of electrogastrography. Am. J. Gastroenterol. 1993, 88, 88. [Google Scholar]
- Parkman, H.P.; Hasler, W.L.; Barnett, J.L.; Eaker, E.Y. Electrogastrography: A document prepared by the gastric section of the American Motility Society Clinical GI Motility Testing Task Force. Neurogastroenterol. Motil. 2003, 15, 89–102. [Google Scholar] [CrossRef]
- Oh, S.; Kim, E.-K.; Neu, J. Chapter 21—Technologies for the Evaluation of Enteral Feeding Readiness in Premature Infants. In Gastroenterology and Nutrition: Neonatology Questions and Controversies, 2nd ed.; Neu, J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 339–347. ISBN 978-1-4377-2603-9. [Google Scholar]
- Friesen, C.; Lin, Z.; Hyman, P.E.; Andre, L.; Welchert, E.; Schurman, J.V.; Cocjin, J.T.; Burchell, N.; Pulliam, S.; Moore, A.; et al. Electrogastrography in Pediatric Functional Dyspepsia. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Breen, V.; Kasabov, N.; Du, P.; Calder, S. A Spiking Neural Network for Personalised Modelling of Electrogastrography (EGG). In Proceedings of the Artificial Neural Networks in Pattern Recognition, Ulm, Germany, 28–30 September 2016; Schwenker, F., Abbas, H.M., El Gayar, N., Trentin, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 18–25. [Google Scholar]
- Chen, J.; Pasricha, P.J.; Pasricha, T. Lie Detection via Electrogastrography. U.S. Patent No. 7,967,750, 28 June 2011. [Google Scholar]
- Dennison, M.S.; Wisti, A.Z.; D’Zmura, M. Use of physiological signals to predict cybersickness. Displays 2016, 44, 42–52. [Google Scholar] [CrossRef]
- Poscente, M.D.; Mintchev, M.P. Enhanced electrogastrography: A realistic way to salvage a promise that was never kept? World J. Gastroenterol. 2017, 23, 4517–4528. [Google Scholar] [CrossRef] [PubMed]
- Calder, S.S.S.; O’Grady, G.; Cheng, L.K.; Du, P. Torso-Tank Validation of High-Resolution Electrogastrography (EGG): Forward Modelling, Methodology and Results. Ann. Biomed. Eng. 2018, 46, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, G.; Du, P.; Cheng, L.K.; Egbuji, J.U.; Lammers, W.J.E.P.; Windsor, J.A.; Pullan, A.J. Origin and propagation of human gastric slow-wave activity defined by high-resolution mapping. Am. J. Physiol. Liver Physiol. 2010, 299, G585–G592. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Russo, F.; Indrio, F. Electrogastrography in Adults and Children: The Strength, Pitfalls, and Clinical Significance of the Cutaneous Recording of the Gastric Electrical Activity. BioMed Res. Int. 2013, 2013, 282757. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.; Steffen, R. 5-Physiology of Gastrointestinal Motility. In Pediatric Gastrointestinal and Liver Disease, 4th ed.; Wyllie, R., Hyams, J.S., Eds.; W.B. Saunders: Saint Louis, MI, USA, 2011; pp. 39–49.e2. ISBN 978-1-4377-0774-8. [Google Scholar]
- Sk, C. Electrogastrography in Cyclic Vomiting Syndrome. Dig. Dis. Sci. 1999, 44, 64S–73S. [Google Scholar]
- Tokumaru, O.; Mizumoto, C.; Takada, Y.; Tatsuno, J.; Ashida, H. Vector analysis of electrogastrography during motion sickness. Dig. Dis. Sci. 2003, 48, 498–507. [Google Scholar] [CrossRef]
- Rhee, P.-L.; Lee, J.Y.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Kim, S.; Koh, S.D.; Hwang, S.J.; Sanders, K.M.; Ward, S.M. Analysis of pacemaker activity in the human stomach. J. Physiol. 2011, 589, 6105–6118. [Google Scholar] [CrossRef]
- Himia, N.; Koga, T.; Nakamura, E.; Kobashi, M.; Yamane, M.; Tsujioka, K. Differences in autonomic responses between subjects with and without nausea while watching an irregularly oscillating video. Auton. Neurosci. 2004, 116, 46–53. [Google Scholar] [CrossRef]
- Lien, H.-C.; Sun, W.M.; Chen, Y.-H.; Kim, H.; Hasler, W.; Owyang, C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am. J. Physiol. Liver Physiol. 2003, 284, G481–G489. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.L. Gastric dysrhythmias: A potential objective measure of nausea. Exp. Brain Res. 2014, 232, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, H.T.; Yoo, B. Virtual Reality Sickness: A Review of Causes and Measurements. Int. J. Hum.-Comput. Interact. 2020, 36, 1658–1682. [Google Scholar] [CrossRef]
- Vujic, A.; Tong, S.; Picard, R.; Maes, P. Going with Our Guts: Potentials of Wearable Electrogastrography (EGG) for Affect Detection. In Proceedings of the 2020 International Conference on Multimodal Interaction, Virtual Event, The Netherlands, 21 October 2020; Association for Computing Machinery: New York, NY, USA, 2020; pp. 260–268. [Google Scholar]
- Miljković, N.; Popović, N.B.; Prodanov, M.; Sodnik, J. Assessment of Sickness in Virtual Environments. In Proceedings of the 9th International Conference on Information Society and Technology (ICIST 2019), Kopaonik, Serbia, 10–13 March 2019. [Google Scholar]
- Popović, N.; Miljković, N.; Popović, M.B. Simple gastric motility assessment method with a single-channel electrogastrogram. Biomed. Tech. Eng. 2018, 64, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Bellem, H.; Klüver, M.; Schrauf, M.; Schöner, H.-P.; Hecht, H.; Krems, J.F. Can We Study Autonomous Driving Comfort in Moving-Base Driving Simulators? A Validation Study. Hum. Factors J. Hum. Factors Ergon. Soc. 2016, 59, 442–456. [Google Scholar] [CrossRef]
- Kuiper, O.X.; Bos, J.E.; Diels, C.; Cammaerts, K. Moving base driving simulators’ potential for carsickness research. Appl. Ergon. 2019, 81, 102889. [Google Scholar] [CrossRef]
- Nervtech Simuation Technologies. Available online: https://www.nervtech.com (accessed on 29 April 2019).
- Fanatec ClubSport Pedals V3. Available online: https://fanatec.com/eu-en/pedals/clubsport-pedals-v3 (accessed on 12 August 2020).
- Fanatec ClubSport Wheel Base V2.5. Available online: https://fanatec.com/eu-en/racing-wheels-wheel-bases/wheel-bases/clubsport-wheel-base-v2.5 (accessed on 12 August 2020).
- Oculus Rift Oculus. Available online: https://www.oculus.com/rift/ (accessed on 12 August 2020).
- AVSimulation SCANeR Studio. Available online: https://www.avsimulation.com/scanerstudio/ (accessed on 12 August 2020).
- SAE International Releases Updated Visual Chart for Its “Levels of Driving Automation” Standard for Self-Driving Vehicles. Available online: https://www.sae.org/ (accessed on 19 July 2019).
- Universal Interface Module|UIM100C|Research|BIOPAC. BIOPAC Systems, Inc. Available online: https://www.biopac.com/product/universal-interface-module/ (accessed on 13 May 2019).
- E4 Wristband|Real-Time Physiological Signals|Wearable PPG, EDA, Temperature, Motion Sensors. Available online: https://www.empatica.com/research/e4 (accessed on 12 August 2020).
- Gruden, T.; Stojmenova, K.; Sodnik, J.; Jakus, G. Assessing Drivers’ Physiological Responses Using Consumer Grade Devices. Appl. Sci. 2019, 9, 5353. [Google Scholar] [CrossRef]
- Matlab—MathWorks. Available online: https://www.mathworks.com/products/matlab.html (accessed on 10 September 2020).
- Geldof, H.; Van Der Schee, E.J.; Van Blankenstein, M.; Grashuis, J.L. Electrogastrographic study of gastric myoelectrical activity in patients with unexplained nausea and vomiting. Gut 1986, 27, 799–808. [Google Scholar] [CrossRef]
- Curilem, M.; Ulloa, S.; Flores, M.; Zanelli, C.; Chacón, M. Recognition of Nausea Patterns by Multichannel Electrogastrography. In Pattern Recognition Techniques Applied to Biomedical Problems; Ortiz-Posadas, M.R., Ed.; STEAM-H: Science, Technology, Engineering, Agriculture, Mathematics & Health; Springer International Publishing: Cham, Switzerland, 2020; pp. 91–108. ISBN 978-3-030-38021-2. [Google Scholar]
- McClure, J.A.; Fregly, A.R.; Molina, E.; Graybiel, A. Response from Arousal and Thermal Sweat Areas During Motion Sickness; Naval Aerospace Medical Research Laboratory, Naval Aerospace Medical Institute, Naval Aerospace and Regional Medical Center: Pensacola, FL, USA, 1971. [Google Scholar]





| Activity | Duration (min) |
|---|---|
| Introduction | |
| Brief overview of: - the purpose of experiment - experiment procedure - participant’s tasks | 2 |
| Sign a consent form | 1 |
| Place EGG electrodes (to establish stable impedance) and sensors | 3 |
| Questionnaire 1: demographic data and anthropometric characteristics | 2 |
| Questionnaire 2a: Simulator Sickness Pre-questionnaire | 2 |
| Questionnaire 3a: Georgia Tech Simulator Sickness Screening Protocol Pre-questionnaire | 2 |
| Verification of the measuring system | 4 |
| Total | 16 |
| Experiment | |
| Baseline measurement (Trial 1) | 15 |
| Test drive in the simulated AV using VR. | 5 |
| Questionnaire 3b: Georgia Tech Simulator Sickness Screening Protocol Post-questionnaire | 2 |
| AV simulation (Trial 2) | 15 |
| Resting measurement (Trial 3) | 15 |
| Total | 52 |
| Post study | |
| Questionnaire 2b: Simulator Sickness Post-questionnaire | 2 |
| Detaching EGG electrodes and sensors | 1 |
| Total | 3 |
| Total session duration | 71 min |
| Parameter Set | Parameter | Expected Trend | Reference | Resulting Trend |
|---|---|---|---|---|
| EGG | RMS | Increase | [32,58] | Increase, not significant |
| MF | Increase | [31] | Increase, not significant | |
| MFM | Increase | [57] | Increase, not significant | |
| DF | Increase | [31] | Increase, significant with p < 0.1 | |
| CF | Decrease | [57] | Decrease, significant | |
| FSD | Increase | [57] | Increase, significant | |
| GSR | Mean | Increase | [26] | Increase, significant |
| St. dev. | Increase | [27] | Increase, not significant | |
| HR | Mean | Increase | [27] | Increase, significant * |
| St. dev. | Increase | [27] | Increase, not significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruden, T.; Popović, N.B.; Stojmenova, K.; Jakus, G.; Miljković, N.; Tomažič, S.; Sodnik, J. Electrogastrography in Autonomous Vehicles—An Objective Method for Assessment of Motion Sickness in Simulated Driving Environments. Sensors 2021, 21, 550. https://doi.org/10.3390/s21020550
Gruden T, Popović NB, Stojmenova K, Jakus G, Miljković N, Tomažič S, Sodnik J. Electrogastrography in Autonomous Vehicles—An Objective Method for Assessment of Motion Sickness in Simulated Driving Environments. Sensors. 2021; 21(2):550. https://doi.org/10.3390/s21020550
Chicago/Turabian StyleGruden, Timotej, Nenad B. Popović, Kristina Stojmenova, Grega Jakus, Nadica Miljković, Sašo Tomažič, and Jaka Sodnik. 2021. "Electrogastrography in Autonomous Vehicles—An Objective Method for Assessment of Motion Sickness in Simulated Driving Environments" Sensors 21, no. 2: 550. https://doi.org/10.3390/s21020550
APA StyleGruden, T., Popović, N. B., Stojmenova, K., Jakus, G., Miljković, N., Tomažič, S., & Sodnik, J. (2021). Electrogastrography in Autonomous Vehicles—An Objective Method for Assessment of Motion Sickness in Simulated Driving Environments. Sensors, 21(2), 550. https://doi.org/10.3390/s21020550







